Investigation and Identification of Cyst Nematodes in the Bashang Region of Hebei, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Isolation of Cyst Nematodes
2.2. Morphological Characterization
2.3. Molecular Identification
2.4. Identification of SCN Races
2.5. Koch’s Postulates Test and the Host Range Experiment of the SBCN
3. Results
3.1. Isolation of Cyst Nematodes
3.2. Morphological Identification
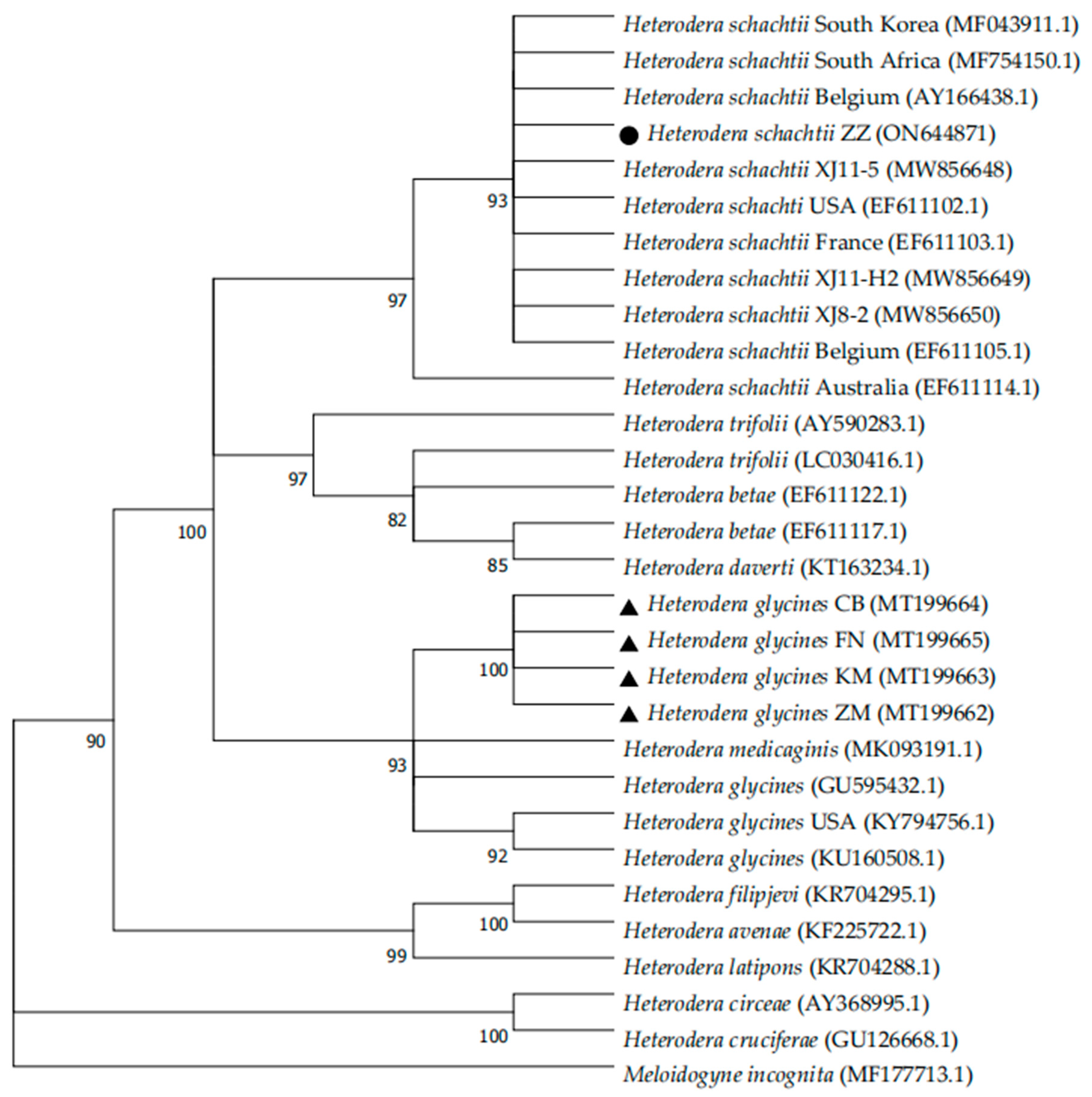
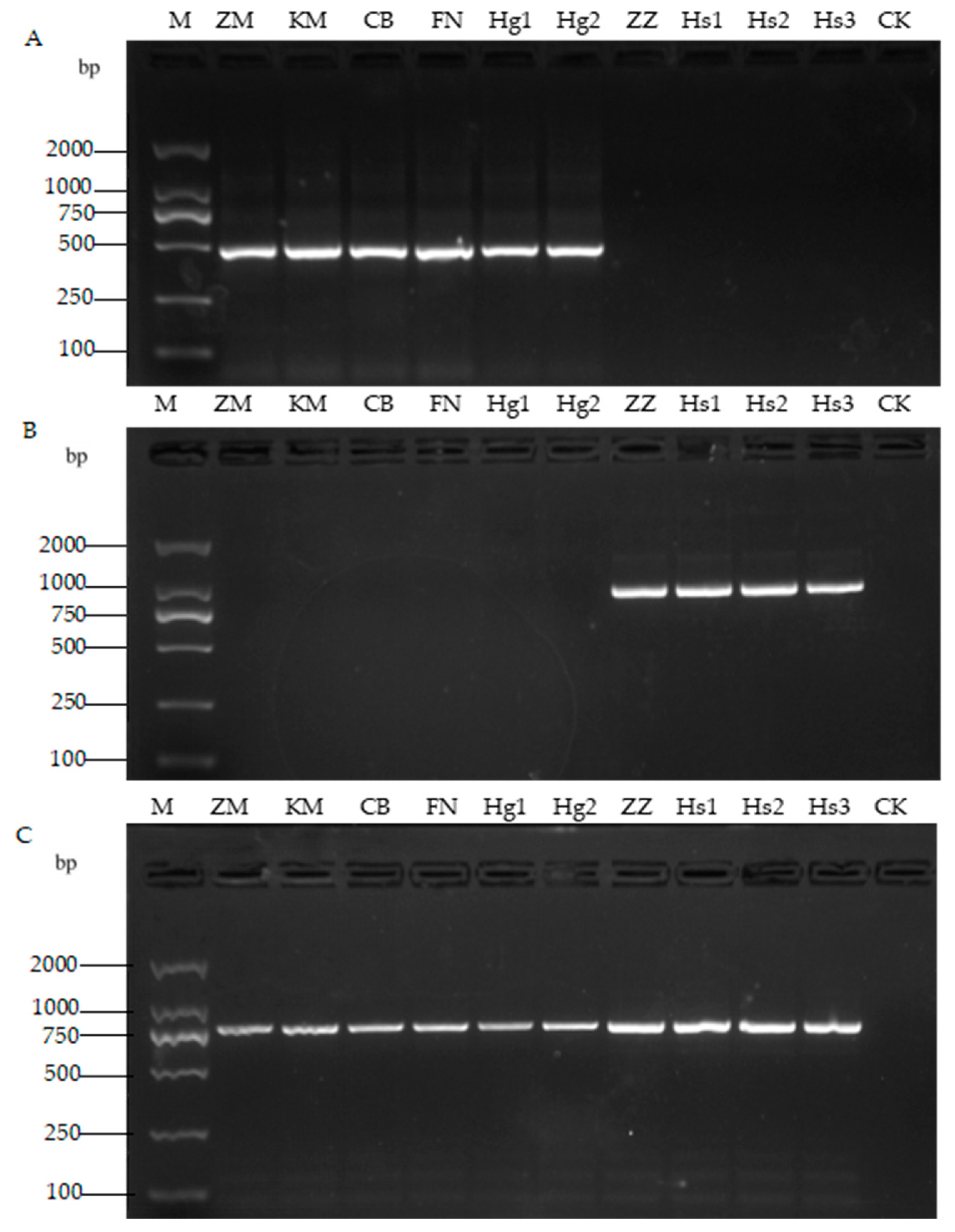
3.3. Molecular Identification
3.4. Identification of SCN Races
3.5. Koch’s Postulates Test and the Host Range Experiment of the SBCN
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, J.W.; Zhang, Y.; Li, X.; Zhao, L.; Chen, S. First report of the soybean cyst nematode Heterodera glycines on soybean in zhejiang, Easten China. Plant Dis. 2009, 93, 319. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zheng, J.W. First report of soybean cyst nematode (Heterodera glycines) on tobacco in Henan, central China. Plant Dis. 2013, 97, 852. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.L.; Peng, H.; Wu, D.Q.; Huang, W.K.; Cui, J.K. First report of soybean cyst nematode (Heterodera glycines) on soybean from gansu and ningxia China. Plant Dis. 2015, 100, 229. [Google Scholar] [CrossRef]
- Chen, J.S.; Zhou, Y.Y.; Wang, Y.Y.; Fan, H.Y.; Liu, X.Y.; Wang, D.; Zhao, D.; Duan, Y.X.; Zhu, X.F.; Chen, L.J. Characterization of virulence phenotypes of Heterodera glycines in Heilongjiang, Northeast China. Plant Dis. 2021, 105, 2056–2060. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Duan, Y.X.; Wang, Y.Y.; Zhu, X.F.; Chen, L.J.; Liu, X.Y.; Chen, J.S. First report of soybean cyst nematode, Heterodera glycines, on soybean from Guangxi, Guizhou, and Jiangxi Province, China. Plant Dis. 2015, 99, 893. [Google Scholar] [CrossRef]
- Zhang, J.L.; Peng, D.L.; Cao, K.Q. Molecular identification and distribution of Heterodera glycines in Hebei. Plant Prot. 2005, 31, 40–43. [Google Scholar]
- Lu, W.G.; Gai, J.Y.; Li, W.D. Sampling survey and identification of races of soybean cyst nematode (Heterodera glycines Ichinohe) in Huang-Huai Valleys. Agric. Sci. China 2006, 5, 615–621. [Google Scholar] [CrossRef]
- Ross, J.P. Physiological strains of Heterodera glycines. Plant Dis. Rep. 1962, 46, 766–769. [Google Scholar]
- Golden, A.M.; Epps, J.M.; Riggs, R.D.; Duclos, L.A.; Fox, J.A.; Bernard, R.L. Terminology and identity of infra-specific forms of the soybean cyst nematode (Heterodera glycines). Plant Dis. Rep. 1970, 54, 544–546. [Google Scholar]
- Riggs, R.D.; Schmitt, D.P. Complete characterization of the race scheme for Heterodera glycines. J. Nematol. 1988, 20, 392–395. [Google Scholar]
- Xing, H.; Zhao, J.R.; Zhang, M.K.; Li, P.T.; Gai, J.Y. Identification of races of soybean cyst nematode (Heterodera glycines Ichinohe) from Shandong province. China Oil 1997, 19, 61–65. [Google Scholar]
- Chen, S.; Pan, F.J.; Zhou, C.J.; Li, C.J.; Hua, C.; Mao, Y.Z.; Hu, Y.F.; Tian, Z.Y.; Jiao, X.G.; Wang, C.L. Genetic Variation of Soybean Cyst Nematode Races in Anda Area of Heilongjiang Province. Soil Crop. 2015, 4, 42–47. [Google Scholar]
- Liu, H.Q.; Liu, Q.C.; Zhao, H.H. Comparative analysis of the parasitism between soybean and tobacco populations of Heterodera glycines. Plant Prot. 2016, 42, 68–73. [Google Scholar]
- Ma, C.W.; Duan, Y.X.; Chen, L.J.; Wang, Y.Y.; Liu, D.W. Identification of physiological race of Heterodera glycines in Liaoning province. Soybean Sci. 2009, 28, 85–289. [Google Scholar]
- Yan, Q.S.; Chen, P.S.; Wang, L.Z. The verification of race 4 of soybean cyst nematode in suburban Beijing. Soybean Sci. 1995, 14, 355–359. [Google Scholar]
- Subbotin, S.A.; Mundoocampo, M.; Baldwin, J.G.; Subbotin, S.A.; Mundoocampo, M.; Baldwin, J.G. Systematics of Cyst Nematodes (Nematoda: Heteroderinae). In Nematology Monographs and Perspectives Volume 8B; Hunt, D.J., Perry, R.N., Eds.; Koninklijke Brill NV: Leiden, The Netherlands, 2010; Volume 61, pp. 35–42. [Google Scholar]
- Nelson, B.D.; Bolton, M.D.; Lopez-Nicora, H.D.; Niblack, T.L.; del Rio Mendoza, L. First confirmed report of sugar beet cyst nematode, Heterodera schachtii, in North Dakota. Plant Dis. 2012, 96, 772. [Google Scholar] [CrossRef]
- Westphal, A.; Institut, J.K. Vertical distribution of Heterodera schachtii under susceptible, resistant, or tolerant sugar beet cultivars. Plant Dis. 2013, 97, 101–106. [Google Scholar] [CrossRef]
- Escobar-Avila, I.M.; Cruz-Alvarado, Y.; Tovar-Soto, A.; Subbotin, A.S. First report of sugar beet cyst nematode, Heterodera schachtii on beetroot and broccoli in Mexico. Plant Dis. 2019, 103, 1434. [Google Scholar] [CrossRef]
- Peng, H.; Liu, H.; Gao, L.; Jiang, R.; Li, G.K.; Gao, H.F.; Wu, W.; Wang, J.; Zhang, Y.; Huang, W.K.; et al. Identification of Heterodera schachtii on sugar beet in Xinjiang Uygur Autonomous Region of China. J. Integr. Agric. 2022, 21, 1694–1702. [Google Scholar]
- Kim, J.; Kim, T.; Lee, Y.C.; Chun, J.Y.; Kern, E.M.; Jung, J.; Park, J.K. Characterization of 15 microsatellite loci and genetic analysis of Heterodera schachtii (Nematoda: Heteroderidae) in South Korea. Biochem. Syst. Ecol. 2016, 64, 97–104. [Google Scholar] [CrossRef]
- Lafi, H.A.; Al-Banna, L.; Sadder, M.T.; Migdadi, H.M. Morphological and morphometrical analysis of Heterodera spp. populations in Jordan. Saudi J. Biol. Sci. 2016, 23, 108–114. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sekimoto, S.; Hisai, J.; Iwahori, H. First report of the sugar beet cyst nematode, Heterodera schachtii, on Brassica sp. in Japan. Plant Dis. 2019, 103, 1433. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Koike, S.T.; Logan, G.D.; Drozd, C.; Silva, J.D.O.; Colindres, N.B.; Peacock, B.B.; Becker, J.S.; Loffredo, A.; Wu, H.Y.; et al. Detection of Nematophagous fungi from Heterodera schachtii females using a baiting experiment with soils cropped to Brassica species from California’s central coast. Pytofrontiers 2021, 1, 4–12. [Google Scholar] [CrossRef]
- Peng, H.; Gao, H.F.; Zhang, Y.D.; Li, H.K.; Wu, W.; Wang, S.L.; Peng, D.L. Study on the rapid molecular detection of SCAR of the sugar beet cyst nematode. In Proceedings of the 2019 Academic Annual Meeting of Chinese Society of Plant Protection, Guiyang, China, 23 October 2019. [Google Scholar]
- Whitehead, A.G.; Hemming, J.R. A comparison of some quantitative methods of extracting small vermiform nematodes from soil. Ann. Appl. Biol. 1965, 55, 25–38. [Google Scholar] [CrossRef]
- Golden, A.M.; Maqbool, M.A.; Shahina, F. Redescription of Heterodera fici (Nematoda: Heteroderidae) with SEM observations. J. Nematol. 1988, 20, 381–391. [Google Scholar] [PubMed]
- Ou, S.Q.; Peng, D.L.; Liu, X.M.; Li, Y.; Moens, M. Identification of Heterodera glycines using PCR with sequence characterised amplified region (SCAR) primers. Nematology 2008, 10, 397–403. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Joyce, S.A.; Reid, A.; Driver, F.; Curran, J. Application of polymerase chain reaction (PCR) methods to identification of entomopathogenic nematodes. In COST 812 Biotechnology: Genetics of Entomopathogenic Nematode-Bacterium Complexes; Burnell, A.M., Ehlers, R.U., Masson, J.P., Eds.; European Commission: Brussels, Belgium, 1994; pp. 178–187. [Google Scholar]
- Jiang, C.; Zhang, Y.D.; Yao, K.; Abdulsalam, S.; Li, G.K.; Gao, H.F.; Li, K.M.; Huang, W.K.; Kong, L.A.; Peng, D.L.; et al. Development of a species-specific SCAR-PCR assay for dirtect detection of sugar beet nematode (Heterodera schachtii) from infected roots and soil samples. Life 2021, 11, 1358. [Google Scholar] [CrossRef]
- Subbotin, S.A.; Peng, D.L.; Moens, M. A rapid method for the identification of the soybean cyst nematode Heterodera glycines using duplex PCR. Nematology 2001, 3, 365–371. [Google Scholar] [CrossRef]
- Vanholme, B.; Thuyne, W.V.; Vanhouteghem, K.; Meutter, J.D.; Cannoot, B.; Gheysen, G. Molecular characterization and functional importance of pectate lyase secreted by the cyst nematode Heterodera schachtii. Mol. Plant Pathol. 2007, 8, 267–278. [Google Scholar] [CrossRef]
- Qiao, J.S.; Peng, D.L.; Liu, H.; Feng, X.D.; Gao, H.F.; Li, G.K.; Hu, X.Q.; Peng, H. The host range and life story of Heterodera schachtii in China. Plant Prot. 2021, 47, 177–183. [Google Scholar]
- Tylka, G.L.; Marett, C.C. Known distribution of the soybean cyst nematode, Heterodera glycines, in the United States and Canada in 2020. Plant Health Prog. 2021, 22, 72–74. [Google Scholar] [CrossRef]
- Howland, A.; Monnig, N.; Mathesius, J.; Nathan, M.; Mitchum, M.G. Survey of Heterodera glycines population densities and virulence phenotypes during 2015-2016 in Missouri. Plant Dis. 2018, 102, 2407–2410. [Google Scholar] [CrossRef] [PubMed]
- Ichinohe, M. Studies on the morphology and ecology of the soy bean cyst nematode, Heterodera glycines, in Japan. Rep. Hok. Natl. Agric. Exp. Sta. 1955, 48, 59–64. [Google Scholar]
- Cui, J.K.; Erginbas-Orakci, G.; Peng, H.; Huang, W.K.; Liu, S.M.; Qiao, F.; Elekcioglu, H.; Imren, M.; Dababat, A.A.; Peng, D.L. First report of sugar beet nematode, Heterodera schachtii Schmidt, 1871 (Nemata: Heteroderidae) in sugar beet growing areas of Sanliurfa, Turkey. Turk. J. Entomol. 2016, 40, 303–314. [Google Scholar] [CrossRef]
- Mwamula, A.O.; Ko, H.R.; Kim, Y.; Kim, Y.H.; Lee, J.K.; Lee, D.W. Morphological and molecular characterization of Heterodera schachtii and the newly recorded cyst nematode, H. trifolii associated with Chinese cabbage in Korea. Plant Pathol. J. 2018, 34, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Riggs, R.D.; Schmitt, D.P. Optimization of the Heterodera glycines Race Test Procedure. J. Nematol. 1991, 23, 149–154. [Google Scholar]
- Tian, Z.Y.; Gao, G.J.; Zhou, C.J.; Du, Z.Q.; Wu, Y.K.; Wang, M.Z.; Yang, L.; Li, J.Y. Study on the variation of soybean cyst nematode. Soybean Sci. 2007, 26, 290–292. [Google Scholar]
- Chowdhury, I.A.; Yan, G.P.; Plaisance, A.; Markell, S. Characterization of virulence phenotypes of soybean cyst nematode (Heterodera glycines) populations in North Dakota. Phytopathology 2021, 111, 2100–2109. [Google Scholar] [CrossRef]
- Peterka, H.; Budahn, H.; Schrader, O.; Ahne, R. Transfer of resistance against the beet cyst nematode from radish (Raphanus sativus) to rape (Brassica napus) by monosomic chromosome addition. Theor. Appl. Genet. 2004, 109, 30–41. [Google Scholar] [CrossRef]
- Budahn, H.; Peterka, H.; Mousa, M.A.A.; Ding, Y.H.; Zhang, S.S.; Li, J.B. Molecular mapping in oil radish (Raphanus sativus L.) and QTL analysis of resistance against beet cyst nematode (Heterodera schachtii). Theor. Appl. Genet. 2009, 118, 775–782. [Google Scholar] [CrossRef] [PubMed]


| Primer Name | Sequence 5′-3′ | Types | Reference |
|---|---|---|---|
| TW81 | 5′-GTTTCCGTAGGTGAACCTGC-3′ | rDNA-ITS universal primers | [30] |
| AB28 | 5′-ATATGCTTAAGTTCAGCGGGT-3′ | ||
| SCNFI | 5′-GGACCCTGACCAAAAAGTTTCCGC-3′ | SCAR primers | [28] |
| SCNRI | 5′-GGACCCTGACGAGTTATGGGCCCG-3′ | ||
| OPA06-HsF | 5′-GGACCCTGACGACCAGAATA-3′ | SCAR primers | [31] |
| OPA06-HsR | 5′-GACAACACGAAGGAGCGAGC-3′ | ||
| D2A | 5′-ACAAGTACCGTGAGGGAAAGTTG-3′ | 28S-rDNA universal primers | [32] |
| D3B | 5′-TCGGAAGGAACCAGCTACTA-3′ |
| Location | Total Number of Plot Inspected | Number of Plot Inspected | Population | Density of Cysts (NO./200 cc soil) |
|---|---|---|---|---|
| Zhangbei county | 94 | 1 | ZM | 57 |
| 1 | ZZ | 94 | ||
| Kangbao county | 17 | 1 | KM | 41 |
| Guyuan county | 11 | 0 | - | - |
| Shangyi county | 11 | 0 | - | - |
| Chabei management district | 4 | 1 | CB | 103 |
| Saibei management district | 4 | 0 | - | - |
| Weichang manchu autonomous county | 12 | 0 | - | - |
| Fengning manchu autonomous county | 5 | 1 | FN | 31 |
| Populations | Survey Item | Soybean Differential Hosts | Race | ||||
|---|---|---|---|---|---|---|---|
| Peking | Pickett | PI88788 | PI90763 | Lee | |||
| ZM | No. of cysts | 124.4 ± 9.8 | 112.2 ± 11.3 | 108.6 ± 11.1 | 103.4 ± 4.8 | 144.0 ± 10.2 | 4 |
| FI (%) | 86.4 | 77.9 | 75.4 | 71.8 | 100 | ||
| Reaction | + | + | + | + | |||
| KM | No. of cysts | 77.6 ± 5.1 | 84.2 ± 4.3 | 57.4 ± 5.5 | 115.6 ± 7.2 | 125.8 ± 5.9 | 4 |
| FI (%) | 61.7 | 66.9 | 45.6 | 91.9 | 100 | ||
| Reaction | + | + | + | + | |||
| CB | No. of cysts | 123.6 ± 8.3 | 91.0 ± 9.4 | 82.6 ± 4.6 | 103.8 ± 4.8 | 151.6 ± 3.4 | 4 |
| FI (%) | 81.5 | 60.0 | 54.5 | 68.5 | 100 | ||
| Reaction | + | + | + | + | |||
| FN | No. of cysts | 0.4 ± 0.5 | 6.4 ± 1.9 | 12.4 ± 3.0 | 2.6 ± 1.3 | 157.2 ± 5.6 | 3 |
| FI (%) | 0.3 | 4.1 | 7.9 | 1.7 | 100 | ||
| Reaction | - | - | - | - | |||
| Family | Tested Plants | Cultivar | No. of Second-Stage Juveniles | No. of Third-Stage Juveniles | No. of Fourth-Stage Juveniles | No. of Females |
|---|---|---|---|---|---|---|
| Brassicaceae | Chinese cabbage | Linglonghuang012 | 43.2 ± 4.15 b | 80.2 ± 5.50 a | 125.6 ± 12.34 a | 37.8 ± 4.87 a |
| Cabbage | Chunwang | 21 ± 3.16 d | 19.8 ± 5.26 c | 9.8 ± 2.28 b | 13.8 ± 2.39 b | |
| canola | Lvguan1 | 14.4 ± 3.36 e | 0 d | 0 c | 0 c | |
| Leguminosae | Soybean | Zhonghuang13 | 33.2 ± 2.95 c | 34.8 ± 2.59 b | 6.8 ± 1.48 b | 0 c |
| Kidney bean | Baifeng | 126.6 ± 7.83 a | 0 d | 0 c | 0 c | |
| Jiulibai | 15.4 ± 2.41 e | 0 d | 0 c | 0 c | ||
| Baibulao | 5.2 ± 1.30 f | 0 d | 0 c | 0 c | ||
| Cucurbitaceae | Zucchini | Jinghu12 | 6.0 ± 2.24 f | 0 d | 0 c | 0 c |
| Zaoqing1 | 5.6 ± 1.82 f | 0 d | 0 c | 0 c | ||
| Cucumber | Zhongnong18 | 4.4 ± 1.14 f | 0 d | 0 c | 0 c | |
| Solanaceae | Tomato | Zhongza9 | 11.4 ± 2.30 e | 0 d | 0 c | 0 c |
| Potato | Helan15 | 0 g | 0 d | 0 c | 0 c | |
| Eggplant | Junlang | 0 g | 0 d | 0 c | 0 c | |
| Umbelliferae | Celery | Yuhuang | 0 g | 0 d | 0 c | 0 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Peng, H.; Liu, S.; Shao, H.; Li, Y.; Zhang, Y.; Li, Y.; Liu, D.; Peng, D. Investigation and Identification of Cyst Nematodes in the Bashang Region of Hebei, China. Agronomy 2022, 12, 2227. https://doi.org/10.3390/agronomy12092227
Wu Y, Peng H, Liu S, Shao H, Li Y, Zhang Y, Li Y, Liu D, Peng D. Investigation and Identification of Cyst Nematodes in the Bashang Region of Hebei, China. Agronomy. 2022; 12(9):2227. https://doi.org/10.3390/agronomy12092227
Chicago/Turabian StyleWu, Yuhuan, Huan Peng, Shiming Liu, Hudie Shao, Yunqing Li, Yingdong Zhang, Yaning Li, Daqun Liu, and Deliang Peng. 2022. "Investigation and Identification of Cyst Nematodes in the Bashang Region of Hebei, China" Agronomy 12, no. 9: 2227. https://doi.org/10.3390/agronomy12092227
APA StyleWu, Y., Peng, H., Liu, S., Shao, H., Li, Y., Zhang, Y., Li, Y., Liu, D., & Peng, D. (2022). Investigation and Identification of Cyst Nematodes in the Bashang Region of Hebei, China. Agronomy, 12(9), 2227. https://doi.org/10.3390/agronomy12092227







