Effects and Molecular Mechanism of Mycorrhiza on the Growth, Nutrient Absorption, Quality of Fresh Leaves, and Antioxidant System of Tea Seedlings Suffering from Salt Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. AMF Processing and Plant Culture
2.3. Variable Determinations
2.4. Statistical Analysis
3. Results
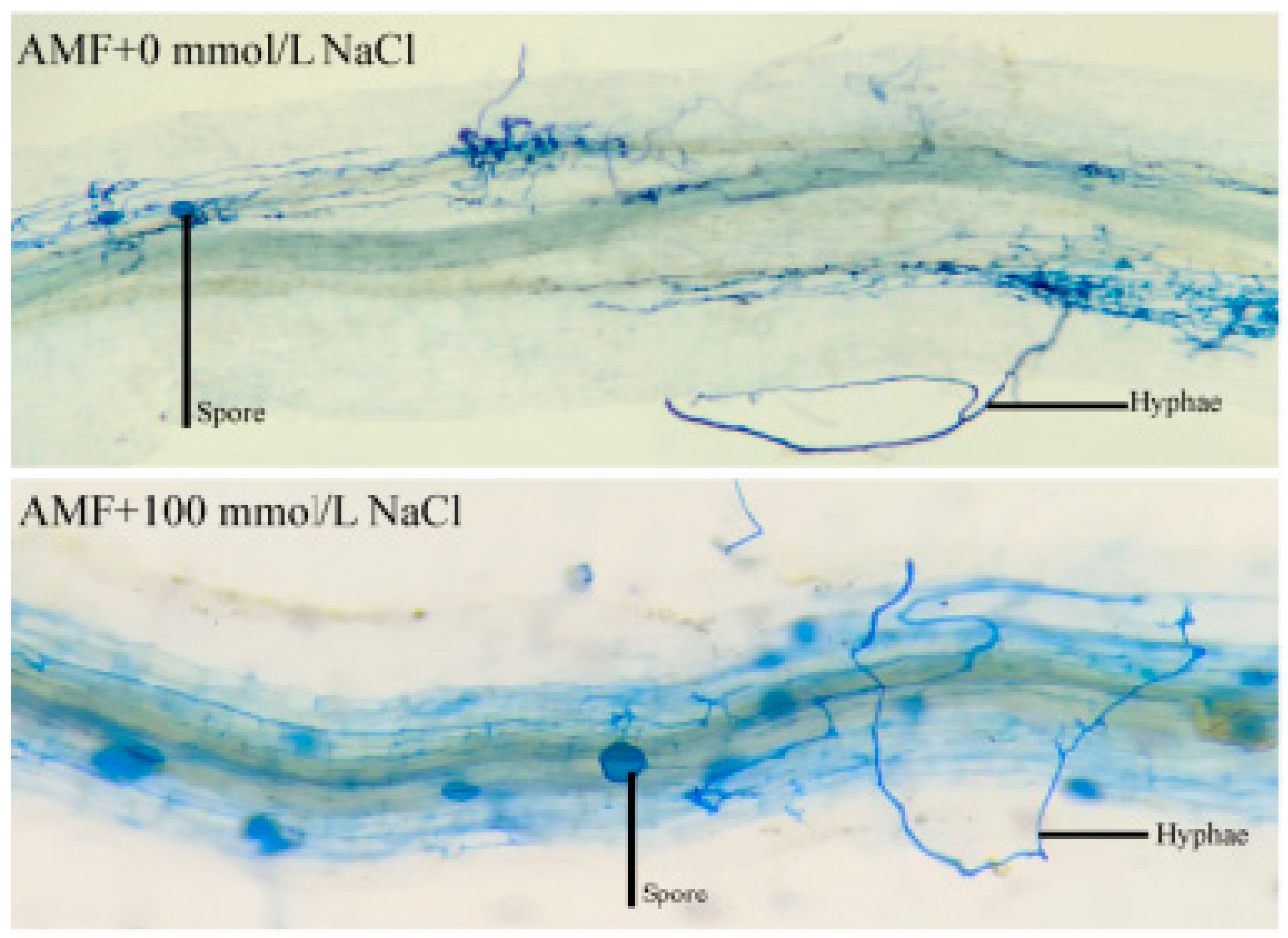
3.1. Plant Growth Performance and Root Mycorrhizal Colonization
3.2. Changes in the Leaves’ Nutrients
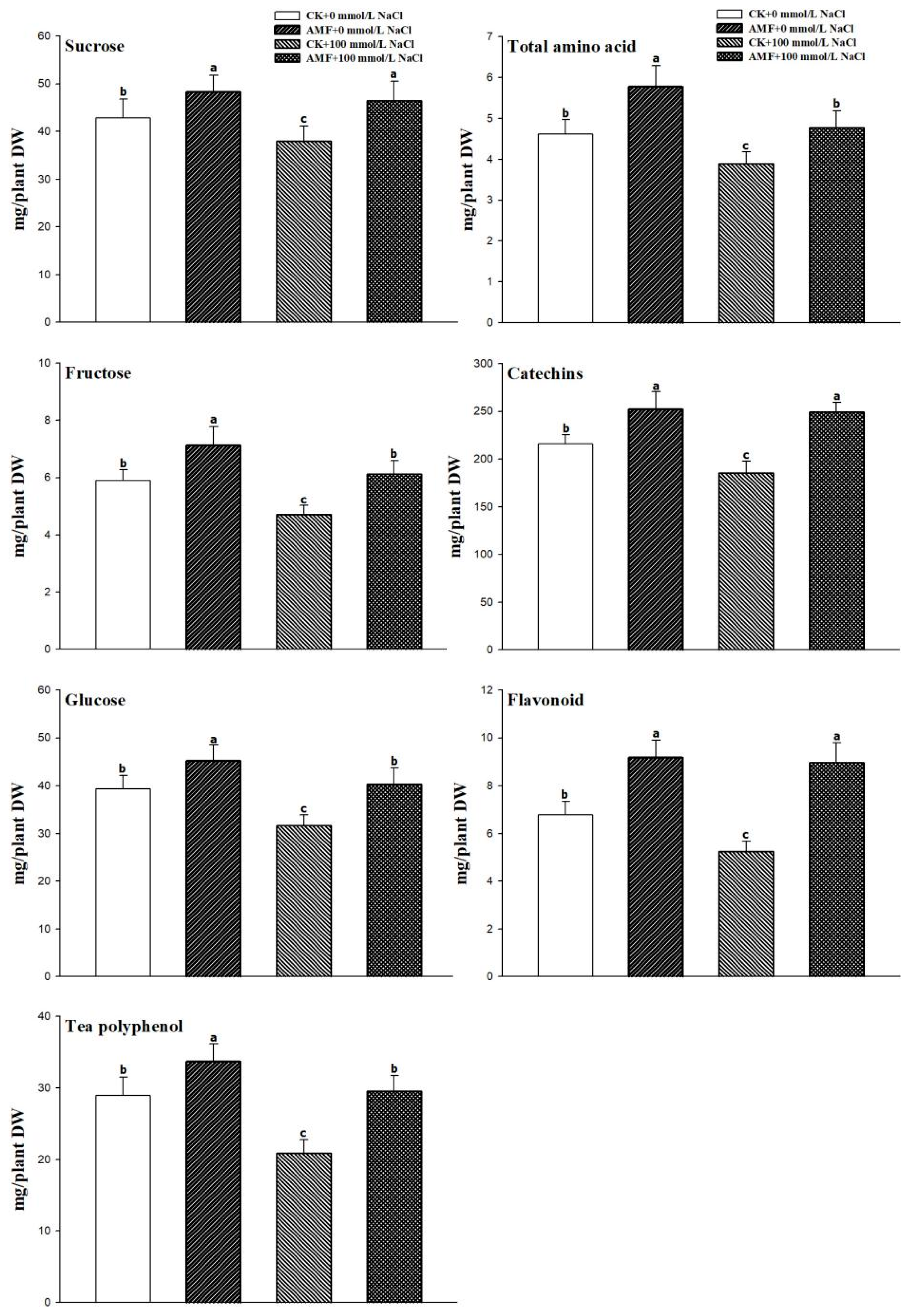
3.3. Changes in the Leaves’ Food Quality
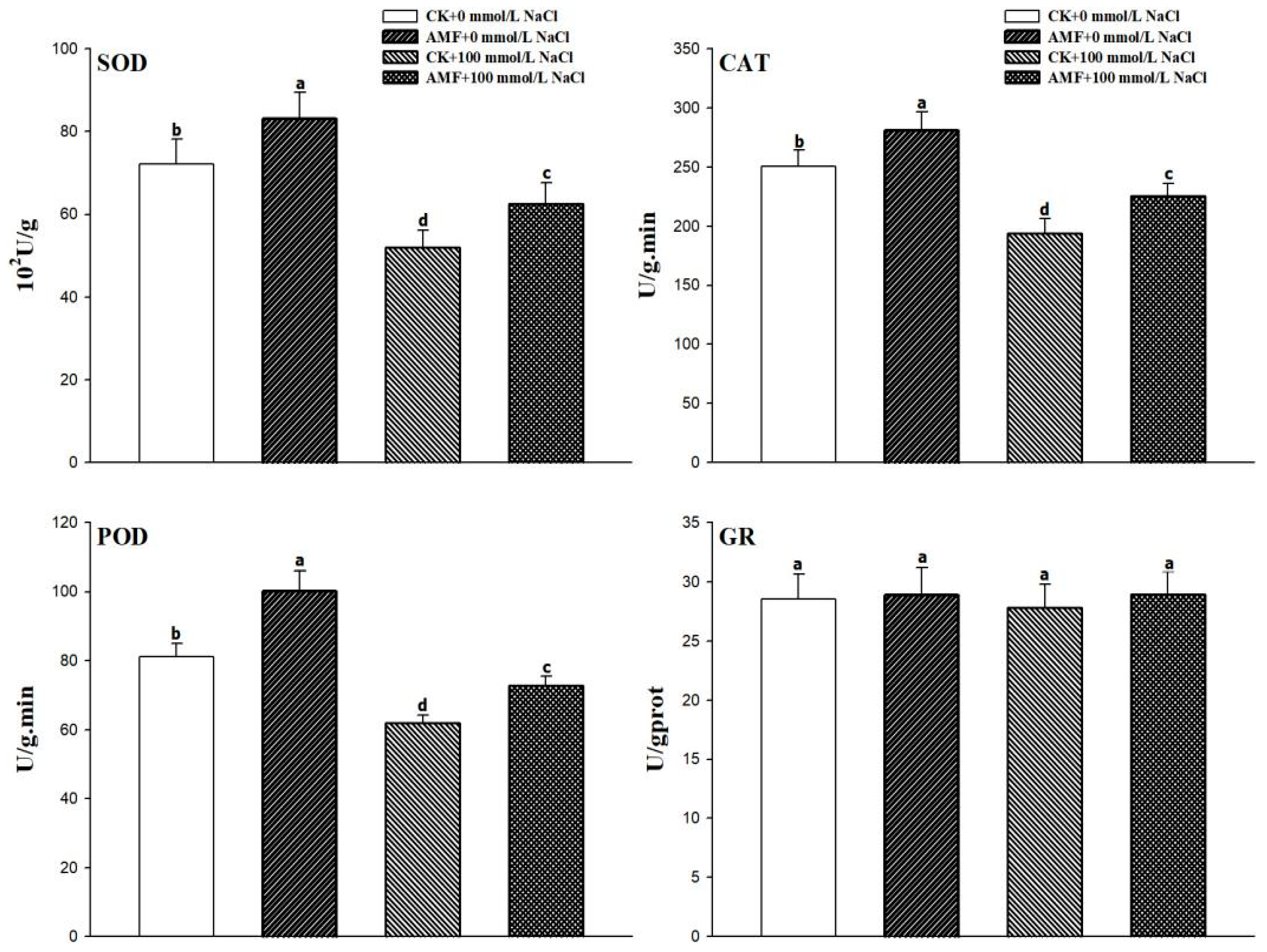
3.4. Changes in the Antioxidant Enzyme Activities
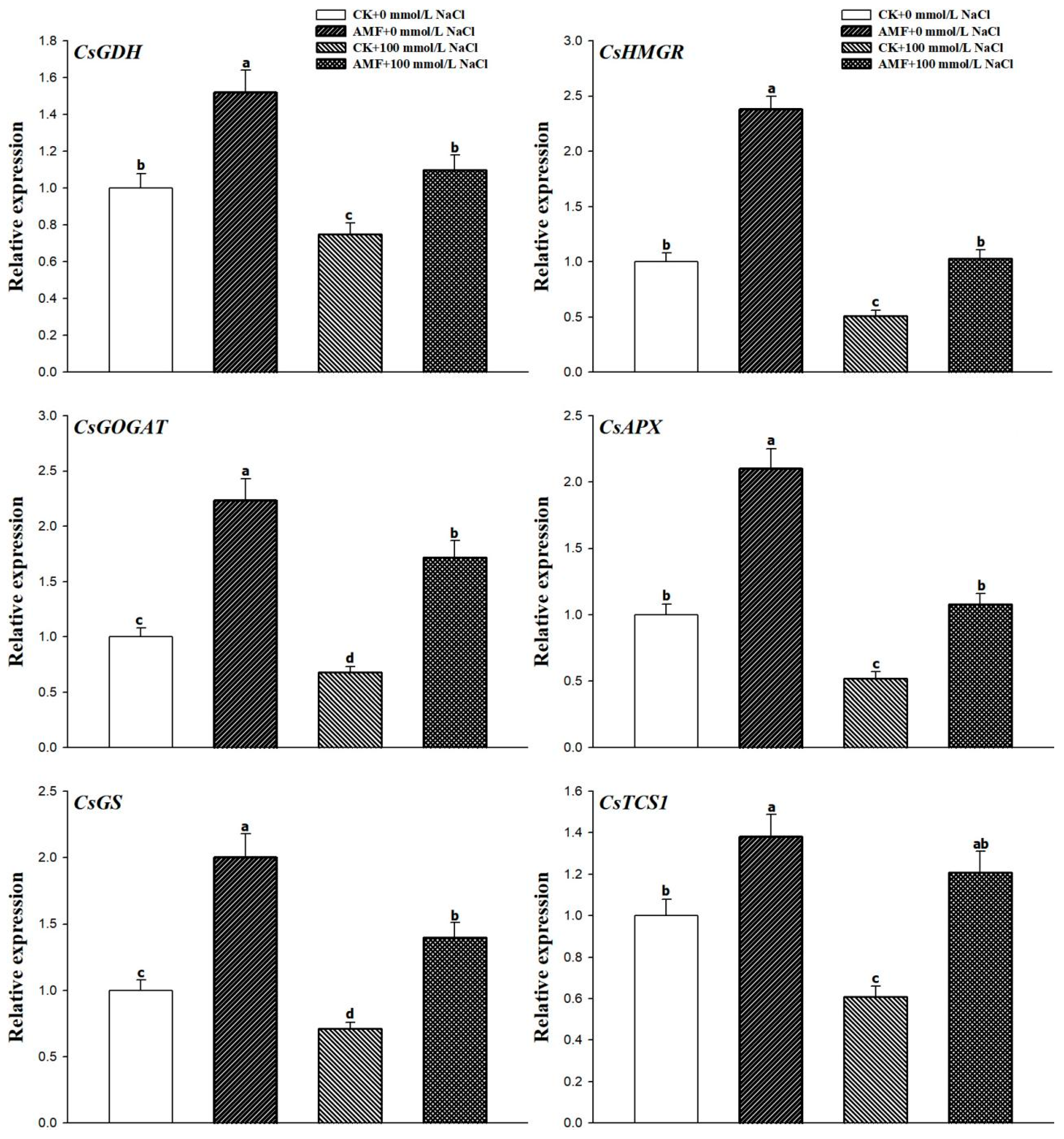
3.5. Changes in Leaf CsHMGR, CsAPX, CsGS, CsGDH, CsGOGAT, and CsTCS1 Expression Levels
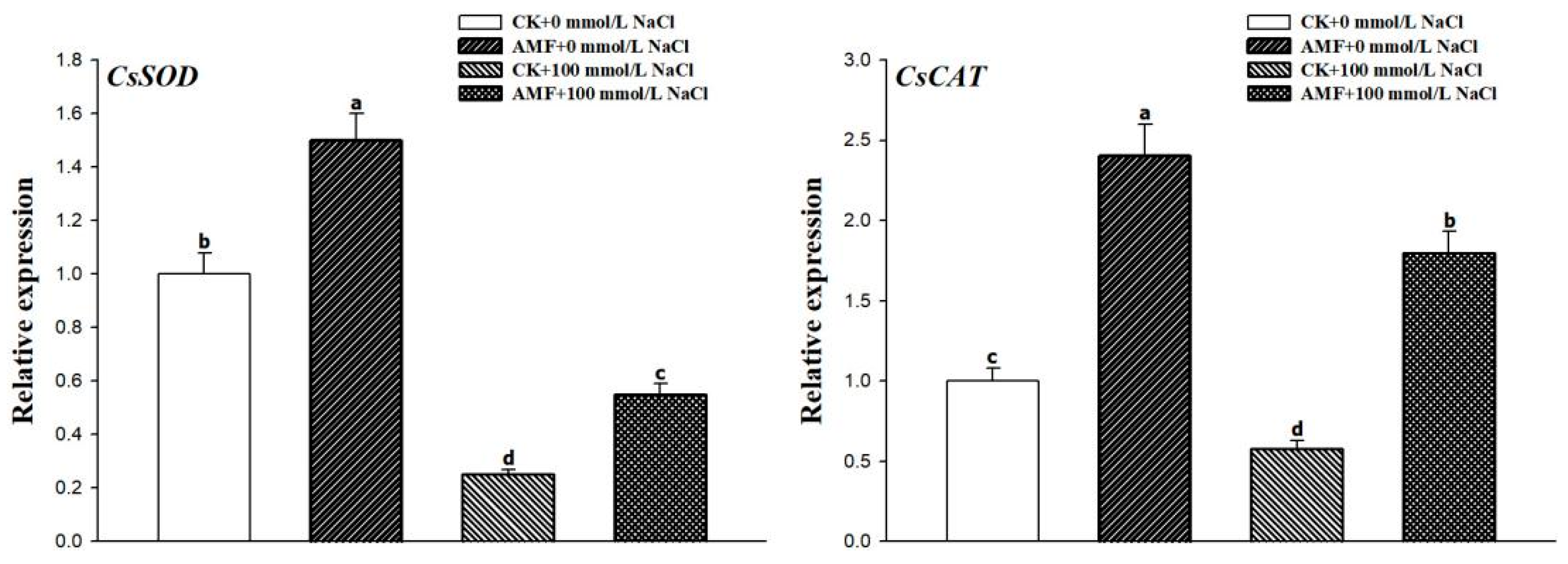
3.6. Changes in Leaf CsCAT and CsSOD Expression Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ding, Z.T.; Jia, S.S.; Wang, Y.; Xiao, J.; Zhang, Y.F. Phosphate stresses affect ionome and metabolome in tea plants. Plant Physiol. Biochem. 2017, 120, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Wang, Y.J.; Wu, Q.S.; Yang, T.Y.; Kuča, K. Arbuscular mycorrhizal fungi improve the antioxidant capacity of tea (Camellia sinensis) Seedlings Under Drought Stress. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 1993–2005. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, K.; Wang, J.; Ding, Z.T.; Wang, H.; Bi, C.H.; Zhang, Y.W.; Sun, H.W. Proteomic analysis of Camellia sinensis (L.) reveals a aynergistic network in the response to drought stress and recovery. J. Plant Physiol. 2017, 219, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Kayang, H. Effects of arbuscular mycorrhizal fungi (AMF) on Camellia sinensis (L.) O. Kuntze under greenhouse conditions. J. Exp. Biol. 2017, 5, 235–241. [Google Scholar] [CrossRef]
- Wan, S.Q.; Wang, W.D.; Zhou, T.S.; Zhang, Y.H.; Chen, J.F.; Xiao, B.; Yang, Y.J.; Yu, Y.B. Transcriptomic analysis reveals the molecular mechanisms of Camellia sinensis in response to salt stress. Plant Growth Regul. 2018, 84, 481–492. [Google Scholar] [CrossRef]
- Hu, C.H.; Zheng, Y.; Tong, C.L.; Zhang, D.J. Effects of exogenous melatonin on plant growth, root hormones and photosynthetic characteristics of trifoliate orange subjected to salt stress. Plant Growth Regul. 2022, 97, 551–558. [Google Scholar] [CrossRef]
- Upadhyaya, H.; Panda, S.K. Abiotic stress responses in tea [Camellia sinensis L (O) Kuntze]: An overview. Rev. Agric. Sci. 2013, 1, 1–10. [Google Scholar] [CrossRef]
- Bothe, H. Arbuscular mycorrhiza and salt tolerance of plants. Symbiosis 2012, 58, 7–16. [Google Scholar]
- Malik, J.A.; Alqarawi, A.; Dar, B.A.; Hashem, A.; Alshahrani, T.S.; AlZain, M.N.; Habib, M.M.; Javed, M.M.; Abd_Allah, E.F. Arbuscular mycorrhizal fungi isolated from highly saline “Sabkha Habitat” soil alleviated the nacl-induced stress and improved Lasiurus scindicus Henr. growth. Agriculture 2022, 12, 337. [Google Scholar] [CrossRef]
- Wu, Q.S.; Shao, Y.D.; Gao, X.B.; Xia, T.J.; Kuca, K. Characterization of AMF-diversity of endosphere versus rhizosphere of tea (Camellia sinensis) crops. Indian J. Agric. Sci. 2019, 89, 348–352. [Google Scholar]
- Qiao, S.; Feng, Y.; Yan, J.; Li, K.; Xu, H. Overexpression of tomato SlTpx improves salt stress tolerance in transgenic tobacco plants by scavenging H2O2. Plant Cell Tissue Organ Cult. 2022, 1–13. [Google Scholar] [CrossRef]
- Nguyen, H.; Bhowmik, S.D.; Long, H.; Cheng, Y.; Mundree, S.; Hoang, L. Rapid accumulation of proline enhances salinity tolerance in Australian wild rice Oryza australiensis Domin. Plants 2021, 10, 2044. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.T.; Pardo, J.M.; Batelli, G.; Van, O.M.J.; Bressan, R.A.; Li, X. The Salt Overly Sensitive (SOS) Pathway: Established and Emerging Roles. Mol. Plant 2013, 6, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.S.; Zou, Y.N.; He, X.H. Mycorrhizal Symbiosis Enhances Tolerance to NaCl Stress Through Selective Absorption but not Selective Transport of K+ Over Na+ in Trifoliate Orange. Sci. Hortic. 2013, 160, 366–374. [Google Scholar] [CrossRef]
- Iwaniuk, P.; Lozowicka, B. Biochemical compounds and stress markers in lettuce upon exposure to pathogenic Botrytis cinerea and fungicides inhibiting oxidative phosphorylation. Planta 2022, 255, 61. [Google Scholar] [CrossRef]
- Hashem, A.; Alqarawi, A.A.; Al-Huqail, A.A.; AbdAllah, E.F. Biodiversity of Arbuscular Mycorrhizal Fungi Associated with Acacia Gerrardii Benth in Different Habitats of Saudi Arabia. Pak. J. Bot. 2018, 50, 1211–1217. Available online: https://www.cabdirect.org/cabdirect/abstract/20183259381 (accessed on 2 July 2020).
- Zou, Y.N.; Wu, H.H.; Giri, B.; Wu, Q.S.; Kuca, K. Mycorrhizal Symbiosis Down-Regulates or Does not Change Root Aquaporin Expression in Trifoliate Orange under Drought Stress. Plant Physiol. Biochem. 2019, 144, 292–299. [Google Scholar] [CrossRef]
- Cheng, X.-F.; Wu, H.-H.; Zou, Y.-N.; Wu, Q.-S.; Kuča, K. Mycorrhizal Response Strategies of Trifoliate Orange under Well-Watered, Salt Stress, and Waterlogging Stress by Regulating Leaf Aquaporin Expression. Plant Physiol. Biochem. 2021, 162, 27–35. [Google Scholar] [CrossRef]
- Mathur, S.; Tomar, R.S.; Jajoo, A. Arbuscular Mycorrhizal Fungi (AMF) Protects Photosynthetic Apparatus of Wheat under Drought Stress. Photosynth. Res. 2019, 139, 227–238. [Google Scholar] [CrossRef]
- Zou, Y.N.; Wang, P.; Liu, C.Y.; Ni, Q.D.; Zhang, D.J.; Wu, Q.S. Mycorrhizal Trifoliate Orange has Greater Root Adaptation of Morphology and Phytohormones in Response to Drought Stress. Sci. Rep. 2017, 7, 41134. [Google Scholar] [CrossRef]
- Mathur, S.; Sharma, M.P.; Jajoo, A. Improved Photosynthetic Efficacy of Maize (Zea mays) Plants with Arbuscular Mycorrhizal Fungi (AMF) under High Temperature Stress. J. Photochem. Photobiol. B Biol. 2018, 180, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.D.; Zhang, D.J.; Hu, X.C.; Wu, Q.S.; Jiang, C.J.; Xia, T.J.; Gao, X.B.; Kuča, K. Mycorrhiza-Tnduced Changes in Root Growth and Nutrient Absorption of Tea Plants. Plant Soil Environ. 2018, 64, 283–289. [Google Scholar] [CrossRef]
- Sun, M.F.; Yuan, D.; Hu, X.C.; Zhang, D.J.; Li, Y.Y. Effects of Mycorrhizal Fungi on Plant Growth, Nutrient Absorption and Phytohormones Levels in Tea Under Shading Condition. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 2006–2020. [Google Scholar] [CrossRef]
- Zhang, D.J.; Yang, Y.J.; Liu, C.Y.; Zhang, F.; Hu, W.; Gong, S.B.; Wu, Q.S. Auxin modulates root-hair growth through its signaling pathway in Citrus. Sci. Hortic. 2018, 236, 73–78. [Google Scholar] [CrossRef]
- Shao, Y.-D.; Zhang, D.-J.; Hu, X.-C.; Wu, Q.-S.; Jiang, C.-J.; Gao, X.-B.; Kuča, K. Arbuscular Mycorrhiza Improves Leaf Food Quality of Tea Plants. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 608–614. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-time Quantitative PCR and 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Adillah, A.; Widada, J.; Kurniasih, B. Root Growth and Yield of Rice Plant Associated with Arbuscular Mycorrhizal Symbiosis under Salt Stress. In Proceedings of the 4th International Conference on Sustainable Agriculture (ICoSA 2021), Yogyakarta, Indonesia, 25–26 August 2021. [Google Scholar] [CrossRef]
- Kusale, S.P.; Attar, Y.C.; Sayyed, R.Z.; En, S.H.E.; Hanapi, S.Z.; Ilyas, N.; Ilyas, N.; Elgorban, A.; Bahkali, A.; Marraiki, N. Inoculation of Klebsiella Variicola Alleviated Salt Stress and Improved Growth and Nutrients in Wheat and Maize. Agronomy 2021, 11, 927. [Google Scholar] [CrossRef]
- Shahvali, R.; Shiran, B.; Ravash, R.; Fallahi, H.; Eri, B.B. Effect of Symbiosis with Arbuscular Mycorrhizal Fungi on Salt Stress Tolerance in gf677 (Peach × Almond) Rootstock. Sci. Hortic. 2020, 272, 109535. [Google Scholar] [CrossRef]
- Mokhtar, M.; Baslam, M.; Ben-Laouane, R.; Anli, M.; Abdelilah, M. Alleviation of Detrimental Effects of Salt Stress on Date Palm (Phoenix dactylifera L.) by the Application of Arbuscular Mycorrhizal Fungi and/or Compost. Front. Sustain. Food Syst. 2020, 4, 131. [Google Scholar] [CrossRef]
- He, J.D.; Li, J.L.; Wu, Q.S. Effects of Rhizoglomus Intraradices on plant growth and root endogenous hormones of trifoliate orange under salt stress. J. Anim. Sci. 2020, 29, 245–250. [Google Scholar]
- Alavi, S.A.; Ghehsareh, A.M.; Soleymani, A.; Panahpour, E. Enhanced nutrient uptake in salt-stressed mentha piperita using magnetically treated water. Protoplasma 2021, 258, 403–414. [Google Scholar] [CrossRef]
- Bellingrath-Kimura, S.D. Biochar amendments improve licorice (Glycyrrhiza uralensis Fisch.) growth and nutrient uptake under salt stress. Plants 2021, 10, 2135. [Google Scholar] [CrossRef]
- Khare, T.; Kumar, V.; Kishor, P. Na+ and Cl− ions show additive effects under NaCl stress on induction of oxidative stress and the responsive antioxidative defense in rice. Protoplasma 2015, 252, 1149–1165. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kumar, J.; Singh, V.P.; Prasad, S.M. Plant tolerance mechanism against salt stress: The nutrient management approach. Biochem. Pharmacol. 2014, 3, 165. [Google Scholar] [CrossRef]
- Zou, Y.N.; Zhang, F.; Srivastava, A.K.; Wu, Q.S.; Kuča, K. Arbuscular mycorrhizal fungi regulate polyamine homeostasis in roots of trifoliate orange for improved adaptation to soil moisture deficit stress. Front. Plant Sci. 2021, 11, 600792. [Google Scholar] [CrossRef]
- Wu, Q.S.; Srivastava, A.K.; Zou, Y.N. AMF-induced tolerance to drought stress in citrus: A review. Sci. Hortic. 2013, 164, 77–87. [Google Scholar] [CrossRef]
- Piyasena, K.G.N.P.; Hettiarachchi, L.S.K.; Jayawardhane, S.A.D.P.S.; Edirisinghe, E.N.U.; Jayasinghe, W.S. Evaluation of inherent fructose, glucose and sucrose concentrations in tea leaves (Camellia sinensis L.) and in black tea. Appl. Food Res. 2022, 2, 100100. [Google Scholar] [CrossRef]
- Wu, Q.S.; Lou, Y.G.; Li, Y. Plant growth and tissue sucrose metabolism in the system of trifoliate orange and arbuscular mycorrhizal fungi. Sci. Hortic. 2015, 181, 189–193. [Google Scholar] [CrossRef]
- Cely, M.V.T.; Oliveira, A.G.; Freitas, V.F.; Luca, M.B.; Barazetti, A.R.; Santos, I.M.O.; Gionco, B.; Garcia, G.V.; Prete, C.E.C.; Andrade, G. Inoculant of Arbuscular Mycorrhizal Fungi (Rhizophagus clarus) Increase Yield of Soybean and Cotton under Field Conditions. Front. Microbiol. 2016, 7, 720. [Google Scholar] [CrossRef]
- Singh, S.; Pandey, A.; Kumar, B.; Palni, L.M.S. Enhancement in Growth and Quality Parameters of Tea [Camellia sinensis (L.) O. Kuntze] through Inoculation with Arbuscular Mycorrhizal Fungi in an Acid Soil. Biol. Fertil. Soils 2010, 46, 427–433. [Google Scholar] [CrossRef]
- Vijay, S.T.; Karishma, G.; Sanjay, G. The Chemopreventive and Chemotherapeutic Potentials of Tea Polyphenols. Curr. Pharm. Biotechnol. 2012, 13, 191–199. [Google Scholar] [CrossRef]
- Kerio, L.C.; Wachira, F.N.; Wanyoko, J.K.; Rotich, M.K. Total Polyphenols, Catechin Profiles and Antioxidant Activity of Tea Products from Purple Leaf Coloured Tea Cultivars. Food Chem. 2013, 136, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.H.; Sun, L.T.; Wang, Y.; Ding, Z.T.; Li, M. Effects of Arbuscular Mycorrhizal Fungi and Nitrogen Regimes on Plant Growth, Nutrient Uptake and Tea Quality in Camellia sinensis (L.) O. Kuntze. Plant Physiol. J. 2014, 50, 164–170, (In Chinese with English abstract). [Google Scholar]
- Tchameni, S.N.; Nwaga, D.; Wakam, L.N.; Ngonkeu, E.L.M.; Etoa, F.X. Growth Enhancement, Amino Acid Synthesis and Reduction in Susceptibility Towards Phytophthora Megakarya by Arbuscular Mycorrhizal Fungi Inoculation in Cocoa Plants. J. Phytopathol. 2012, 160, 220–228. [Google Scholar] [CrossRef]
- Zubek, S.; Rola, K.; Szewczyk, A.; Majewska, M.L.; Turnau, K. Enhanced concentrations of elements and secondary metabolites in Viola tricolor L. induced by arbuscular mycorrhizal fungi. Plant Soil 2015, 390, 129–142. [Google Scholar] [CrossRef]
- Gachomo, E.; Allen, J.W.; Pfeffer, P.E.; Govindarajulu, M.; Bücking, H. Germinating Spores of Glomus Intraradices can use Internal and Exogenous Nitrogen Sources for de Novo Biosynthesis of Amino Acids. New Phytol. 2009, 184, 399–411. [Google Scholar] [CrossRef]
- Pei, Y.; Evan, S.; Tian, B.; Ding, J. Root flavonoids are related to enhanced amf colonization of an invasive tree. AoB Plants 2020, 12, plaa002. [Google Scholar] [CrossRef]
- Lin, Z.H.; Zhong, Q.S.; Chen, C.S. Molecular Cloning and Quantitative Analysis of GDH, GS and GOGAT Genes from Leave of Tea Plant. J. Tea Sci. 2012, 32, 523–529. Available online: https://www.cabdirect.org/cabdirect/abstract/20133097775 (accessed on 4 July 2020).
- Elbasan, F.; Ozfidan-Konakci, C.; Yildiztugay, E.; Kucukoduk, M. Rare-earth element scandium improves stomatal regulation and enhances salt and drought stress tolerance by up-regulating antioxidant responses of Oryza sativa. Plant Physiol. Biochem. 2020, 152, 157–169. [Google Scholar] [CrossRef]
- Jin, J.Q.; Yao, M.Z.; Ma, C.L.; Ma, J.Q.; Chen, L. Natural Allelic Variations of TCS1 Play a Crucial Role in Caffeine Biosynthesis of Tea Plant and its Related Species. Plant Physiol. Biochem. 2016, 100, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Ellouzi, H.; Oueslati, S.; Hessini, K.; Rabhi, M.; Abdelly, C. Seed-priming with H2O2 alleviates subsequent salt stress by preventing ROS production and amplifying antioxidant defense in cauliflower seeds and seedlings. Sci. Hortic. 2021, 288, 110360. [Google Scholar] [CrossRef]
- Zhu, X.; Song, F.; Liu, S. Arbuscular Mycorrhiza Impacts on Drought Stress of Maize Plants by Lipid Peroxidation, Proline Content and Activity of Antioxidant System. J. Food Agric. Environ. 2011, 9, 583–587. Available online: https://www.researchgate.net/publication/260055159 (accessed on 4 July 2020).
- He, J.D.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Mycorrhizas Enhance Drought Tolerance of Trifoliate Orange by Enhancing Activities and Gene Expression of Antioxidant Enzymes. Sci. Hortic. 2020, 262, 108745. [Google Scholar] [CrossRef]
- Zou, Y.N.; Wu, Q.S.; Kuča, K. Unraveling the role of arbuscular mycorrhizal fungi in mitigating the oxidative burst of plants under drought stress. Plant Biol. 2021, 1, 50–57. [Google Scholar] [CrossRef]
- Zhang, F.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Arbuscular mycorrhizas modulate root polyamine metabolism to enhance drought tolerance of trifoliate orange. Environ. Exp. Bot. 2020, 171, 103962. [Google Scholar] [CrossRef]
- González-Guerrero, M.; Oger, E.; Benabdellah, K.; Azcón-Aguilar, C.; Lanfranco, L.; Ferro, N. Characterization of a CuZn superoxide dismutase gene in the arbuscular mycorrhizal fungus Glomus intraradices. Curr. Genet. 2010, 56, 265–274. [Google Scholar] [CrossRef]

| Gene Name | Accession | Sequence (5′-3′)-Forward | Sequence (5′-3′)-Reverse |
|---|---|---|---|
| CsHMGR | KJ946250 | CTCTTCCTCCTCCTCCTC | CTTTGTGCCCTTGGATAG |
| CsAPX | EU547804 | TTCTATCAGTTGGCTGGAGT | ATGGTCACATCCCTTATCG |
| CsGS | JN602372 | CTCAGAAGCAAAGCAAGGAC | ACATCAGGGTGGCTGAAAAT |
| CsGDH | JN602371 | AGCGGCAAATCATCCTACT | TCGTCCCACATGAAACCTT |
| CsGOGAT | JN602373 | GCTTCAGGACGTTTTGGTG | CATGATGTGGAGGTGGGGAT |
| CsTCS1 | AB031280 | TCCGTGTTATGTGATGGGAG | ATGATGTGGAGGTGGGGATA |
| CsCAT | KR819178.1 | TTTGATCTGGTGGGAAACAA | TCCAATTCTCCTGGATGTGA |
| CsSOD | AY694187.1 | TTTCAATCCTGCTGGCAAAGA | ATGGACAACAACGGCCCTACC |
| CsGADPH | TEA003029 | TGGCATCGTTGAGGGTC | CAGTGGGAACACGGAAAG |
| Treatment | Mycorrhizal Colonization Rate (%) | Plant Height (cm) | Leaf Area (cm2) | Taproot Length (cm) | Root Biomass (g FW/Plant) | Shoot Biomass (g FW/Plant) |
|---|---|---|---|---|---|---|
| Control + 0 mmol/L NaCl | / | 15.11 ± 1.23 b | 20.21 ± 1.49 b | 14.12 ± 1.08 b | 1.56 ± 0.12 b | 2.11 ± 0.18 b |
| AMF + 0 mmol/L NaCl | 40.56 ± 2.39 a | 18.52 ± 0.98 a | 23.96 ± 2.08 a | 16.58 ± 1.19 a | 1.89 ± 0.14 a | 2.51 ± 0.19 a |
| Control + 100 mmol/L NaCl | / | 13.32 ± 1.14 c | 17.98 ± 1.18 c | 13.24 ± 1.11 bc | 1.35 ± 0.11 c | 1.88 ± 0.15 c |
| AMF + 100 mmol/L NaCl | 20.23 ± 1.18 b | 16.63 ± 1.41 b | 21.02 ± 1.85 b | 13.98 ± 1.12 b | 1.52 ± 0.11 b | 2.19 ± 0.21 b |
| ANOVA | NaCl | *** | *** | *** | *** | *** |
| AMF | *** | *** | *** | ** | *** | |
| NaCl × AMF | *** | ns | ns | ns | ns |
| Treatment | N | P | K | Ca | M | Fe |
|---|---|---|---|---|---|---|
| Control + 0 mmol/L NaCl | 14.65 ± 1.12 b | 0.67 ± 0.04 b | 5.81 ± 0.28 b | 2.35 ± 0.21 b | 0.82 ± 0.06 b | 0.16 ± 0.01 a |
| AMF + 0 mmol/L NaCl | 19.53 ± 1.81 a | 0.89 ± 0.07 a | 6.51 ± 0.42 a | 2.97 ± 0.22 a | 0.98 ± 0.07 a | 0.17 ± 0.01 a |
| Control + 100 mmol/L NaCl | 12.18 ± 1.08 c | 0.59 ± 0.03 c | 5.01 ± 0.25 c | 2.01 ± 0.15 c | 0.73 ± 0.05 c | 0.15 ± 0.01 a |
| AMF + 100 mmol/L NaCl | 14.97 ± 1.22 b | 0.71 ± 0.03 b | 5.89 ± 0.29 b | 2.88 ± 0.24 a | 0.91 ± 0.08 a | 0.16 ± 0.01 a |
| ANOVE | NaCl | *** | *** | *** | ** | ** |
| AMF | *** | *** | *** | *** | *** | |
| NaCl × AMF | ns | ** | ns | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-W.; Tong, C.-L.; Sun, M.-F. Effects and Molecular Mechanism of Mycorrhiza on the Growth, Nutrient Absorption, Quality of Fresh Leaves, and Antioxidant System of Tea Seedlings Suffering from Salt Stress. Agronomy 2022, 12, 2163. https://doi.org/10.3390/agronomy12092163
Li Y-W, Tong C-L, Sun M-F. Effects and Molecular Mechanism of Mycorrhiza on the Growth, Nutrient Absorption, Quality of Fresh Leaves, and Antioxidant System of Tea Seedlings Suffering from Salt Stress. Agronomy. 2022; 12(9):2163. https://doi.org/10.3390/agronomy12092163
Chicago/Turabian StyleLi, Yue-Wei, Cui-Ling Tong, and Mu-Fang Sun. 2022. "Effects and Molecular Mechanism of Mycorrhiza on the Growth, Nutrient Absorption, Quality of Fresh Leaves, and Antioxidant System of Tea Seedlings Suffering from Salt Stress" Agronomy 12, no. 9: 2163. https://doi.org/10.3390/agronomy12092163
APA StyleLi, Y.-W., Tong, C.-L., & Sun, M.-F. (2022). Effects and Molecular Mechanism of Mycorrhiza on the Growth, Nutrient Absorption, Quality of Fresh Leaves, and Antioxidant System of Tea Seedlings Suffering from Salt Stress. Agronomy, 12(9), 2163. https://doi.org/10.3390/agronomy12092163




