Abstract
The worldwide saline-affected area is expanding day by day, and soil salinity restricts crop development and productivity, including rice. Considering this, the current study explored the response of gallic acid (GA) in conferring salinity tolerance in rice seedlings. Fourteen-day-old rice (Oryza sativa L. cv. BRRI dhan52) seedlings were treated with 200 mM NaCl alone or combined with 1 mM GA. Salt stress resulted in osmotic, ionic, and oxidative stress in rice seedlings. Osmotic stress increased proline accumulation and osmotic potential, which decreased the relative water content, chlorophyll contents, and dry weight. Ionic stress interrupted ion homeostasis by Na+ accumulation and K+ leakage. Osmotic and ionic stress, concomitantly, disrupted antioxidant defense and glyoxalase systems by higher production of reactive oxygen species (ROS) and methylglyoxal (MG), respectively. It resulted in oxidative damage indicated by the high amount of malondialdehyde (MDA). The supplementation of GA in salt-treated rice seedlings partially recovered salt-induced damages by improving osmotic and ionic homeostasis by increasing water balance and decreasing Na+ content and Na+/K+ ratio. Supplemental GA enhanced the antioxidant defense system in salt-treated rice seedlings by increasing ascorbate (AsA), glutathione (GSH), and phenolic compounds and the activities of AsA-GSH cycle enzymes, including monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), and glutathione reductase (GR) enzymes that accelerated ROS detoxification and decreased oxidative damage. Gallic acid also enhanced the detoxification of MG by triggering glyoxalase enzyme activities in salt-treated rice seedlings. The present findings elucidated that supplemental GA reversed salt-induced damage in rice seedlings through improving osmotic and ionic homeostasis and upregulating the ROS and MG detoxification system.
1. Introduction
Soil salinization is one of the most significant environmental challenges, threatening global agricultural productivity and food sustainability [1]. The alarming point is that soil salinization is increasing daily, and around 20–50% of global irrigated land is already salt-affected [2]. It is also assumed that about half of the agricultural land will be salt affected by 2050 [3]. So, the production of the crop in saline soil is a major challenge to maintaining agricultural sustainability as maximum crops are sensitive to salt. Considering the tolerance ability, rice is also considered as salt-sensitive crop, as growth and yield are negatively affected by salinity [3,4,5]. However, the negative effect of salinity on plants exists from seedling emergence to death. Salinity limits growth and productivity and even causes the death of plants by disturbing the physiological and molecular features of the plant [4,5,6]. The primary response of salinity on the plant is osmotic stress and ionic toxicity. Osmotic stress is very much immediate that hinders water uptake due to stomatal closure and disrupts osmotic balance, which subsequently reduces cell division and expansion [6,7]. Salinity also induces physiological drought within the plants, reduces photosynthetic efficiency by degrading photosynthetic pigments, and decreases CO2 assimilation, which enhances the generation of reactive oxygen species (ROS) [8]. Ionic toxicity is a secondary effect of salinity in plants and is slower compared with osmotic stress. Ionic stress is induced in plants through higher Na+ and Cl− uptake that results in mineral imbalance. Salinity disturbs the translocation of solutes, causes photosynthesis inhibition, and many other physiological and metabolic dysfunction [3]. A higher amount of salt in plant growth medium results in higher Na+ uptake and K+ efflux by depolarizing root plasma membrane that disrupts ion homeostasis [9,10] and alters redox homeostasis, which also leads to higher production of ROS [3]. However, salinity-induced osmotic and ionic stress disrupt plant metabolism and many other physiological and biochemical processes that trigger ROS production [6,11,12]. The overproduced ROS disrupts the equilibrium between ROS and ROS, detoxifying antioxidant defense system and leading to oxidative stress. Oxidative stress limits the growth and productivity of plants and even causes death through lipid peroxidation, damaging proteins and altering carbohydrate metabolism [6,13]. In addition, methylglyoxal (MG), a cytotoxic compound, degrades protein synthesis and causes oxidative stress under abiotic stress, including salinity [14].
However, plants can overcome oxidative damage by detoxifying ROS and MG by utilizing their natural antioxidant defense and glyoxalase systems, respectively [15]. The antioxidant defense system comprises non-enzymatic components, including ascorbate (AsA), glutathione (GSH), phenolic compounds, flavonoids, alkaloids, and enzymatic components, including catalase, ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), glutathione reductase (GR), that play a role in detoxifying ROS [6]. The glyoxalase system also works in the detoxification of MG through the activities of glyoxalase I (Gly I) and glyoxalase II (Gly II) [15].
So, enhancing antioxidant defense and the glyoxalase system by reducing ROS production are important mechanisms to improve oxidative stress tolerance [6,15]. Improving ion homeostasis by reducing Na+ uptake and increasing K+ accumulation through deactivating guard cell outward rectifying potassium channels (GORK) is also a salinity tolerance mechanism [16]. Detoxification of ROS can play a role in ion homeostasis by deactivating non-selective cationic channels (NSCC), which is a pathway of Na+ influx. Overproduced ROS can activate NSCC, which promotes Na+ accumulation and disrupt ion homeostasis [9].
Gallic acid (GA), a component of phenolic compounds, plays an effective role in scavenging ROS by regulating the antioxidant defense system in rice [17] and wheat [18] under abiotic stress conditions. Gallic acid also plays role in developing resistance against pests, such as filbert aphid in hazel [19]. However, external application of GA partially overcomes oxidative stress in the plant by scavenging ROS through the enhanced antioxidant defense system [20]. Ion homeostasis, ROS detoxification, and osmotic balance under salt stress were attained by supplemental GA application in Lepidium sativum [21]. Moreover, the response of the glyoxalase system and coordination of ion homeostasis, antioxidant defense, and glyoxalase system under GA supplementation has not yet been studied. Considering the potential role of GA, the current study was carried out to assess the role of supplemental GA in conferring salt stress tolerance by upregulating antioxidant defense, glyoxalase system, and ion homeostasis in rice seedlings.
2. Materials and Methods
2.1. Plant Materials and Treatments
Healthy and sterilized rice (Oryza sativa L. cv. BRRI dhan52) seeds were soaked in double distilled water for imbibition. The soaked seeds were kept in an incubator for 2 days, maintaining the temperature of 28 ± 2 °C. The imbibed seeds were washed thoroughly in distilled water, sown on a plastic net floated on a plastic pot having 250 mL distilled water, and kept in the same place for 3 days. After keeping 60 germinated seeds, plastic pots were transferred to the cultivation chamber (light, 350 µmol photons m−2 s−1; temperature, 25 ± 2 °C; relative humidity, 65–70%), providing 7500 times diluted commercial hydroponic nutrient solution (Hyponex, Osaka, Japan). The nutrient solutions were renewed twice every week. NaCl (200 mM) were applied to 14-day-old rice seedlings with or without 1 mM gallic acid (C7H6O5). Control seedlings were exposed to nutrient solution only. Data were measured and recorded after 3 days of treatment. The experiment was replicated thrice under the same condition. All chemicals were procured from Fujifilm, Tokyo, Japan, unless it was stated otherwise.
2.2. Measurement of Leaf Relative Water Content
The relative water content (RWC) of the leaf was measured as per Barrs and Weatherly [22] by weighing fresh weight (FW), dry weight (DW), and turgid weight (TW). Immediately after the collection of leaves, FW was measured and then immersed in distilled water for 24 h to measure TW. The turgid leaves were oven dried at 70 °C for 48 h to determine DW. However, the RWC of the leaf was measured as per the following equation:
RWC (%) = (FW − DW)/(TW − DW) × 100
2.3. Measurement of Proline Content and Osmotic Potential
Proline (Pro) content was measured as per Bates et al. [23] by homogenizing fresh leaves in 3% sulfosalicylic acid. The homogenized leaf sample was centrifuged for 12 min at 11,500× g. The collected supernatant was incubated at 100 °C for 1 h using acid ninhydrin and glacial acetic acid. After cooling the incubated mixture in ice, the developed color was extracted by toluene to observe the optical density spectrophotometrically at 520 nm.
The osmotic potential was determined as per Ozfidan-Konakci et al. [17] with a modification using K-7400 semi-micro osmometer. Ground fresh leaves were centrifuged twice for 10 min at 12,000× g. Osmolarity was determined from collected supernatant and converted to osmotic potential by Van’t Hoff equation as per the following
ΨΠ (MPa) = −c (mOsmol kg−1) × 2.58 × 10−3
2.4. Measurement of Chlorophyll Content
Chlorophyll (chl) content was determined by following the method of Arnon [24]. Fresh leaves were ground with 80% acetone and centrifuged at 9000× g for 10 min. Chlorophyll a, chl b and carotenoid content were measured spectrophotometrically at wavelengths of 663, 645, and 470 nm, respectively.
2.5. Measurement of Na+ and K+ Content
Sodium and K+ ion contents were measured by an atomic absorption spectrophotometer (Hitachi Z-5000; Tokyo, Japan). After oven drying, the dry roots and shoots were digested in a mixture of nitric acid and perchloric acid. The digested samples were used for the spectrophotometric determination of Na+ and K+ content.
2.6. Histochemical Detection of O2•− Generation in the Leaves
Localization of O2•− in the leaves was identified with a modification by Chen et al. [25]. Fresh leaves were incubated at room temperature for 12 h after dipping in 0.1% Nitroblue tetrazolium chloride (NBT) solution. The incubated leaves were decolorized by dipping them in boiling ethanol to reveal the dark blue spots produced by the reaction of NBT and O2•−.
2.7. Measurement of Lipid Peroxidation and Hydrogen Peroxide Levels
The level of lipid peroxidation was determined according to Heath and Packer [26] by estimating malondialdehyde (MDA) content. Malondialdehyde content was determined using an extinction coefficient of 155 mM−1 cm−1 and observing the difference in absorbance at 532 nm.
Hydrogen peroxide (H2O2) content was measured following Yu et al. [27] using an extinction coefficient of 0.28 µM−1 cm−1 by observing the absorbance at 410 nm.
2.8. Measurement of Phenolic Compounds
According to Ashraf et al. [28], total phenol contents were measured by homogenizing leaf samples in acetone. The homogenized leaf samples were centrifuged at 10,000× g for 10 min, and supernatants were mixed with Folin phenol reagent and Na2CO3. Total phenolic content was measured spectrophotometrically at 750 nm wavelength by comparing it with a gallic acid standard.
Flavonoid content was determined with a modification of Zhishen et al. [29]. Fresh leaf samples were homogenated in aqueous ethanol and centrifuged at 10,000× g for 20 min at room temperature. The diluted supernatant was mixed with NaNO2 and incubated after mixing with AlCl3. Finally, incubated mixture was diluted with distilled water and 1 M NaOH. Subsequently, after vigorous vortex, the flavonoid content was determined spectrophotometrically at 510 nm wavelength by comparing it with the quercetin standard.
2.9. Measurement of AsA and GSH
Ascorbate content was measured as per Nahar et al. [30] by homogenizing leaf samples in a mixture of 5% meta-phosphoric acid and 1 mM EDTA. After 15 min centrifuging (at 11,500× g at 4 °C), the collected supernatant was neutralized by K-P buffer (pH 7.0), and the oxidized fraction was reduced by dithiothreitol. Total and reduced ascorbate content was measured spectrophotometrically at 265 nm in K-P buffer containing ascorbate oxidase compared with the AsA standard. Dehydroascorbate (DHA) was measured by subtracting the reduced AsA from the total ascorbate.
Total GSH and oxidized glutathione (GSSG) content were measured as per Griffiths [31] by comparing them with known concentrations of GSH and GSSG. Reduced GSH was calculated by subtracting GSSG from total GSH.
2.10. Measurement of Protein
As per Bradford [32], protein concentration was estimated using bovine serum albumin (BSA) as a protein standard.
2.11. Enzyme Extraction and Assays
Fresh rice leaf samples were ground in K-P buffer (pH 7.0), having KCl, ascorbate, β-mercaptoethanol, and glycerol in an ice-cooled mortar and pestle. After centrifuging (11,500× g for 15 min at 4 °C) the ground samples, supernatants were used to determine protein and enzyme activities.
Ascorbate peroxidase (APX, EC: 1.11.1.11) activity was estimated according to Nakano and Asada [33] using an extinction coefficient of 2.8 mM−1 cm−1. Monodehydroascorbate reductase (MDHAR, EC: 1.6.5.4) activity was determined according to Hossain et al. [34] using an extinction coefficient of 6.2 mM−1 cm−1. Dehydroascorbate reductase (DHAR, EC: 1.8.5.1) activity was measured by the method of Nakano and Asada [33] using an extinction coefficient of 14 mM−1 cm−1. Glutathione reductase (GR, EC: 1.6.4.2) activity was estimated as per Foyer and Halliwell [35] using an extinction coefficient of 6.2 mM−1 cm−1.
Glyoxalase I (Gly I, EC: 4.4.1.5) and glyoxalase II (Gly II, EC: 3.1.2.6) activities were determined, as described by Principato et al. [36] using an extinction coefficient of 3.37 and 13.6 mM−1 cm−1, respectively.
2.12. Measurement of Methylglyoxal Content
Methylglyoxal (MG) content was estimated according to Wild et al. [37]. Leaf samples were homogenized in perchloric acid and centrifuged at 11,000× g for 10 min. Supernatants were decolorized by charcoal and neutralized by saturated Na2CO3. Neutralized supernatant was incubated for 10 min after adding sodium dihydrogen phosphate and N-acetyl-L-cysteine. Finally, MG content was measured at 288 nm wavelength in comparison with the known concentration of MG.
2.13. Statistical Analysis
All the recorded data were analyzed by CoStat v.6.400 computer-based software (CoHort Software, Monterey, CA, USA) [38]. Tukey’s HSD test was used for comparison of the mean differences at 5% level of significance. Means (±SD) were calculated from three replicates for each treatment.
3. Results
3.1. Growth and Phenotypic Appearance
The growth and phenotypic appearance of rice seedlings deteriorated due to salt stress. Salinity resulted in the yellowing of the whole plant, burning, and rolling of leaf tips (Figure 1). In response to 200 mM NaCl stress, the DW of rice seedlings decreased by 22% compared with the untreated control (Table 1). Supplementation with GA in salt-treated rice seedlings eliminated salt-induced damage and improved the phenotypic appearance (Figure 1). Exogenous application of GA increased the DW of salt-treated rice seedlings by 16% compared with salt-treated alone (Table 1). Nevertheless, the phenotypic appearance and DW of non-stressed rice seedlings remain unchanged due to external application of GA.

Figure 1.
Phenotypic appearance of rice (O. sativa L. cv. BRRI dhan52) seedlings as influenced by GA under salt stress. 1 mM gallic acid and 200 mM NaCl are specified by GA and salt, correspondingly.

Table 1.
Dry weight, leaf RWC, proline content, osmotic potential, MDA content, and H2O2 content of salt-treated rice (O. sativa L. cv. BRRI dhan52) seedlings as influenced by GA supplementation.
3.2. Effect on Leaf RWC, Pro Content and Osmotic Potential
Salinity exposure resulted in osmotic stress in rice seedlings, rising osmotic potential, Pro content, and lowering RWC of leaf (Table 1). In salt-treated rice seedlings, osmotic potential and Pro accumulation rose by 215 and 1052%, respectively, compared to the control. Conversely, the RWC of salt-treated rice leaves was reduced by 23% compared to the control. However, supplementation of GA eradicated osmotic stress in salt-treated rice seedlings by increasing the RWC of leaves and diminishing osmotic potential and Pro accumulation (Table 1). Osmotic stress indicating parameters remain unchanged in control seedlings due to supplementation of GA (Table 1).
3.3. Effect on Photosynthetic Pigments
The photosynthetic pigments in the leaves of rice seedlings decreased by salt exposure but were considerably reinstated by GA supplementation (Table 2). Salinity lessened chl a, chl b, chl (a + b) and carotenoid contents by 36, 42, 38 and 41%, correspondingly, comparing control seedlings. Supplementation with GA in salt-affected rice seedlings restored photosynthetic pigments (chl a, chl b, chl (a + b) and carotenoid contents by 33, 48, 36 and 38%, correspondingly) compared with salt-treated alone. However, the application of GA acid had no effect on photosynthetic pigments on the control treatment (Table 2).

Table 2.
Photosynthetic pigments of salt-treated rice (O. sativa L. cv. BRRI dhan52) seedlings as influenced by GA supplementation.
3.4. Effect on Ion Regulation
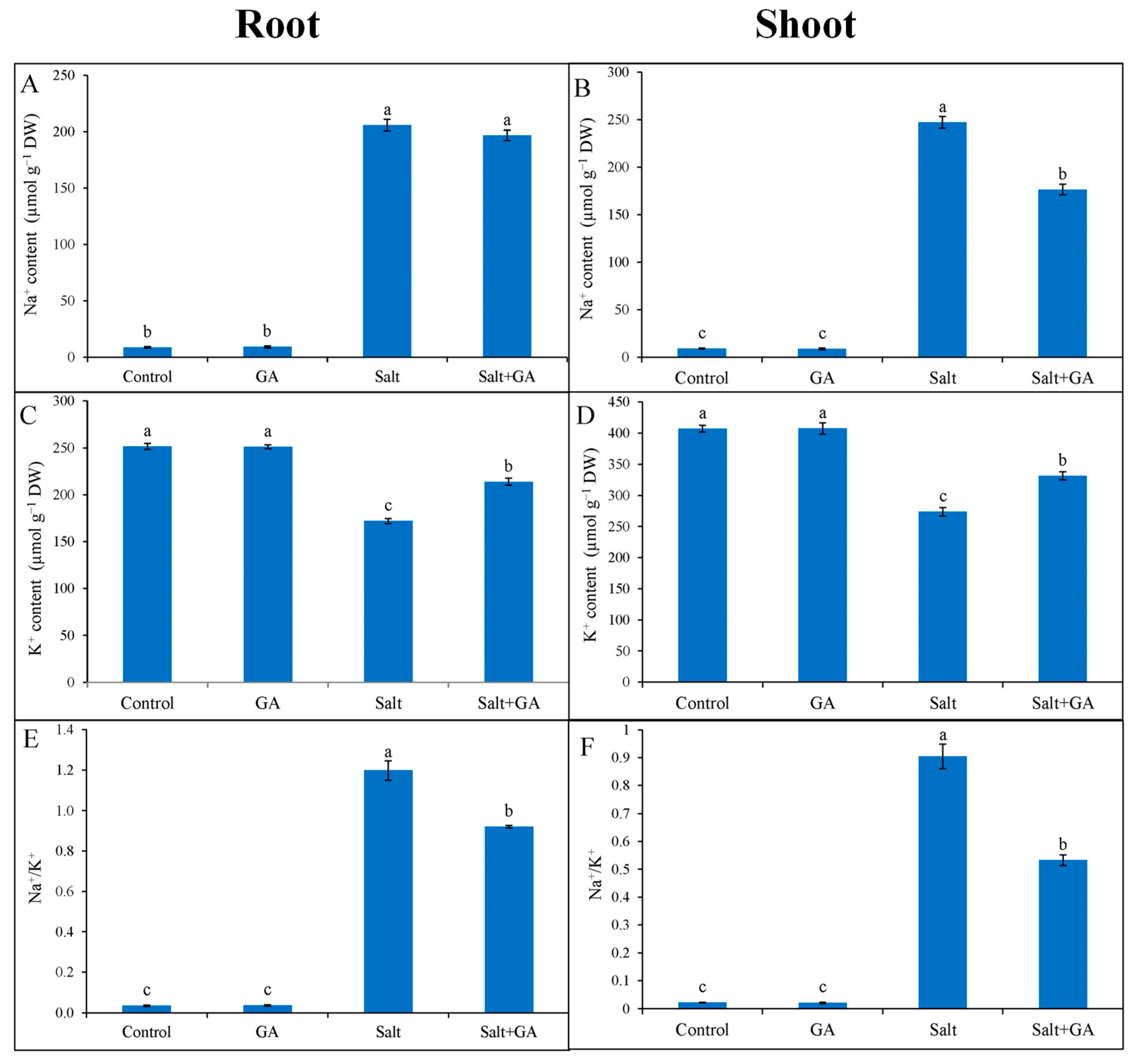
The salt-induced stress dislocated ion homeostasis by rising Na+ accumulation and Na+/K+ ratio and lowering K+ uptake in root and shoot of rice seedlings compared with untreated control (Figure 2). In 200 mM NaCl-treated rice seedlings, accumulation of Na+ was lower in the root than shoot. In comparison with salt treatment alone, supplementation of GA decreased 29% Na+ accumulation in the shoot of salt-treated rice seedlings (Figure 2B). Exogenous application of GA increased K+ accumulation and decreased Na+/K+ ratio in the root and shoot of salt-treated rice seedlings compared with salt-treated alone (Figure 2).
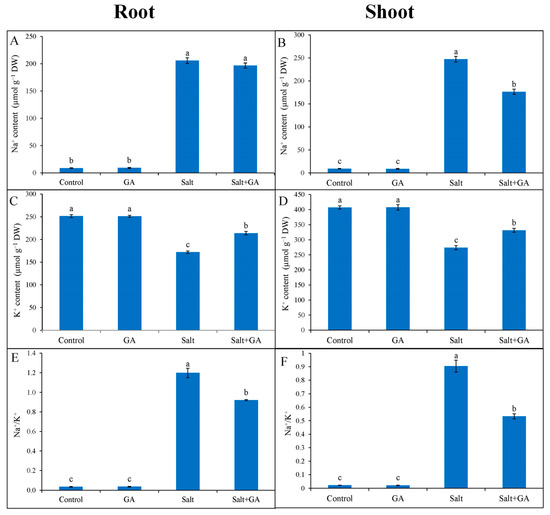
Figure 2.
Ion regulation in root (A,C,E) and shoot (B,D,E) of salt-treated rice (O. sativa L. cv. BRRI dhan52) seedlings as influenced by GA supplementation. 1 mM gallic acid and 200 mM NaCl are specified by GA and Salt, correspondingly. Values with different letters are significantly different at p ≤ 0.05 applying the Tukey’s HSD test.
3.5. Effect on ROS Generation and Lipid Peroxidation
Salinity resulted in oxidative stress in rice seedlings, indicated by overproduction of ROS and lipid peroxidation. The imposition of 200 mM NaCl increased H2O2 content in rice seedlings by 96% compared with untreated control (Table 1). Histochemical staining revealed the overproduction of superoxide radical (O2•−) indicated by dark blue spots in stained rice leaves (Figure 3). External application of GA in salt-treated rice seedlings reversed the generation of ROS (Table 1; Figure 3). Compared with the control, generation of H2O2 was lowered by 34% in salt-treated rice seedlings due to GA supplementation (Table 1). Nevertheless, the external application of GA to control rice seedlings did not affect ROS production.

Figure 3.
Histochemical detection of O2•− (indicated by dark blue spots) in the leaf of salt-treated rice (O. sativa L. cv. BRRI dhan52) seedlings as influenced by GA supplementation. 1 mM gallic acid and 200 mM NaCl are specified by GA and Salt, correspondingly.
Salinity-induced oxidative stress increased lipid peroxidation (indicated by MDA content) in rice seedlings. Exposure to 200 mM NaCl in rice seedlings increased MDA content by 147% compared to the control (Table 1). A remarkable decrease in MDA content was recorded in salt-treated rice seedlings by GA supplementation, which was 37% lower compared with salt-treated alone (Table 1).
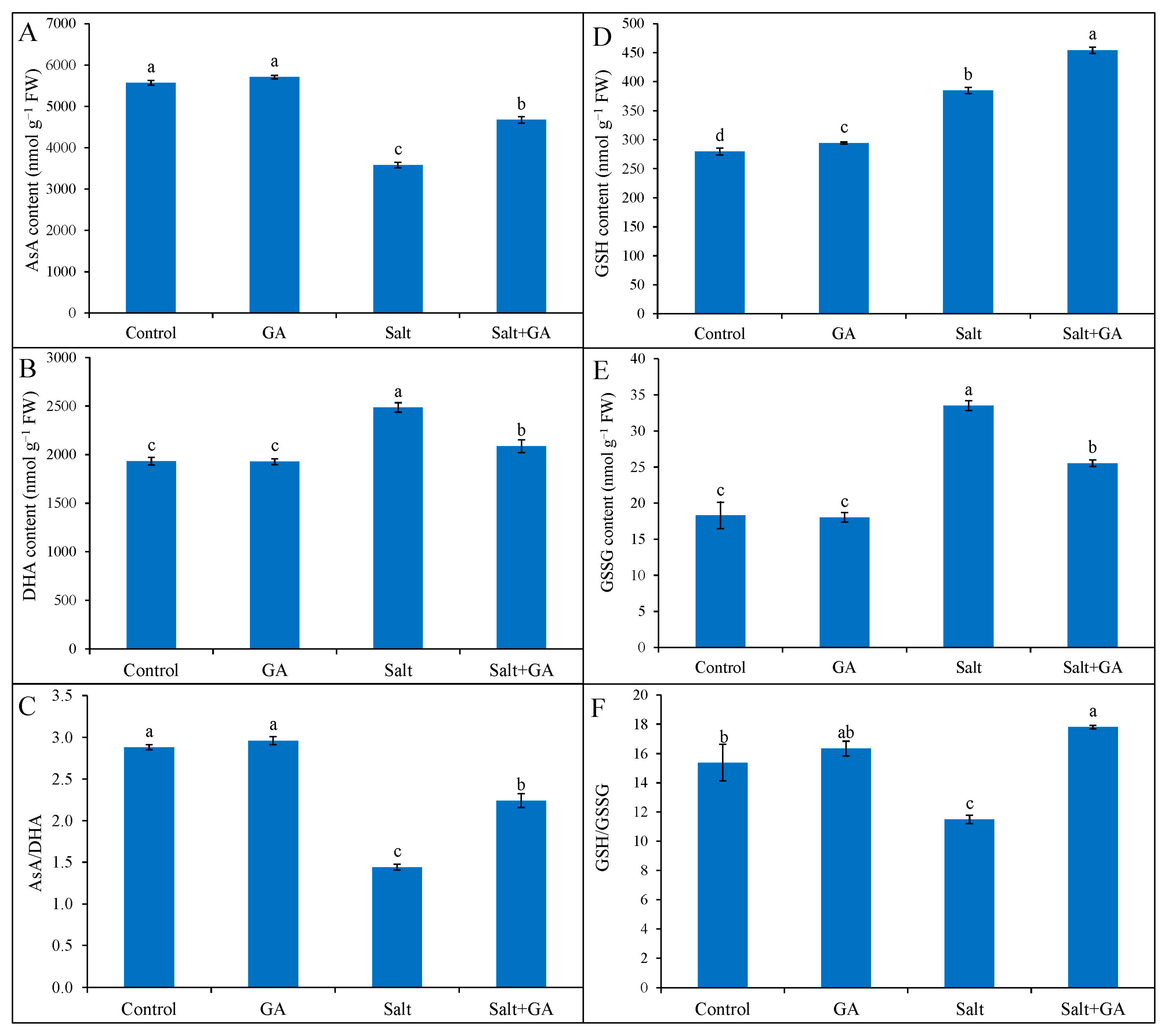
3.6. Effect on AsA and GSH Pool
Exposure of rice seedlings to salt stress lessened AsA accumulation and AsA/DHA ratio, but fostered DHA accumulation in rice seedlings. In response to 200 mM NaCl stress, AsA generation and AsA/DHA ratio were declined by 36 and 50%, respectively, compared to the control seedlings (Figure 4). However, the addition of GA in salt-treated rice seedlings uplifted AsA generation and AsA/DHA ratio by 30 and 56%, correspondingly, compared to salt-treated alone (Figure 4).
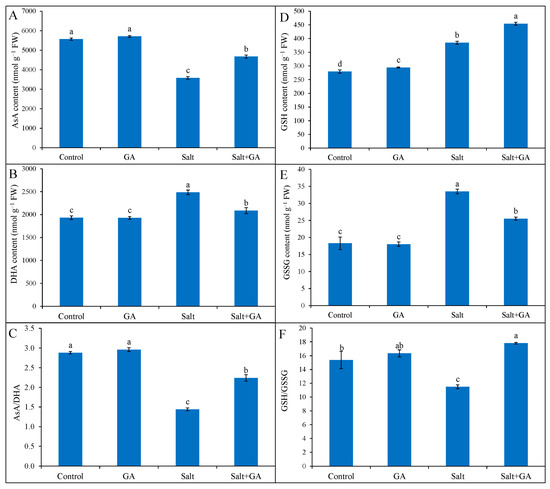
Figure 4.
Ascorbate (A–C) and GSH (D–F) pool of salt-treated rice (O. sativa L. cv. BRRI dhan52) seedlings as influenced by GA supplementation. 1 mM gallic acid and 200 mM NaCl are specified by GA and Salt, correspondingly. Values with different letters are significantly different at p ≤ 0.05 applying the Tukey’s HSD test.
Reduced glutathione and GSSG content were multiplied in 200 mM NaCl-treated rice seedlings by 38 and 83%, correspondingly, contrasted with the control (Figure 4). The ratio of GSH/GSSG declined in salt-treated rice seedlings by 25%, contrasted with the control. However, the treatment of GA in salt-treated rice seedlings further uplifted GSH generation by 18% and diminished GSSG content by 24%, compared to salt-treated alone (Figure 4). Moreover, GA supplementation in salt-treated rice seedlings boosted the GSH/GSSG ratio by 55%, contrasted with salt-treated seedlings alone.
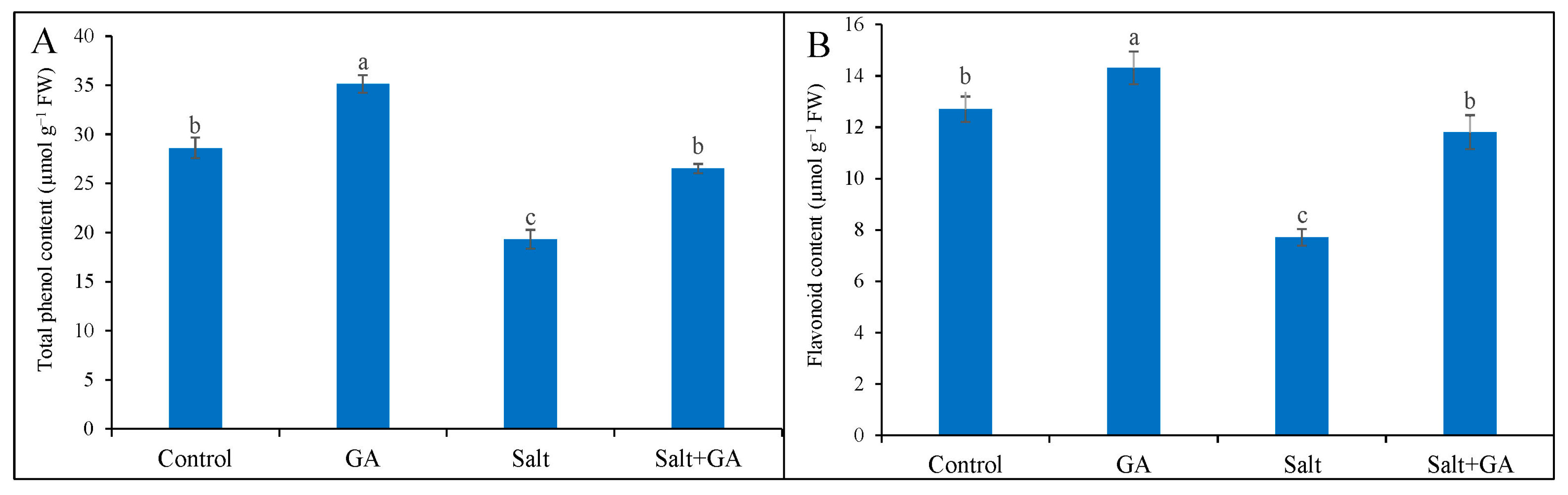
3.7. Effect on Phenolic Compounds
Total phenol and flavonoid accumulation declined in salt-treated rice seedlings by 32 and 39%, correspondingly, contrasted with the untreated control (Figure 5). The addition of GA in salt-treated rice seedlings boosted total phenol and flavonoid content by 37 and 53%, correspondingly, contrasted with salt-treated alone (Figure 5). Supplementation of GA in seedlings without salt application also significantly increased total phenol and flavonoid contents.
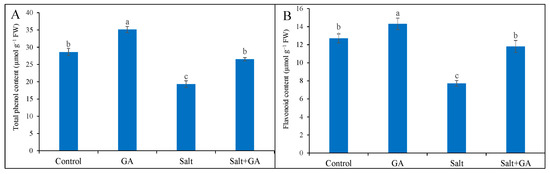
Figure 5.
Phenolic compounds (total phenol, (A) and flavonoid contents, (B)) of salt-treated rice (O. sativa L. cv. BRRI dhan52) seedlings as influenced by GA supplementation. 1 mM gallic acid and 200 mM NaCl are specified by GA and salt, correspondingly. Values with different letters are significantly different at p ≤ 0.05 applying the Tukey’s HSD test.
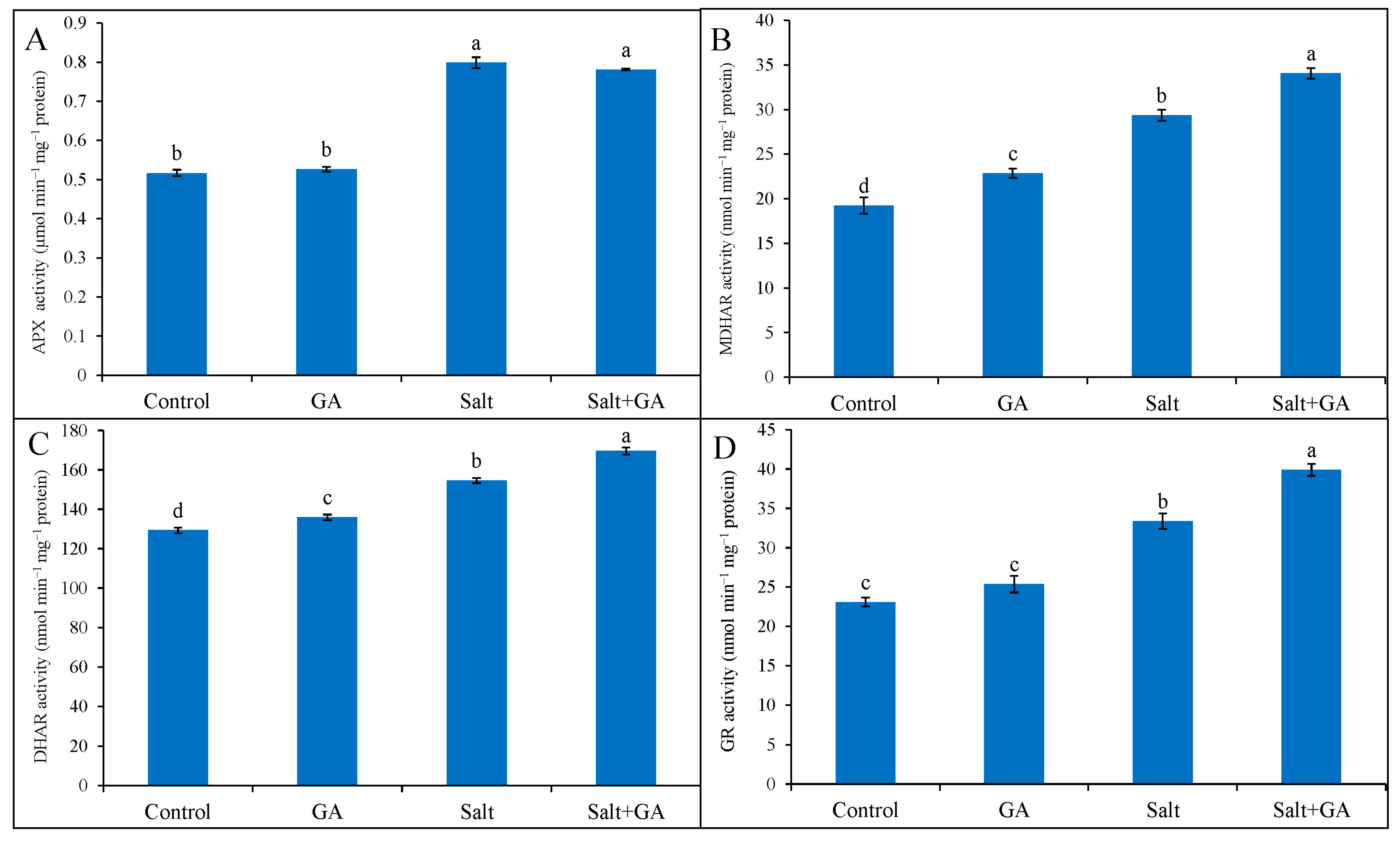
3.8. Effect on Antioxidant Enzymes
External application of GA on control rice seedlings did not influence antioxidant enzymes, but had a significant impact under salt stress conditions. In rice seedlings exposed to 200 mM NaCl, the activities of antioxidant enzymes including APX, MDHAR, DHAR, and GR was boosted by 54, 53, 20, and 44%, correspondingly, contrasted with control seedlings (Figure 6). Nevertheless, the exogenous treatment of GA in salt-treated rice seedlings further upgraded the activities of MDHAR, DHAR, and GR by 16, 10 and 20%, correspondingly, contrasted with salt-treated seedlings alone (Figure 6).
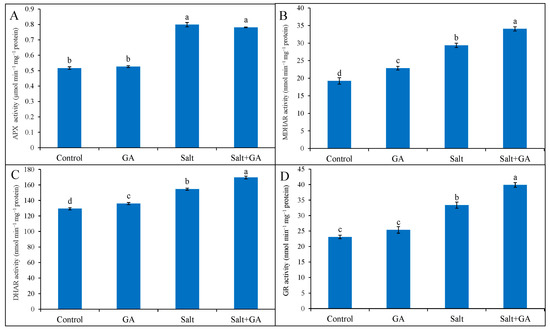
Figure 6.
Activities of APX (A), MDHAR (B) DHAR (C) and GR (D) of salt-treated rice (O. sativa L. cv. BRRI dhan52) seedlings as influenced by GA supplementation. 1 mM gallic acid and 200 mM NaCl are specified by GA and salt, correspondingly. Values with different letters are significantly different at p ≤ 0.05 applying the Tukey’s HSD test.
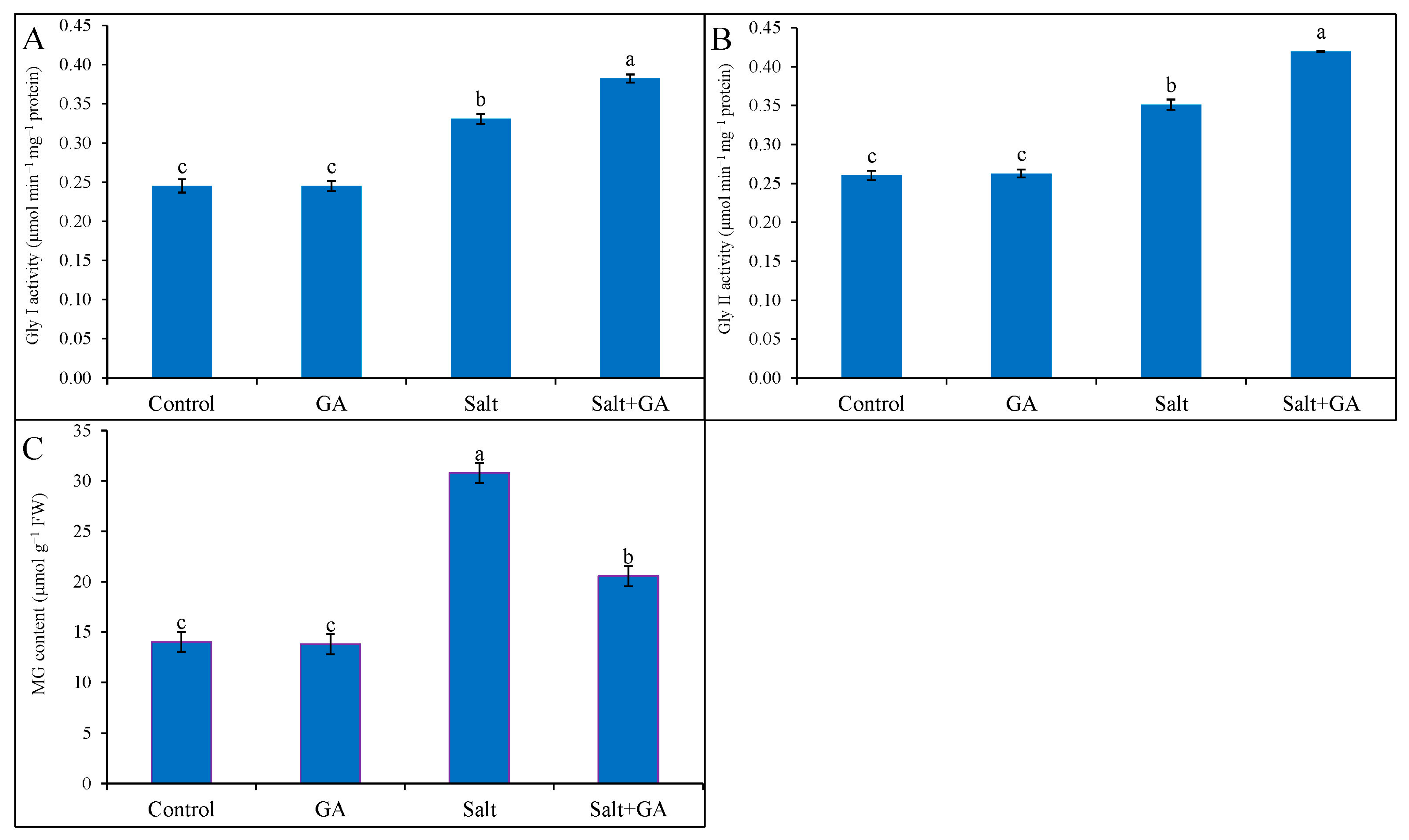
3.9. Effect on Glyoxalase System
Compared with the control, 200 mM NaCl stress sharply replicated MG accumulation, Gly I, and Gly II activities in rice by 119, 33 and 35%, respectively (Figure 7). Applying GA in salt-treated rice seedlings decreased MG accumulation by 33% compared to salt-treated seedlings alone (Figure 7). Supplementations of GA in salt-treated rice seedlings boosted the activities of Gly I and Gly II enzymes by 15 and 20%, correspondingly, compared to salt-treated seedlings alone (Figure 7). However, the application of GA did not influence the glyoxalase system of control rice seedlings.
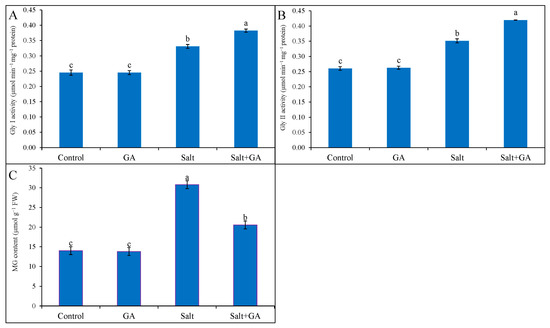
Figure 7.
Methylglyoxal accumulation (C) and the activities of GA on Gly I (A) and Gly II (B) enzymes in salt-treated rice (O. sativa L. cv. BRRI dhan52) seedlings as influenced by GA supplementation. 1 mM gallic acid and 200 mM NaCl are specified by GA and salt, correspondingly. Values with different letters are significantly different at p ≤ 0.05 applying the Tukey’s HSD test.
4. Discussion
The preliminary response of salinity on the plant is osmotic stress and ionic toxicity. Among them, osmotic stress is very much immediate. It disturbs water uptake and causes stomatal closure, which is one of the reasons for reducing cell division and enlargement, as well as plant growth [6,12]. In the current study, exposure to salinity resulted in osmotic stress that was indicated by greater osmotic potential, Pro accumulation, and poor leaf RWC. Salinity also reduced the growth of rice seedlings, as indicated by decreasing DW. These results are in aligned with previous results [17,39,40,41,42], which reported salinity-induced osmotic, ionic, and oxidative stress on the plant and decreased growth. Hasanuzzaman et al. [6] stated that salinity augmented osmotic potential, Pro content, and declined RWC by reducing the water retention dimension for osmotic modification. Conversely, the external application of GA restored the growth and water balance of salt-affected rice seedlings by decreasing osmotic potential, Pro accumulation, and increasing DW and RWC. Similar results were also illustrated in earlier studies [17,18,21], which reported that the external application of GA restored the growth and water uptake capacity by reducing osmotic stress. Exposure to salinity creates physiological drought conditions within the plants, which hampers stomatal closure, slows down photosynthetic CO2 assimilation, degrades chl content and photosynthesis efficiency [43], and leads to higher ROS generation, as well as reducing growth [8,44]. Saha et al. [45] reported that salt-induced stress shrunken photosynthetic pigments and increased ROS production by activating the chlorophyllase enzyme. Our study revealed that salt stress sharply decreased the photosynthetic pigments of rice seedlings, which were considerably restored by GA supplementation. The present results are in line with earlier illustrations [18,21], which noted that GA supplementation in plants promoted the phenolic pool that reduced ROS and restored photosynthetic pigments under abiotic stress conditions.
Salt-induced ionic toxicity disrupts ion homeostasis and cellular function, disturbing nutrient uptake and translocation [6,46]. Ionic toxicity activates GORK channels by depolarizing the plasma membrane, resulting in higher Na+ and lower K+ accumulation in the plant [16,47]. The present study showed that salt stress unsettled ion homeostasis by escalating Na+ accumulation and Na+/K+ ratio and declining K+ uptake in the root and shoot of rice seedlings. Accumulation of Na+ was greater in shoot than root in salt-affected rice seedlings. Related ionic imbalance by salinity in rice was also narrated in earlier studies [39,42,48]. Conversely, supplemental GA restored the ion homeostasis by decreasing Na+ accumulation in the shoot and increasing K+ accumulation in the root and shoot of salt-treated rice seedlings. This finding corroborates with Babaei et al. [21], who reported that supplemental GA upregulates ion homeostasis by declining Na+ accumulation and replicating K+ accumulation. Under salt stress, overproduced ROS activate NSCC, the major pathway of Na+ uptake in plants [9,16]. External GA in salt-treated rice seedlings of the present study diminished the ROS levels (discussed in the later part) and contributed to improving ion homeostasis.
Salt-induced osmotic stress degrades chl content and photosynthesis efficiency [40] and leads to higher ROS generation [8,44]. Salinity-induced Na+ influx and K+ efflux disrupt electron flow from the central transport chain to the oxygen reduction pathway in the cell and lead to higher ROS generation [3]. The salinity-induced osmotic and ionic stress concomitantly disturbs plant metabolism and causes accumulation of ROS [11,12] and leads to oxidative stress by lipid peroxidation, protein oxidation, and enzyme inhibition [13,49]. The current study presented that salinity resulted in oxidative stress in rice seedlings, signified by greater ROS (H2O2 and O2•−) production, which caused lipid peroxidation (specified by MDA content). In agreement with our results, previous findings registered that salinity-induced osmotic and ionic stress resulted in oxidative damage in plants through more ROS production and lipid peroxidation [3,15]. However, supplemental GA reversed the production of ROS and lipid peroxidation in rice seedlings under salt stress. Treatment of GA in salt-treated rice seedlings increased phenolic compounds, including total phenol and flavonoid contents, which might work in scavenging overproduced ROS. The present observation agrees with earlier studies [18,50], which determined that GA as a potential antioxidant reduces lipid peroxidation and oxidative damage in the plant by scavenging ROS and improving the antioxidant defense system and metabolism.
The role of AsA, GSH, phenolic compounds, and flavonoids in maintaining cellular redox potential under abiotic stress by detoxifying ROS has been well documented. Ascorbate and GSH directly quench and scavenge ROS by reacting and activating antioxidant enzymes [13,49]. The current study opined that salinity augmented DHA content in rice seedlings, resulting in lower AsA content and AsA/DHA ratio. The salt-induced toxic ROS production and augmented oxidation might be facilitated by lesser AsA accumulation and AsA/DHA ratio. These findings corroborate earlier illustrations [15,51,52], which documented lower AsA content and AsA/DHA ratio by salinity. Gallic acid addition in rice seedlings boosted AsA production, as well as AsA/DHA ratio, under salinity. The increment of AsA production by supplementing GA in salt-treated rice seedlings might be due to the upgradation of phenolic compounds, which scavenged overproduced ROS and uplifted MDHAR activities. The present work delineated that salt-induced oxidative stress promoted GSH production and declined GSH/GSSG ratio in rice seedlings. The overproduced ROS triggered the oxidation of GSH, which confirmed higher production of GSSG and lowered GSH/GSSG ratio in salt-treated rice seedlings. Related verdicts were generated in earlier studies [41,48,53], which recorded that salinity increased the production of GSSG, as well as lowered GSH/GSSG ratio, in rice seedlings. However, supplementing GA in salt-treated rice seedlings increased the GSH/GSSG ratio by further incrementing GSH production and reducing GSSG content. The increment of reduced GSH production and GSH/GSSG ratio in GA-supplemented salt-treated rice seedlings might be due to the upgradation of phenolic compounds, which scavenged overproduced ROS and enhanced GR activities. These verdicts are steady with Colak et al. [18], who confirmed that GA increased GSH content by enhancing GR activities under abiotic stress.
Phenolic compounds and flavonoids are potent antioxidants and secondary metabolites that can be regulated under abiotic stress and reveal the antioxidant potential by ROS detoxification and improving antioxidant enzyme activities [6]. In our study, salt exposure in rice seedlings decreased total phenols and flavonoid contents. In agreement with the present demonstration, Rahman et al. [52] stated that salt stress decreased total phenols and flavonoid contents in salt-treated rice seedlings. However, supplementing GA reversed the production of phenolic compounds and increased total phenols and flavonoid accumulation in salt-affected rice seedlings. Related results are illustrated in earlier demonstrations [20,21], which differed that supplementation of GA increased phenolic compounds that played a role in ROS detoxification under abiotic stress.
The antioxidant enzymes APX, MDHAR, DHAR, and GR play a crucial role in scavenging overproduced ROS and regenerating AsA and GSH through the AsA-GSH cycle [49,51,54]. Glutathione reductase also regenerates AsA and GSH together with MDHAR and DHAR; these all take part in scavenging overproduced ROS under abiotic stress conditions [49]. In the current study, salinity exposure in rice triggered the activities of APX, MDHAR, DHAR, and GR enzymes. Overproduced ROS and lesser-produced AsA might be accountable for higher APX, MDHAR, and DHAR activities, while more ROS and GSH might increase GR activity. Similar results were stated in previous findings [48,52], which reported that activities of AsA-GSH cycle enzymes increased in plants under salt stress. However, supplementing GA further uplifted the activities of MDHAR, DHAR, and GR enzymes. Colak et al. [18] mentioned that supplemental GA upregulates the antioxidant enzyme activities in wheat under ionizing radiation stress by reducing ROS and increasing phenolic compounds. Babaei et al. [21] also displayed that supplemental GA increased APX and GR activities in Lepidium sativum L. under salinity.
Detoxification of MG in rice plants by augmentation of Gly I and Gly II activities is well documented [48,52,53]. By utilizing GSH, Gly I converted MG to S-D-lactoylglutathione (SLG) through the production of intermediate product hemithioacetal. Then, Gly II hydrolyzes SLG and is converted to D-lactate, and subsequently, GSH is regenerated [14,55]. In the current study, salinity replicated MG content and triggered Gly I and Gly II enzymes’ activities. In agreement with the present illustration, earlier illustration [48,52] also revealed that salt stress increased MG production with the enhancement of glyoxalase enzyme activities. External application of GA in rice further upgraded the activities of Gly I and Gly II with a reduction of MG production and an increment of GSH content. Hence, this upgradation of glyoxalase system components by supplemental GA might play a role in MG detoxification and confer tolerance in salt-treated rice seedlings. In corroborate with these results, previous studies [48,52,53] demonstrated that an upregulated glyoxalase system increased salt stress tolerance by detoxifying MG and increasing GSH production.
5. Conclusions
Salt stress in rice seedlings leads to osmotic and ionic stress by disrupting water balance and ion homeostasis. Salinity also induced oxidative stress by interrupting ROS and MG detoxification systems. Conversely, supplementing GA in salt-treated rice seedlings restored salt-induced damage. Gallic acid addition in salt-treated rice seedlings replicated antioxidant (AsA, GSH, and phenolic compounds) production and activities of AsA-GSH cycle enzymes that maintain equilibrium among ROS and antioxidant defense systems. The glyoxalase system was also boosted by GA supplementation and maintained MG detoxification. Detoxification of ROS by GA supplementation worked in ion homeostasis and osmoregulation. However, ionic homeostasis, ROS, and MG detoxification system worked coordinately to restore salt-induced damage by GA supplementation.
Author Contributions
Conceptualization, A.R., M.H. and M.F.; methodology, A.R. and M.U.A.; formal analysis, M.H.; investigation, A.R., M.U.A., M.S.H., J.A.M. and K.N.; resources, M.F.; writing—original draft preparation, A.R.; writing—review and editing, M.S.H., J.A.M., K.N. and M.H.; visualization, A.R. and K.N.; supervision, M.H. and M.F.; project administration, M.F.; funding acquisition, M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
Data Availability Statement
All data are available in this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sun, Y.; Mu, C.; Zheng, H.; Lu, S.; Zhang, H.; Zhang, X.; Liu, X. Exogenous Pi supplementation improved the salt tolerance of maize (Zea mays L.) by promoting Na+ exclusion. Sci. Rep. 2018, 8, 16203. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. 2021. Available online: http://www.fao.org/global-soil-partnership/resources/highlights/detail/en/c/1412475/ (accessed on 30 September 2022).
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Mahmud, J.A.; Nahar, K.; Hasanuzzaman, M.; Hossain, M.S.; Fujita, M. Salt stress tolerance in rice: Emerging role of exogenous phytoprotectants. In Advances in International Rice Research; Li, J., Ed.; Intech: Rijeka, Crotia, 2017; pp. 140–174. [Google Scholar]
- Hasanuzzaman, M.; Raihan, M.R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef]
- Munns, R.; Gilliham, M. Salinity tolerance of crops—What is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Jalil, S.U.; Ansari, M.I. Physiological role of gamma-aminobutyric acid in salt stress tolerance. In Salt and Drought Stress Tolerance in Plants; Hasanuzzaman, M., Tanveer, M., Eds.; Springer: Cham, Switzerland, 2020; pp. 337–350. [Google Scholar]
- Shabala, S.; Demidchick, V.; Shabala, L.; Cuin, T.A.; Smith, S.J.; Miller, A.J.; Davies, J.M.; Newman, I.A. Extracellular Ca2+ ameliorates NaCl-induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels. Plant Physiol. 2006, 141, 1653–1665. [Google Scholar] [CrossRef]
- Wu, G.Q.; Wang, S.M. Calcium regulates K+/Na+ homeostasis in rice (Oryza sativa L.) under saline conditions. Plant Soil Environ. 2012, 58, 121–127. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of plant responses to salt stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Yadav, S.K.; Singla-Pareek, S.L.; Reddy, M.K.; Sopory, S.K. Methylglyoxal detoxification by glyoxalase system: A survival strategy during environmental stresses. Physiol. Mol. Biol. Plants 2005, 11, 1. [Google Scholar]
- Hasanuzzaman, M.; Raihan, M.R.H.; Nowroz, F.; Fujita, M. Insight into the mechanism of salt-induced oxidative stress tolerance in soybean by the application of Bacillus subtilis: Coordinated actions of osmoregulation, ion homeostasis, antioxidant defense, and methylglyoxal detoxification. Antioxidants 2022, 11, 1856. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Tester, M. Sodium fluxes through non selective cation channels in the plasma membrane of protoplasts from Arabidopsis roots. Plant Physiol. 2002, 128, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Ozfidan-Konakci, C.; Yildiztugay, E.; Kucukoduk, M. Protective roles of exogenously applied gallic acid in Oryza sativa subjected to salt and osmotic stresses: Effects on the total antioxidant capacity. Plant Growth Regul. 2015, 75, 219–234. [Google Scholar] [CrossRef]
- Colak, N.; Kurt-Celebi, A.; Fauzan, R.; Torun, H.; Ayaz, F.A. The protective effect of exogenous salicylic and gallic acids ameliorates the adverse effects of ionizing radiation stress in wheat seedlings by modulating the antioxidant defence system. Plant Physiol. Biochem. 2021, 168, 526–545. [Google Scholar] [CrossRef]
- Gantner, M.; Najdaz, A.; Piesik, D. Effect of phenolic acid content on acceptance of hazel cultivars by filbert aphid. Plant Prot. Sci. 2019, 55, 116–122. [Google Scholar] [CrossRef]
- Singh, A.; Gupta, R.; Pandey, R. Exogenous application of rutin and gallic acid regulate antioxidants and alleviate reactive oxygen generation in Oryza sativa L. Physiol. Mol. Biol. Plants 2017, 23, 301–309. [Google Scholar] [CrossRef]
- Babaei, M.; Shabani, L.; Hashemi-Shahraki, S. Improving the effects of salt stress by β-carotene and gallic acid using increasing antioxidant activity and regulating ion uptake in Lepidium sativum L. Bot. Stud. 2022, 63, 22. [Google Scholar] [CrossRef]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teari, D. Rapid determination of free proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Arnon, D.T. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, F.; Wu, F.; Mao, W.; Zhang, G.; Zhou, M. Modulation of exogenous glutathione in antioxidant defense system against Cd stress in the two barley genotypes differing in Cd tolerance. Plant Physiol. Biochem. 2010, 48, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.W.; Murphy, T.M.; Lin, C.H. Hydrogen peroxide induced chilling tolerance in mung beans mediated through ABA independent glutathione accumulation. Funct. Plant Biol. 2003, 30, 955–963. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Ashraf, M.; Ali, Q. Response of two genetically diverse wheat cultivars to salt stress at different growth stage: Leaf lipid peroxidation and phenolic contents. Pak. J. Bot. 2010, 42, 559–565. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Rahman, A.; Suzuki, T.; Fujita, M. Polyamine and nitric oxide crosstalk: Antagonistic effects on cadmium toxicity in mung bean plants through upregulating the metal detoxification, antioxidant defense and methylglyoxal detoxification systems. Ecotoxicol. Environ. Saf. 2016, 126, 245–255. [Google Scholar] [CrossRef]
- Griffiths, O.W. Determination of glutathione and glutathione disulphide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Hossain, M.A.; Nakano, Y.; Asada, K. Monodehydroascorbate reductase in spinach chloroplasts and its participation in the regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol. 1984, 25, 385–395. [Google Scholar]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Principato, G.B.; Rosi, G.; Talesa, V.; Govannini, E.; Uolila, L. Purification and characterization of two forms of glyoxalase II from rat liver and brain of Wistar rats. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1987, 911, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Wild, R.; Ooi, L.; Srikanth, V.; Münch, G. A quick: Convenient and economical method for the reliable determination of methylglyoxal in millimolar concentrations: The N-acetyl-L-cysteine assay. Anal. Bioanal. Chem. 2012, 403, 2577–2581. [Google Scholar] [CrossRef] [PubMed]
- CoStat. CoStat-Statistics Software, Version 6.400; CoHort Software: Monterey, CA, USA, 2008.
- Chen, G.; Zheng, D.; Feng, N.; Zhou, H.; Mu, D.; Zhao, L.; Shen, X.; Rao, G.; Meng, F.; Huang, A. Physiological mechanisms of ABA-induced salinity tolerance in leaves and roots of rice. Sci. Rep. 2022, 12, 8228. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, X.; Hu, Y.; Han, W.; Yin, J.; Li, H.; Gong, H. Silicon improves salt tolerance by increasing root water uptake in Cucumis sativus L. Plant Cell Rep. 2015, 34, 1629–1646. [Google Scholar] [CrossRef]
- Zhu, Y.; Jiang, X.; Zhang, J.; He, Y.; Zhu, X.; Zhou, X.; Gong, H.; Yin, J.; Liu, Y. Silicon confers cucumber resistance to salinity stress through regulation of proline and cytokinins. Plant Physiol. Biochem. 2020, 156, 209–220. [Google Scholar] [CrossRef]
- Khan, I.; Muhammad, A.; Chattha, M.U.; Skalicky, M.; Chattha, M.B.; Ayub, M.A.; Anwar, M.R.; Soufan, W.; Hassan, M.U.; Rahman, M.A.; et al. Mitigation of salinity-induced oxidative damage, growth, and yield reduction in fine rice by sugarcane press mud application. Front. Plant Sci. 2022, 13, 840900. [Google Scholar] [CrossRef]
- Puthiyottil, P.; Akkara, Y. Pre treatment with Bacillus subtilis mitigates drought induced photo-oxidative damages in okra by modulating antioxidant system and photochemical activity. Physiol. Mol. Biol. Plants 2021, 27, 945–957. [Google Scholar] [CrossRef]
- Shabani, A.; Sepaskhah, A.R.; Kamgar-Haghighi, A.A. Growth and physiologic response of rapeseed (Brassica napus L.) to deficit irrigation, water salinity and planting method. Int. J. Plant Prod. 2013, 7, 569–596. [Google Scholar]
- Saha, P.; Chatterjee, P.; Biswas, A.K. NaCl pretreatment alleviates salt stress by enhancement of antioxidant defense system and osmolyte accumulation in mungbean (Vigna radita L. Wilczek). Ind J. Exp. Biol. 2010, 48, 593–600. [Google Scholar]
- Rafiq, K.; Akram, M.S.; Shahid, M.; Qaisar, U.; Rashid, N. Enhancement of salt tolerance in maize (Zea mays L.) using locally isolated Bacillus sp. SR-2-1/1. Biologia 2020, 75, 1425–1436. [Google Scholar] [CrossRef]
- Blumwald, E.; Aharon, G.S.; Apse, M.P. Sodium transport in plant cells. Biochim. Biophys. Acta 2000, 1465, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Calcium supplementation improves Na+/K+ ratio, antioxidant defense and glyoxalase systems in salt-stressed rice seedlings. Front. Plant Sci. 2016, 7, 609. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Yildiztugay, E.; Ozfidan-Konakci, C.; Kucukoduk, M. Improvement of cold stress resistance via free radical scavenging ability and promoted water status and photosynthetic capacity of gallic acid in soybean leaves. Soil Sci. Plant Nutr. 2017, 17, 366–384. [Google Scholar] [CrossRef][Green Version]
- Mishra, P.; Bhoomika, K.; Dubey, R.S. Differential responses of antioxidative defense system to prolonged salinity stress in salt-tolerant and salt-sensitive Indica rice (Oryza sativa L.) seedlings. Protoplasma 2013, 250, 3–19. [Google Scholar] [CrossRef]
- Rahman, A.; Hossain, M.S.; Mahmud, J.A.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Manganese-induced salt stress tolerance in rice seedlings: Regulation of ion homeostasis, antioxidant defense and glyoxalase systems. Physiol. Mol. Biol. Plants 2016, 22, 291–306. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Alam, M.M.; Rahman, A.; Hasanuzzaman, M.; Nahar, K.; Fujita, M. Exogenous proline and glycine betaine mediated upregulation of antioxidant defense and glyoxalase systems provides better protection against salt-induced oxidative stress in two rice (Oryza sativa L.) varieties. Biomed Res. Int. 2014, 2014, 757219. [Google Scholar] [CrossRef]
- Asada, K. Ascorbate peroxidase-a hydrogen peroxide-scavenging enzymes in plants. Physiol. Plant 1992, 85, 235–241. [Google Scholar] [CrossRef]
- Singla-Pareek, S.L.; Yadav, S.K.; Pareek, A.; Reddy, M.K.; Sopory, S.K. Enhancing salt tolerance in a crop plant by overexpression of glyoxalase II. Transgen. Res. 2007, 17, 171–180. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).