Effects of Leaf Surface Roughness and Contact Angle on In Vivo Measurement of Droplet Retention
Abstract
:1. Introduction
2. Materials and Methods
2.1. Crops and Culture Environment
2.2. Spray System
2.3. Measurement of Foliar Parameters
2.3.1. Measurement of Leaf Area
2.3.2. Measurement of Surface Roughness
2.3.3. Measurement of Contact Angles
2.4. Measurement of Droplet Retention
3. Results
3.1. Crop-Leaf Parameters
3.2. Droplet Retention on Crop Leaves
3.2.1. Droplet Retention on Tomato Leaves
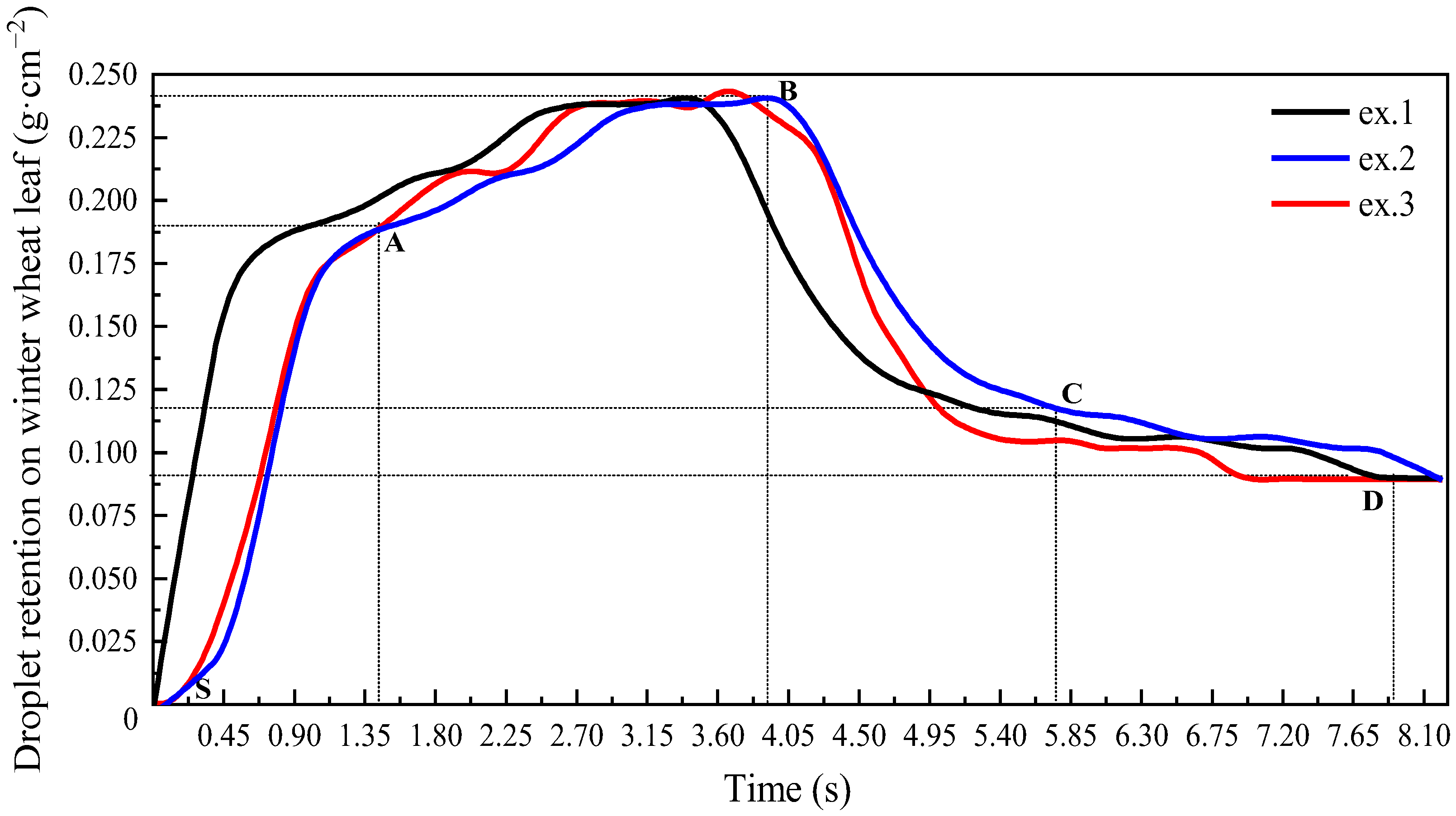
3.2.2. Droplet Retention on Winter Wheat Leaves
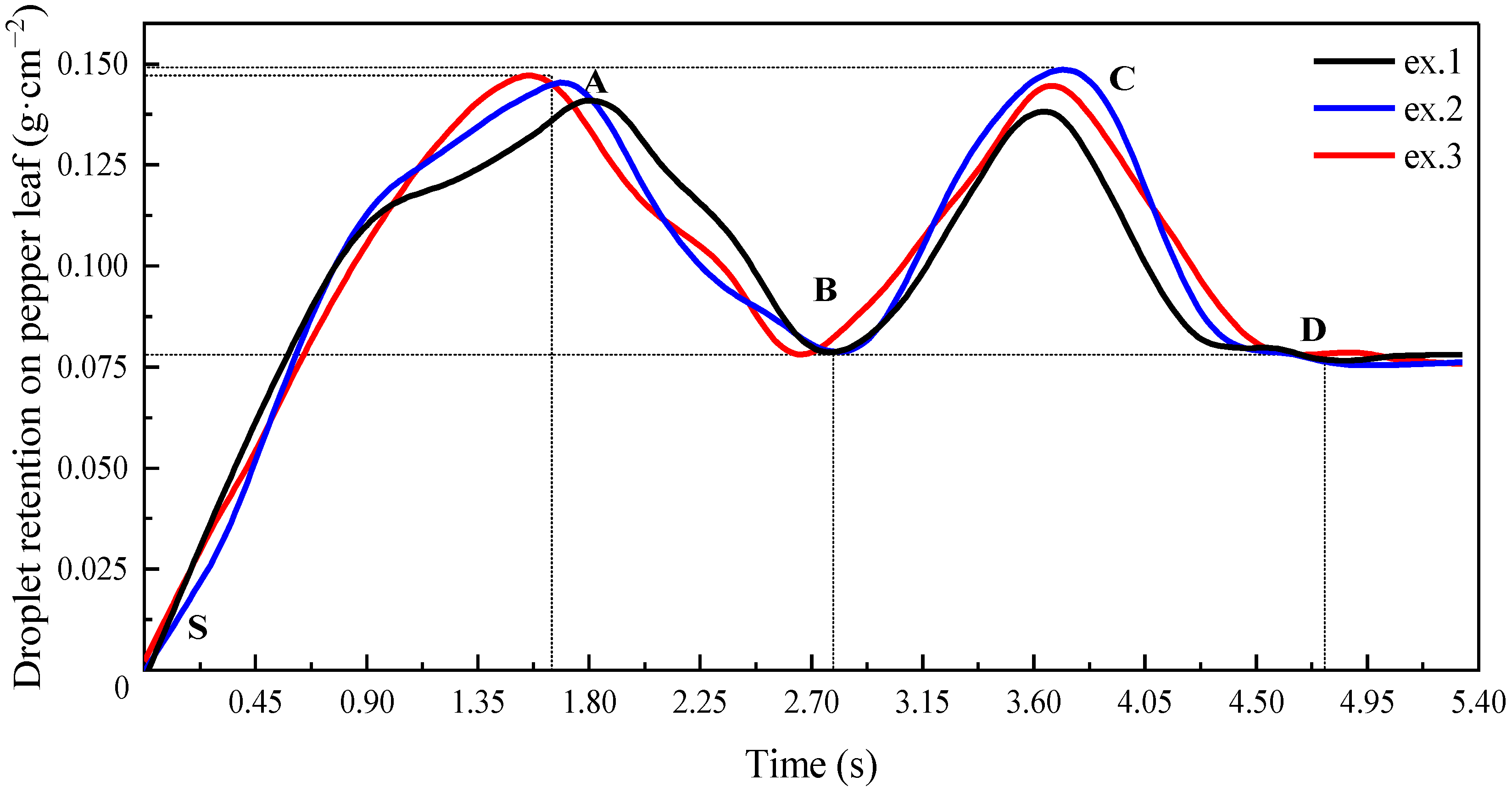
3.2.3. Droplet Retention on Pepper Leaves
4. Discussion
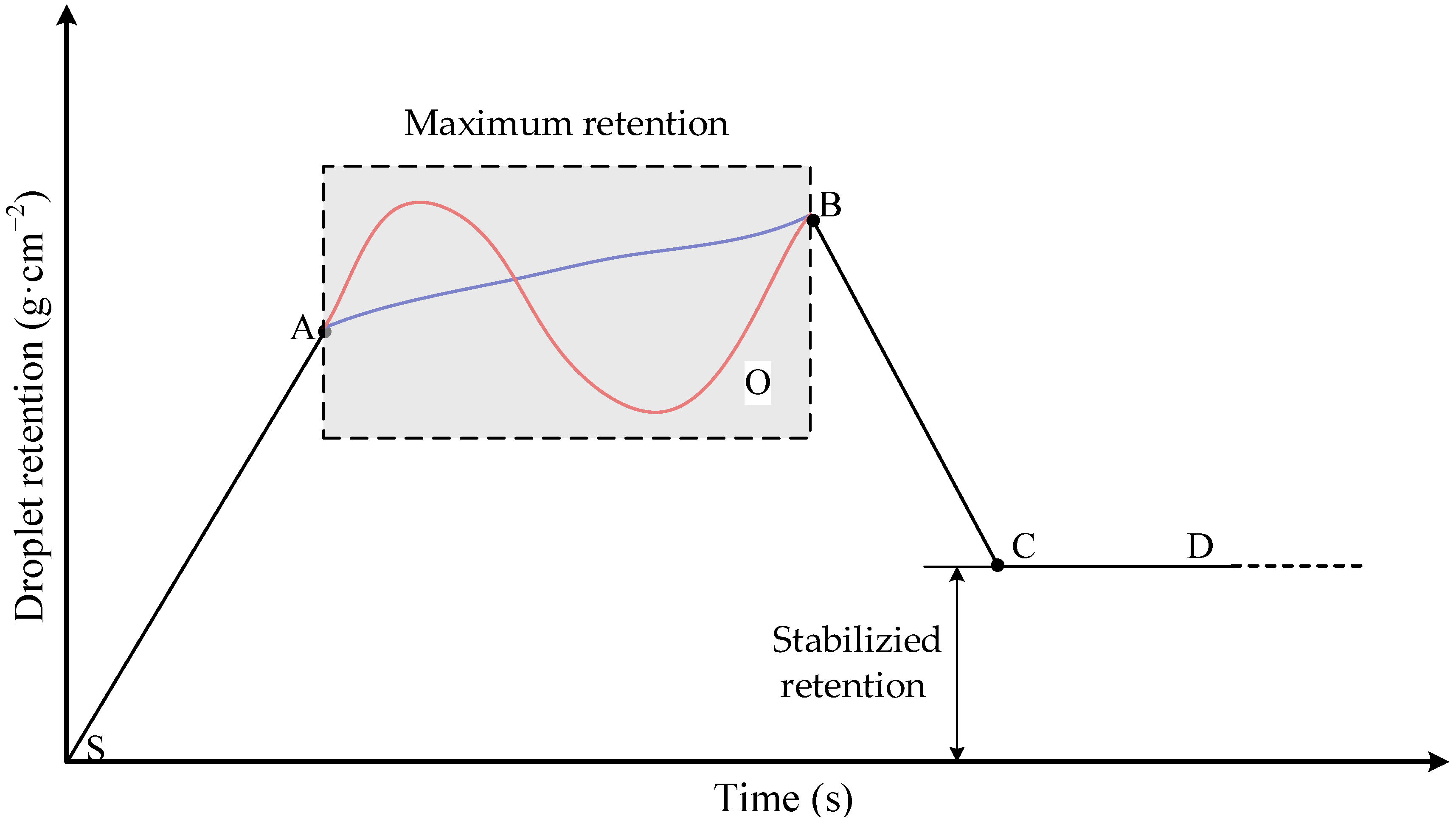
4.1. Model of Droplet Retention on Leaf Surface
4.2. Effects of Leaf Surface Properties on Droplet-Population Retention
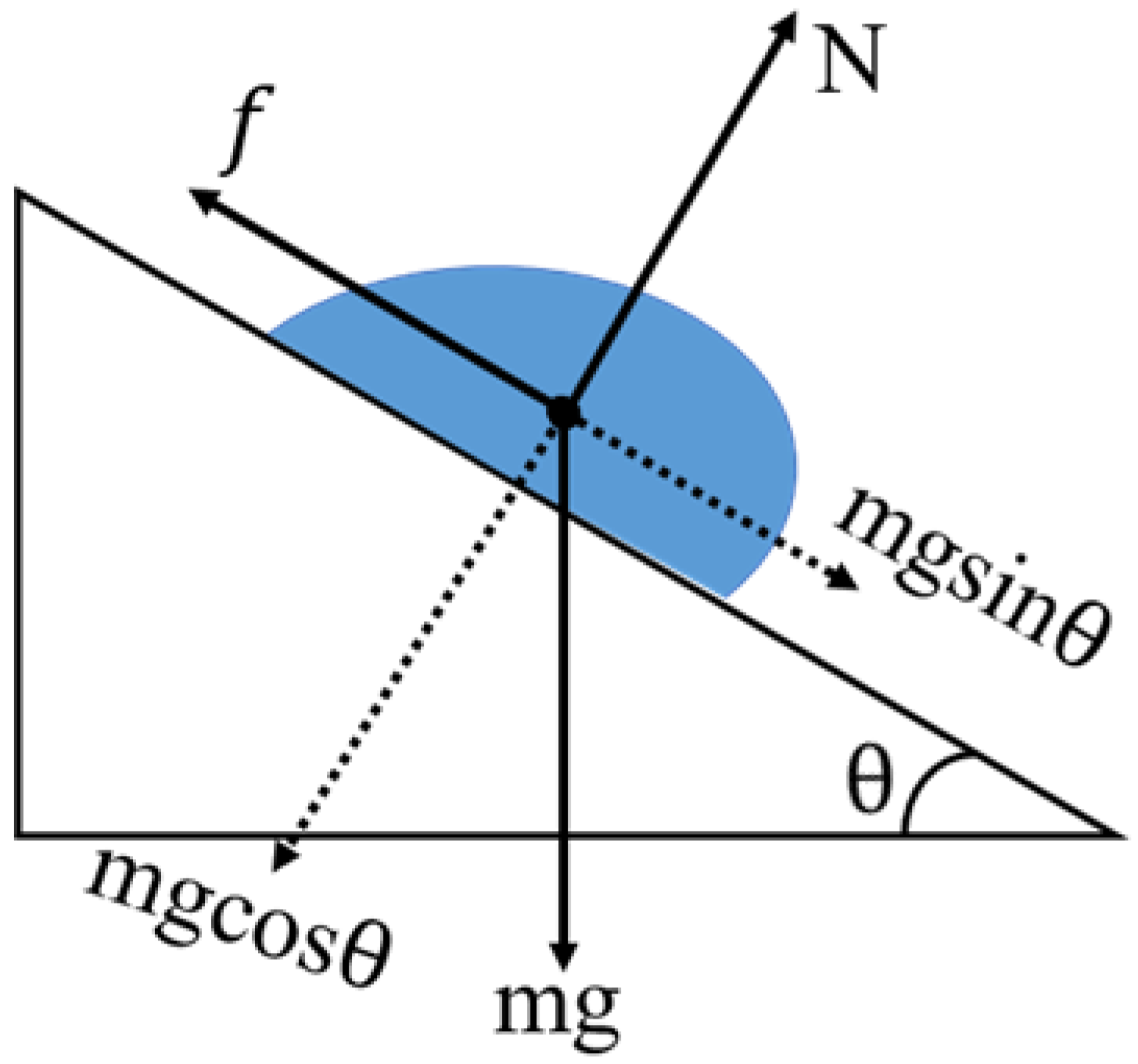
4.3. Effect of the Lateral Retention Force on Droplet Retention
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dorr, G.J.; Wang, S.; Mayo, L.C.; McCue, S.W.; Forster, W.A.; Hanan, J.; He, X. Impaction of Spray Droplets on Leaves: Influence of Formulation and Leaf Character on Shatter, Bounce and Adhesion. Exp. Fluids 2015, 56, 143. [Google Scholar] [CrossRef]
- Park, H.; Kim, S.; Gruszewski, H.A.; Schmale, I.I.I.D.G.; Boreyko, J.B.; Jung, S. Dynamics of splashed droplets impacting wheat leaves treated with a fungicide. J. R. Soc. Interface 2020, 17, 20200337. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.D.; Skurtys, O.; Osorio, F. The Impact of Liquid Drops on Purple Cabbage Leaves (Brassica oleracea L. Var. Capitata). Ing. Investig. 2012, 32, 79–82. [Google Scholar]
- Dong, X.; Zhu, H.; Yang, X. Characterization of Droplet Impact and Deposit Formation on Leaf Surfaces. Pest Manag. Sci. 2015, 71, 302–308. [Google Scholar] [CrossRef]
- Zhang, H.; Dorr, G.J.; Hewitt, A.J. Retention and Efficacy of Ultra-Low Volume Pesticide Applications on Culex Quinquefasciatus (Diptera: Culicidae). Environ. Sci. Pollut. Res. 2015, 22, 16492–16501. [Google Scholar] [CrossRef] [PubMed]
- Papierowska, E.; Mazur, R.; Stańczyk, T.; Beczek, M.; Szewińska, J.; Sochan, A.; Ryżak, M.; Szatyłowicz, J.; Bieganowski, A. Influence of Leaf Surface Wettability on the Drop Splash Phenomenon. Agric. Forest Meteorol. 2019, 279, 107762. [Google Scholar] [CrossRef]
- Shao, F.; Wang, L.; Sun, F.; Li, G.; Yu, L.; Wang, Y.; Zeng, X.; Yan, H.; Dong, L.; Bao, Z. Study on different particulate matter retention capacities of the leaf surfaces of eight common garden plants in Hangzhou, China. Sci. Total Environ. 2019, 652, 939–951. [Google Scholar] [CrossRef]
- Furmidge, C.G.L. Physico-Chemical Studies on Agricultural Sprays. IV—The Retention of Spray Liquids on Leaf Surfaces. J. Sci. Food Agric. 1962, 13, 127–140. [Google Scholar] [CrossRef]
- Yuan, H.; Qi, S.; Yang, D. Study on the Point of Run-off and the Maximum Retention of Spray Liquid on Crop Leaves. Chin. J. Pestic. Sci. 2000, 2, 66–71. [Google Scholar]
- Furmidge, C.G.L. Studies at Phase Interfaces. I. The Sliding of Liquid Drops on Solid Surfaces and a Theory for Spray Retention. J. Colloid Sci. 1962, 17, 309–324. [Google Scholar] [CrossRef]
- Cunningham, G.P.; Harden, J. Reducing Spray Volumes Applied to Mature Citrus Trees. Crop Prot. 1998, 17, 289–292. [Google Scholar] [CrossRef]
- Hall, F.R.; Downer, R.A.; Cooper, J.A.; Ebert, T.A.; Ferree, D.C. Changes in Spray Retention by Apple Leaves during a Growing Season. HortScience 1997, 32, 858–860. [Google Scholar] [CrossRef]
- Lu, J.; Jia, W.; Qiu, B.; Li, P. Experiment on Retention of Spray Liquid on Cucumber Leaves. Trans. Chin. Soc. Agric. Mach. 2010, 41, 60–64. [Google Scholar]
- Dorr, G.J.; Kempthorne, D.M.; Mayo, L.C.; Forster, W.A.; Zabkiewicz, J.A.; McCue, S.W.; Belward, J.A.; Turner, I.W.; Hanan, J. Towards a Model of Spray–Canopy Interactions: Interception, Shatter, Bounce and Retention of Droplets on Horizontal Leaves. Ecol. Modelling 2014, 290, 94–101. [Google Scholar] [CrossRef]
- Dorr, G.J.; Forster, W.A.; Mayo, L.C.; McCue, S.W.; Kempthorne, D.M.; Hanan, J.; Turner, I.W.; Belward, J.A.; Young, J.; Zabkiewicz, J.A. Spray Retention on Whole Plants: Modelling, Simulations and Experiments. Crop Prot. 2016, 88, 118–130. [Google Scholar] [CrossRef]
- Butler Ellis, M.C.; Webb, D.A.; Western, N.M. The Effect of Different Spray Liquids on the Foliar Retention of Agricultural Sprays by Wheat Plants in a Canopy. Pest Manag. Sci. 2004, 60, 786–794. [Google Scholar] [CrossRef]
- Zwertvaegher, I.K.; Verhaeghe, M.; Brusselman, E.; Verboven, P.; Lebeau, F.; Massinon, M.; Nicolaï, B.M.; Nuyttens, D. The Impact and Retention of Spray Droplets on a Horizontal Hydrophobic Surface. Biosyst. Eng. 2014, 126, 82–91. [Google Scholar] [CrossRef]
- Agrawal, G.; Negi, Y.S.; Pradhan, S.; Dash, M.; Samal, S.K. 3—Wettability and contact angle of polymeric biomaterials. In Characterization of Polymeric Biomaterials; Woodhead Publishing: Cambridge, UK, 2017; pp. 57–81. [Google Scholar] [CrossRef]
- Koch, K.; Bhushan, B.; Barthlott, W. Diversity of Structure, Morphology and Wetting of Plant Surfaces. Soft Matter 2008, 4, 1943–1963. [Google Scholar] [CrossRef]
- Qiu, B.; Zhao, X.; Jia, W.; Zhang, Z. Stable Interval Method Based on Images to Measure Drop Contact Angles on Plant Leaf Surfaces. Trans. Chin. Soc. Agric. Mach. 2009, 40, 139–144. [Google Scholar]
- Wang, H.; Feng, H.; Liang, W.; Luo, Y.; Malyarchuk, V. Effect of Surface Roughness on Retention and Removal of Escherichia Coli O157:H7 on Surfaces of Selected Fruits. J. Food Sci. 2009, 74, E8–E15. [Google Scholar] [CrossRef]
- Bediaf, H.; Sabre, R.; Journaux, L.; Cointault, F. Comparison of Leaf Surface Roughness Analysis Methods by Sensitivity to Noise Analysis. Biosyst. Eng. 2015, 136, 77–86. [Google Scholar] [CrossRef]
- Ma, J.; Liu, K.; Chen, C.; Ahmad, F.; Qiu, B. Influence of Plant Leaf Moisture Content on Retention of Electrostatic-Induced Droplets. Sustainability 2021, 13, 11685. [Google Scholar] [CrossRef]
- De Ruiter, H.; Uffing, A.J.M.; Meinen, E.; Prins, A. Influence of Surfactants and Plant Species on Leaf Retention of Spray Solutions. Weed Sci. 1990, 38, 567–572. [Google Scholar] [CrossRef]
- Hunsche, M.; Bringe, K.; Schmitz-Eiberger, M.; Noga, G. Leaf Surface Characteristics of Apple Seedlings, Bean Seedlings and Kohlrabi Plants and Their Impact on the Retention and Rainfastness of Mancozeb. Pest. Manag. Sci. 2006, 62, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Göhl, J.; Mark, A.; Sasic, S.; Edelvik, F. An Immersed Boundary Based Dynamic Contact Angle Framework for Handling Complex Surfaces of Mixed Wettabilities. Int. J. Multiph. Flow 2018, 109, 164–177. [Google Scholar] [CrossRef]
- Holder, C.D.; Lauderbaugh, L.K.; Ginebra-Solanellas, R.M.; Webb, R. Changes in Leaf Inclination Angle as an Indicator of Progression toward Leaf Surface Storage during the Rainfall Interception Process. J. Hydrol. 2020, 588, 125070. [Google Scholar] [CrossRef]
- Guo, M.; Böttcher, F.; Hertkorn, J.; Schmidt, J.-N.; Wenzel, M.; Büchler, H.P.; Langen, T.; Pfau, T. The Low-Energy Goldstone Mode in a Trapped Dipolar Supersolid. Nature 2019, 574, 386–389. [Google Scholar] [CrossRef]
- Bormashenko, E.; Bormashenko, Y.; Stein, T.; Whyman, G.; Bormashenko, E. Why Do Pigeon Feathers Repel Water? Hydrophobicity of Pennae, Cassie-Baxter Wetting Hypothesis and Cassie-Wenzel Capillarity-Induced Wetting Transition. J. Colloid Interface Sci. 2007, 311, 212–216. [Google Scholar] [CrossRef]
- Aboud, D.G.K.; Kietzig, A.-M. Splashing Threshold of Oblique Droplet Impacts on Surfaces of Various Wettability. Langmuir 2015, 31, 10100–10111. [Google Scholar] [CrossRef]
- Tadmor, R.; Chaurasia, K.; Yadav, P.S.; Leh, A.; Bahadur, P.; Dang, L.; Hoffer, W.R. Drop Retention Force as a Function of Resting Time. Langmuir 2008, 24, 9370–9374. [Google Scholar] [CrossRef]
- Cao, C.; Song, Y.-Y.; Zhou, Z.-L.; Cao, L.-D.; Li, F.-M.; Huang, Q.-L. Effect of Adhesion Force on the Height Pesticide Droplets Bounce on Impaction with Cabbage Leaf Surfaces. Soft Matter 2018, 14, 8030–8035. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.S.; Bahadur, P.; Tadmor, R.; Chaurasia, K.; Leh, A. Drop Retention Force as a Function of Drop Size. Langmuir 2008, 24, 3181–3184. [Google Scholar] [CrossRef] [PubMed]


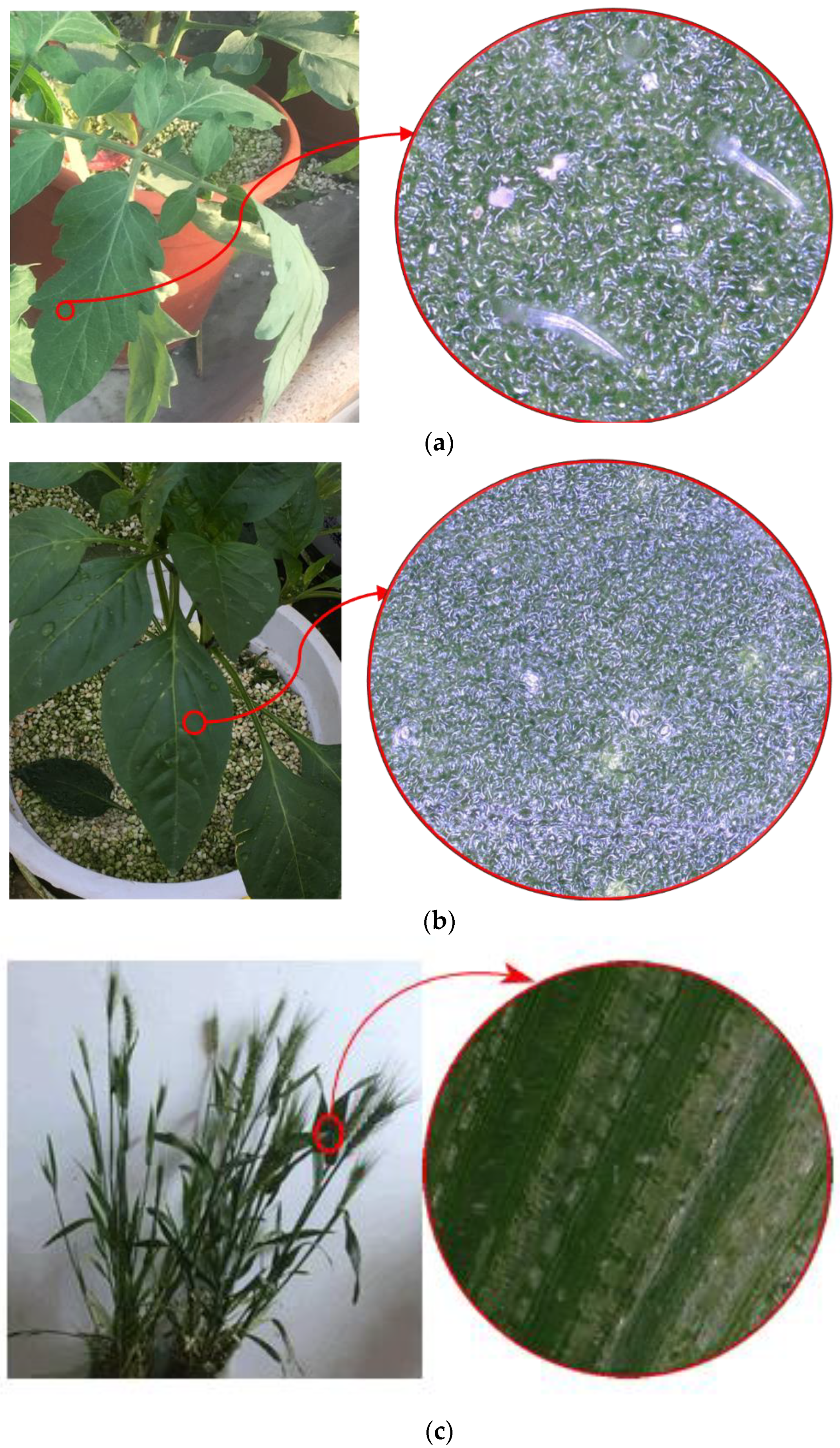

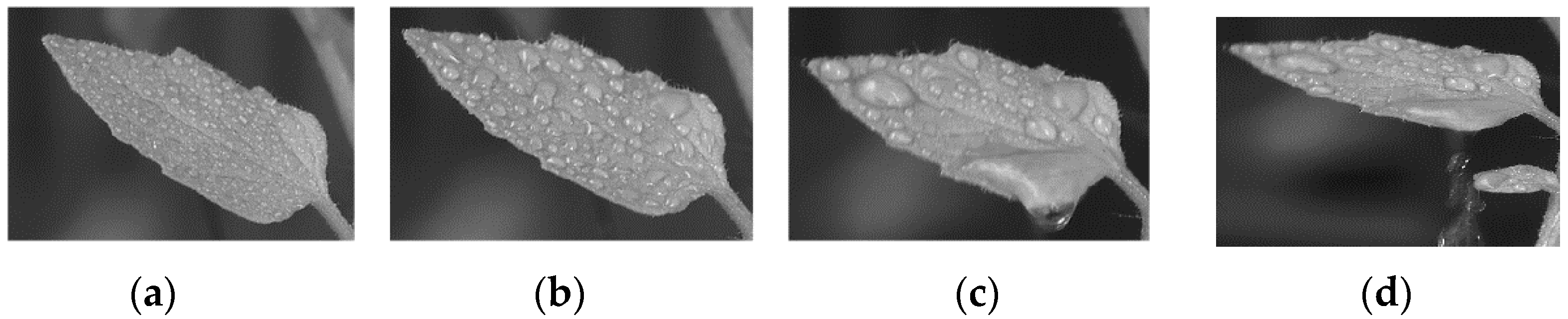

| Crop Species | Surface Properties | Average Leaf Area (cm2) | Surface Roughness () | Surface Hydrophobicity |
|---|---|---|---|---|
| Tomato | Glandular hairs | 14.20 | 24.73 | 97.67° |
| Pepper | Wax | 24.29 | 5.28 | 70.07° |
| Winter wheat | Waxy crystals | 20.90 | 17.59 | 131.98° |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Liu, K.; Dong, X.; Chen, C.; Qiu, B.; Zhang, S. Effects of Leaf Surface Roughness and Contact Angle on In Vivo Measurement of Droplet Retention. Agronomy 2022, 12, 2228. https://doi.org/10.3390/agronomy12092228
Ma J, Liu K, Dong X, Chen C, Qiu B, Zhang S. Effects of Leaf Surface Roughness and Contact Angle on In Vivo Measurement of Droplet Retention. Agronomy. 2022; 12(9):2228. https://doi.org/10.3390/agronomy12092228
Chicago/Turabian StyleMa, Jing, Kuan Liu, Xiaoya Dong, Chenggong Chen, Baijing Qiu, and Songchao Zhang. 2022. "Effects of Leaf Surface Roughness and Contact Angle on In Vivo Measurement of Droplet Retention" Agronomy 12, no. 9: 2228. https://doi.org/10.3390/agronomy12092228
APA StyleMa, J., Liu, K., Dong, X., Chen, C., Qiu, B., & Zhang, S. (2022). Effects of Leaf Surface Roughness and Contact Angle on In Vivo Measurement of Droplet Retention. Agronomy, 12(9), 2228. https://doi.org/10.3390/agronomy12092228








