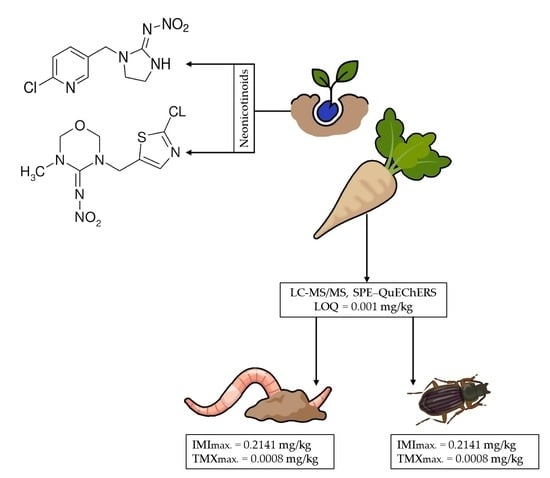
Neonicotinoid Residues in Earthworms and Ground Beetles under Intensive Sugar Beet Production: Preliminary Study in Croatia
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bažok, R.; Kos, T.; Drmić, Z. Važnost trčaka (Coleoptera: Carabidae) za biološku stabilnost poljoprivrednih staništa, osobito u uzgoju šećerne repe. Glas. Bilj. zašt. 2015, 4, 264–277. [Google Scholar]
- Kos, T.; Bažok, R. Korisna fauna u usjevu šećerne repe. In Šećerna Repa: Zaštita od Štetnih Organizama u Sustavu Integrirane Biljne Proizvodnje; Bažok, R., Ed.; Sveučilište u Zagrebu Agronomski Fakultet: Zagreb, Croatia, 2015; pp. 23–28. [Google Scholar]
- Pisa, L.W.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Downs, C.A.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; Mcfield, M.; et al. Effects of Neonicotinoids and Fipronil on Non-Target Invertebrates. Environ. Sci. Pollut. Res. 2014, 22, 68–102. [Google Scholar] [CrossRef]
- Müller, C. Impacts of Sublethal Insecticide Exposure on Insects—Facts and Knowledge Gaps. Basic Appl. Ecol. 2018, 30, 1–10. [Google Scholar] [CrossRef]
- Holland, J.M. Carabid beetles: Their ecology, survival and use in agroecosystems. In The Agroecology of Carabid Beetles; Holland, J.M., Ed.; Intercept Ltd.: Andover, MA, USA, 2002; pp. 1–40. [Google Scholar]
- Thiele, H. Carabid Beetles in Their Environments; Springer: Berlin/Heidelberg, Germany, 1977. [Google Scholar]
- Hole, D.G.; Perkins, A.J.; Wilson, J.D.; Alexander, I.H.; Grice, P.V.; Evans, A.D. Does Organic Farming Benefit Biodiversity? Biol. Conserv. 2005, 122, 113–130. [Google Scholar] [CrossRef]
- Fahrig, L.; Girard, J.; Duro, D.; Pasher, J.; Smith, A.; Javorek, S.; King, D.; Lindsay, K.F.; Mitchell, S.; Tischendorf, L. Farmlands with Smaller Crop Fields Have Higher Within-Field Biodiversity. Agric. Ecosyst. Environ. 2015, 200, 219–234. [Google Scholar] [CrossRef]
- Luo, Y.; Zang, Y.; Zhong, Y.; Kong, Z. Toxicological Study of Two Novel Pesticides on Earthworm Eisenia fetida. Chemosphere 1999, 39, 2347–2356. [Google Scholar] [CrossRef]
- Piearce, T.G.; Lee, K.E. Earthworms, Their Ecology and Relationships with Soils and Land Use. J. Appl. Ecol. 1987, 24, 334. [Google Scholar] [CrossRef][Green Version]
- Pelosi, C.; Bertrand, C.; Daniele, G.; Coeurdassier, M.; Benoit, P.; Nélieu, S.; Lafay, F.; Bretagnolle, V.; Gaba, S.; Vulliet, E.; et al. Residues of Currently Used Pesticides in Soils and Earthworms: A Silent Threat? Agric. Ecosyst. Environ. 2021, 305, 107167. [Google Scholar] [CrossRef]
- Goulson, D. An Overview of the Environmental Risks Posed by Neonicotinoid Insecticides. J. Appl. Ecol. 2013, 50, 977–987. [Google Scholar] [CrossRef]
- Harrison-Dunn, A.-R. Why are Banned ‘Bee-Killer’ Neonicotinoids Still Being Used in Europe? Modern Farmer. Available online: https://modernfarmer.com/2021/03/why-are-banned-bee-killer-neonicotinoids-still-being-used-in-europe/ (accessed on 11 August 2022).
- Dowler, C. Revealed: Europe and the UK’s Vast Shipments of Banned, Bee-Killing ‘Neonics’. Available online: https://unearthed.greenpeace.org/2021/11/18/revealed-europe-and-the-uks-vast-shipments-of-banned-bee-killing-neonics/ (accessed on 8 August 2022).
- Marzaro, M.; Vivan, L.; Targa, A.; Mazzon, L.; Mori, N.; Greatti, M.; Petrucco Toffolo, E.; Di Bernardo, A.; Giorio, C.; Marton, D.; et al. Lethal aerial powdering of honey bees with neonicotinoids from fragments of maize seed coat. Bull. Insectol. 2011, 64, 119–126. [Google Scholar]
- Tapparo, A.; Marton, D.; Giorio, C.; Zanella, A.; Soldà, L.; Marzaro, M.; Vivan, L.; Girolami, V. Assessment of the Environmental Exposure of Honeybees to Particulate Matter Containing Neonicotinoid Insecticides Coming from Corn Coated Seeds. Environ. Sci. Technol. 2012, 46, 2592–2599. [Google Scholar] [CrossRef] [PubMed]
- Krupke, C.H.; Hunt, G.J.; Eitzer, B.D.; Andino, G.; Given, K. Multiple Routes of Pesticide Exposure for Honey Bees Living Near Agricultural Fields. PLoS ONE 2012, 7, e29268. [Google Scholar]
- Viric Gasparic, H.; Grubelic, M.; Uzelac, V.D.; Bazok, R.; Cacija, M.; Drmic, Z.; Lemic, D. Neonicotinoid Residues in Sugar Beet Plants and Soil under Different Agro-Climatic Conditions. Agriculture 2020, 10, 484. [Google Scholar] [CrossRef]
- ISO 23611-1:2006; Soil Quality—Sampling of Soil Invertebrates—Part 1: Hand-Sorting and Formalin Extraction of Earthworms. International Organization for Standardization: Geneva, Switzerland, 2006.
- Bargańska, Ż.; Slebioda, M.; Namieśnik, J. Determination of pesticide residues in honeybees using modified QUEChERS sample work-up and liquid chromatography-tandem mass spectrometry. Molecules 2014, 19, 2911–2924. [Google Scholar] [CrossRef]
- Gylling Data Management Inc. ARM 9® GDM Software, Revision 2019.4; (B = 25105); Gylling Data Management Inc.: Brookings, SD, USA, 2019; Available online: https://gdmdata.com/Products/ARM/Updates/ReleaseNotes/ARM2019 (accessed on 9 August 2022).
- Mullin, C.A.; Frazier, M.; Frazier, J.L.; Ashcraft, S.; Simonds, R.; Vanengelsdorp, D.; Pettis, J.S. High Levels of Miticides and Agrochemicals in North American Apiaries: Implications for Honey Bee Health. PLoS ONE 2010, 5, e9754. [Google Scholar] [CrossRef]
- Gomez-Eyles, J.L.; Svendsen, C.; Lister, L.; Martin, H.; Hodson, M.E.; Spurgeon, D.J. Measuring and Modelling Mixture Toxicity of Imidacloprid and Thiacloprid on Caenorhabditis Elegans and Eisenia fetida. Ecotoxicol. Environ. Saf. 2009, 72, 71–79. [Google Scholar] [CrossRef]
- Agriculture & Environment Research Unit (AERU) at the University of Hertfordshire. Imidacloprid (Ref: BAY NTN 33893). Available online: http://sitem.herts.ac.uk/aeru/ppdb/en/Reports/397.htm (accessed on 11 August 2022).
- European Food Safety Authority. (a) Peer Review of the Pesticide Risk Assessment for Bees for the Active Substance Clothianidin Considering the Uses as Seed Treatments and Granules. EFSA J. 2018, 16, e05177. [Google Scholar] [CrossRef]
- European Food Safety Authority. (b) Peer Review of the Pesticide Risk Assessment for Bees for the Active Substance Imidacloprid Considering the Uses as Seed Treatments and Granules. EFSA J. 2018, 16, e05178. [Google Scholar] [CrossRef]
- European Food Safety Authority. (c) Peer Review of the Pesticide Risk Assessment for Bees for the Active Substance Thiamethoxam Considering the Uses as Seed Treatments and Granules. EFSA J. 2018, 16, e05179. [Google Scholar] [CrossRef]
- Sunderland, K.D. Invertebrate pest control by carabids. In The Agroecology of Carabid Beetles; Holland, J.M., Ed.; Intercept Ltd.: Andover, MA, USA, 2002; pp. 165–214. [Google Scholar]
- Albajes, R.; López, C.; Pons, X. Predatory fauna in cornfields and response to imidacloprid seed treatment. J. Econ. Entomol. 2003, 96, 1805–1813. [Google Scholar] [CrossRef]
- Papachristos, D.P.; Milonas, P.G. Adverse effects of soil applied insecticides on the predatory coccinellid Hippodamia undecimnotata (Coleoptera: Coccinellidae). Biol. Control 2008, 47, 77–81. [Google Scholar] [CrossRef]
- Moser, S.E.; Obrycki, J.J. Non-target effects of neonicotinoid seed treatments; mortality of coccinellid larvae related to zoophytophagy. Biol. Control 2008, 51, 487–492. [Google Scholar] [CrossRef]
- Prabhaker, N.; Castle, S.J.; Naranjo, S.E.; Toscano, N.C.; Morse, J.G. Compatibility of two systemic neonicotinoids, imidacloprid and thiamethoxam, with various natural enemies of agricultural pests. J. Econ. Entomol. 2011, 104, 773–781. [Google Scholar] [CrossRef]
- Khanii, A.; Ahmadi, F.; Ghadamyari, M. Side effects of imidacloprid and abamectin on the Mealybug destroyer, Cryptolaemus montrouzieri. Trakia J. Sci. 2012, 10, 30–35. [Google Scholar]
- Wang, Y.; Cang, T.; Zhao, X.; Yu, R.; Chen, L.; Wu, C.; Wang, Q. Comparative acute toxicity of twenty-four insecticides to earthworm, Eisenia fetida. Ecotoxicol. Environ. Saf. 2012, 79, 122–128. [Google Scholar] [CrossRef]
- Elbert, A.; Becker, B.; Hartwig, J.; Erdelen, C. Imidacloprid—A New Systemic Insecticide; Pflanzenschutz-Nachrichten Bayer: Leverkusen, Germany, 1991. [Google Scholar]
- Volkov, E.M.; Nurullin, L.F.; Nikolsky, E.; Vyskocil, F. Miniature excitatory synaptic ion currents in the earthworm Lumbricus terrestris body wall muscles. Physiol. Res. 2007, 56, 655–658. [Google Scholar] [CrossRef]
| Locality | Four Years Sugar Beet Crop Rotation System | |||||
|---|---|---|---|---|---|---|
| Field | I | II | III | IV | V | |
| Tovarnik | 1. | maize * | sugar beet | soybean | wheat | sugar beet |
| 2. | wheat | maize | sugar beet | wheat | sunflower | |
| 3. | sugar beet | wheat | sunflower | sugar beet | wheat | |
| 4. | soybean | wheat | sunflower | barley | sugar beet | |
| Lukač | 1. | wheat | sugar beet | wheat | sunflower | maize |
| 2. | sugar beet | wheat | sugar beet | maize | maize | |
| 3. | soybean | maize | wheat | sugar beet | bare soil | |
| 4. | maize | oilseed rape | wheat | sunflower | sugar beet | |
| Lukač | |||||||||
| imidacloprid residues | thiamethoxam residues | clothianidin residues | |||||||
| Variant | S1 | S2 | S3 | S1 | S2 | S3 | S1 | S2 | S3 |
| V1 | 0.004 | 0.002 | 0,011 | 0.002 | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 |
| V2 | 0.004 | 0.004 | 0,027 | 0.002 | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 |
| V3 | 0.004 | 0.006 | 0.008 | 0.002 | <0.001 | <0.001 | 0.001 | <0.001 | 0.001 |
| Tovarnik | |||||||||
| imidacloprid residues | thiamethoxam residues | clothianidin residues | |||||||
| S1 | S2 | S3 | S1 | S2 | S3 | S1 | S2 | S3 | |
| V1 | <0.001 | 0.002 | 0.002 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| V2 | 0.001 | 0.008 | 0.003 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| V3 | <0.001 | 0.001 | 0.003 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Active Ingredient | Sample Collection Period | LSD P = 0.05 1 | ||||
|---|---|---|---|---|---|---|
| Crop Rotation | S1 | Crop Rotation | S2 | S3 | ||
| Imidacloprid | sugar beet oilseed rape wheat maize | 0.0321 | wheat maize sugar beet soybean | 0.0184 | 0.0800 | ns 2 |
| 0.0044 b | 0.0049 b | 0.0166 a | 0.00495 | |||
| 0.0493 a | 0.0107 b | 0.0663 a | 0.01929 | |||
| 0.0334 a | 0.0067 b | 0.035 a | 0.01220 | |||
| Thiamethoxam | 0.0005 | 0.0002 | 0.0001 | ns 2 | ||
| 0.001 a | 0.0001 b | 0.0001 b | 0.00007 | |||
| 0.0001 | 0.0003 | 0.0001 | ns | |||
| 0.0001 | 0.0001 | 0.0001 | ns | |||
| Clothianidin | 0.0084 | 0.0037 | 0.0001 | ns 2 | ||
| 0.0077 a | 0.003 b | 0.003 b | 0.00207 | |||
| 0.0054 | 0.0218 | 0.0105 | ns | |||
| 0.0133 | 0.0235 | 0.0070 | ns | |||
| Active Ingredient | Sample collection period | LSD P = 0.05 1 | ||||
|---|---|---|---|---|---|---|
| Crop Rotation | S1 | Crop Rotation | S2 | S3 | ||
| Imidacloprid | sugar beet maize wheat wheat | 0.057 a | maize wheat sugar beet soybean | 0.0234 b | 0.05 b | 0.01609 |
| 0.0128 b | 0.0958 a | 0.1144 a | 0.0289 | |||
| 0.0058 c | 0.0295 b | 0.2141 a | 0.00557 | |||
| - | 0.0275 | 0.1191 | ns | |||
| Thiamethoxam | 0.0008 | 0.0004 | 0 | ns | ||
| 0.0001 | 0.001 | 0.0001 | ns | |||
| 0.0001 | 0.0001 | 0.0001 | ns | |||
| - | 0.0001 | 0.0001 | ns | |||
| Clothianidin | 0.0073 | 0.005 | 0.001 | ns | ||
| 0.0008 | 0.0048 | 0.0048 | ns | |||
| 0.0053 b | 0.018 a | 0.0013 c | 0.00285 | |||
| 0.057 a | 0.0182 | 0.0347 | ns | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viric Gasparic, H.; Lemic, D.; Bazok, R. Neonicotinoid Residues in Earthworms and Ground Beetles under Intensive Sugar Beet Production: Preliminary Study in Croatia. Agronomy 2022, 12, 2102. https://doi.org/10.3390/agronomy12092102
Viric Gasparic H, Lemic D, Bazok R. Neonicotinoid Residues in Earthworms and Ground Beetles under Intensive Sugar Beet Production: Preliminary Study in Croatia. Agronomy. 2022; 12(9):2102. https://doi.org/10.3390/agronomy12092102
Chicago/Turabian StyleViric Gasparic, Helena, Darija Lemic, and Renata Bazok. 2022. "Neonicotinoid Residues in Earthworms and Ground Beetles under Intensive Sugar Beet Production: Preliminary Study in Croatia" Agronomy 12, no. 9: 2102. https://doi.org/10.3390/agronomy12092102
APA StyleViric Gasparic, H., Lemic, D., & Bazok, R. (2022). Neonicotinoid Residues in Earthworms and Ground Beetles under Intensive Sugar Beet Production: Preliminary Study in Croatia. Agronomy, 12(9), 2102. https://doi.org/10.3390/agronomy12092102