Phylogenetic Affinity in the Potential Antagonism of Trichoderma spp. against Moniliophthora roreri
Abstract
1. Introduction
2. Materials and Methods
2.1. Acquisition of Native Strains of Trichoderma spp.
2.2. Acquisition of Moniliophthora roreri
2.3. Mycoparasitism Test
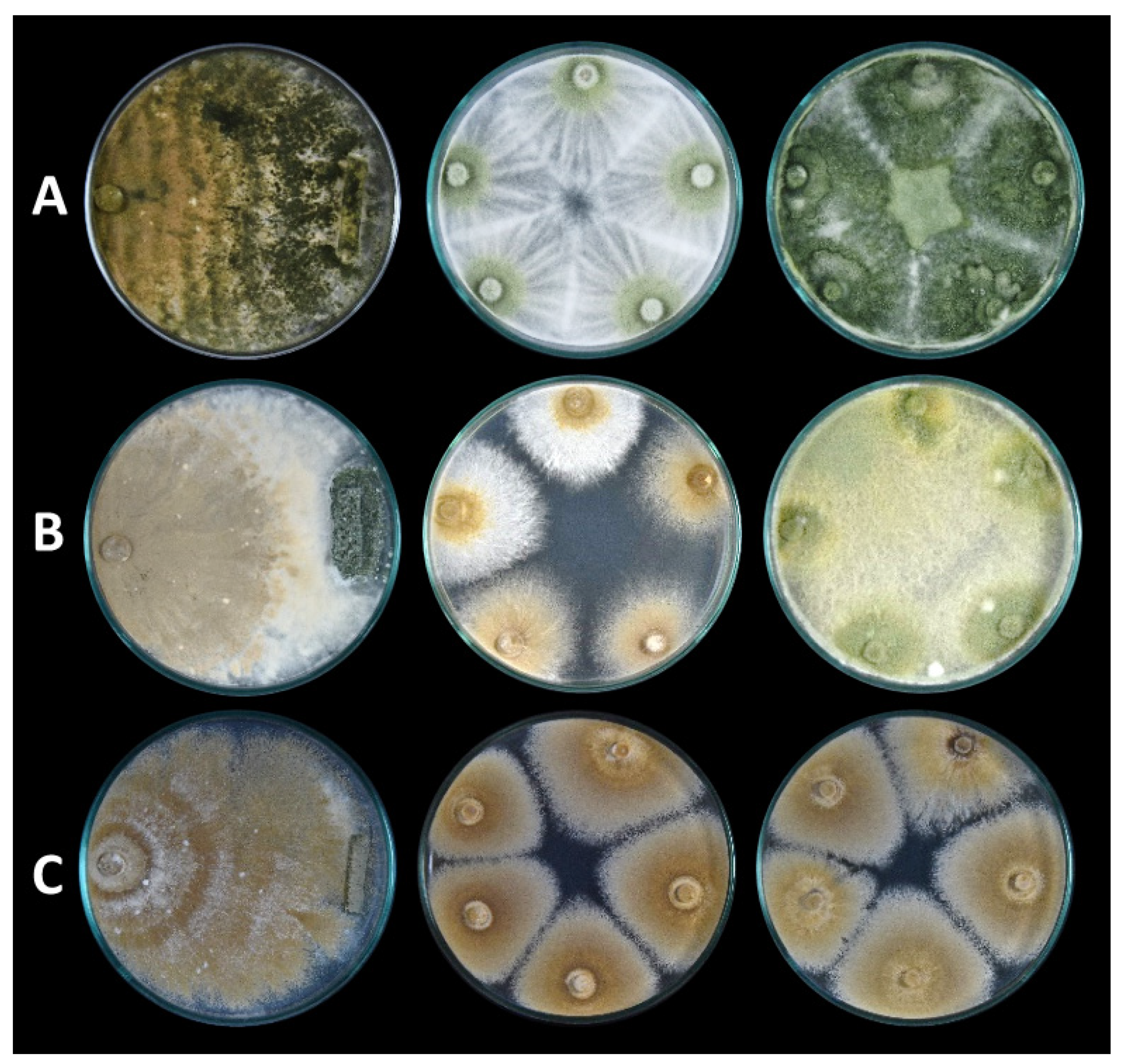
2.4. Antibiosis Test
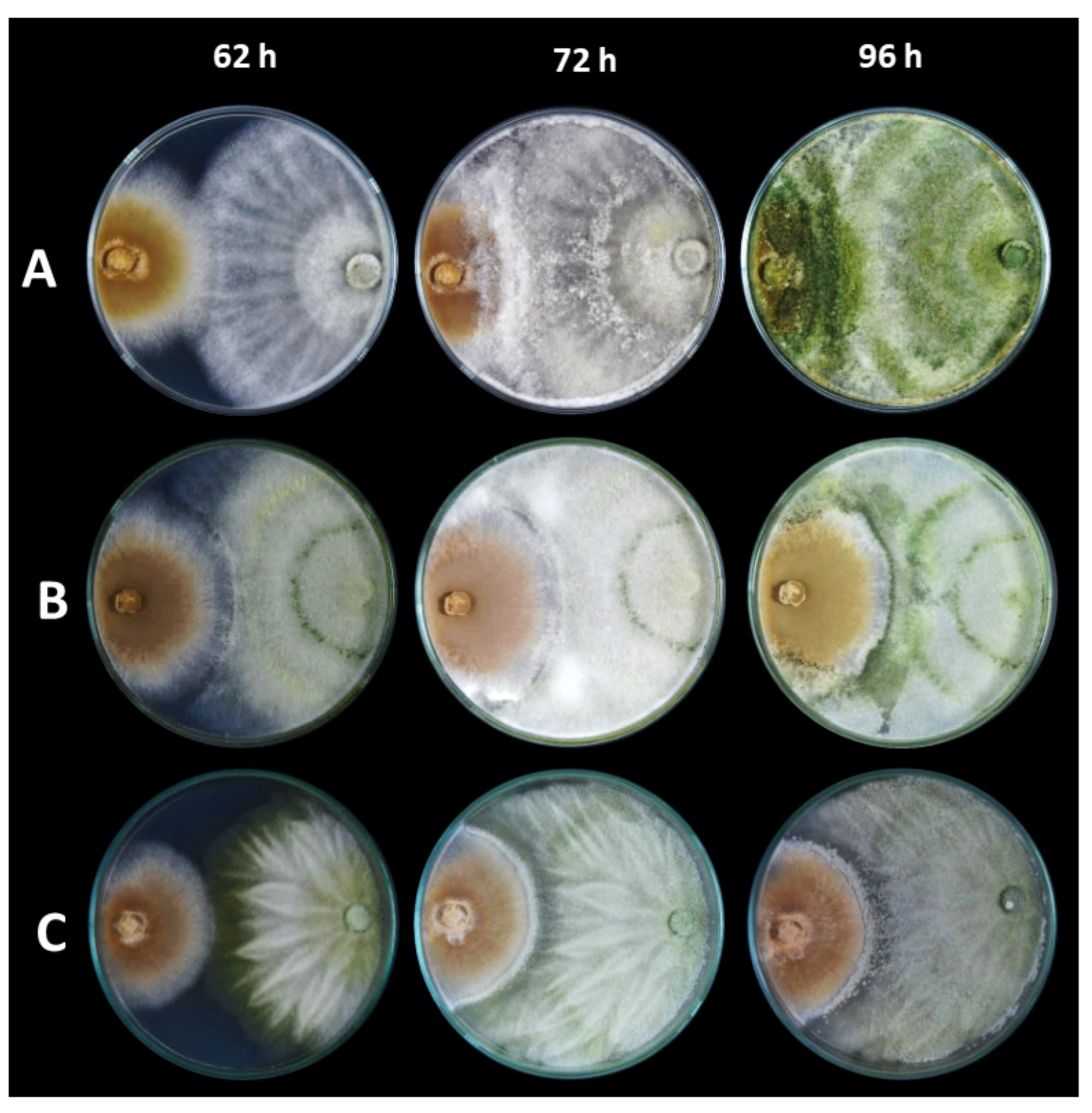
2.5. Potential Antagonism
2.6. Data Analysis
3. Results and Discussion
3.1. Mycoparasitism
3.2. Antibiosis
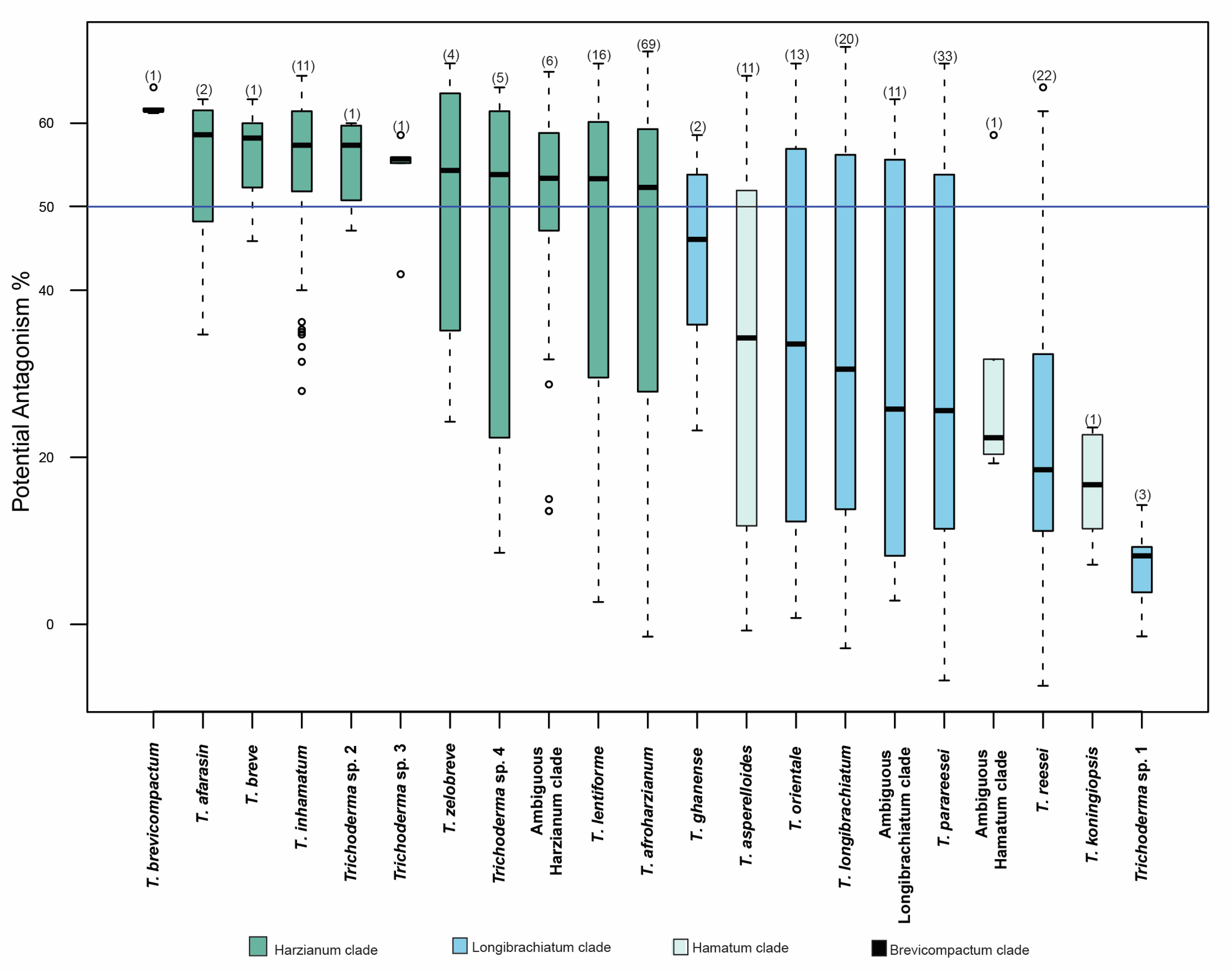
3.3. Potential Antagonism
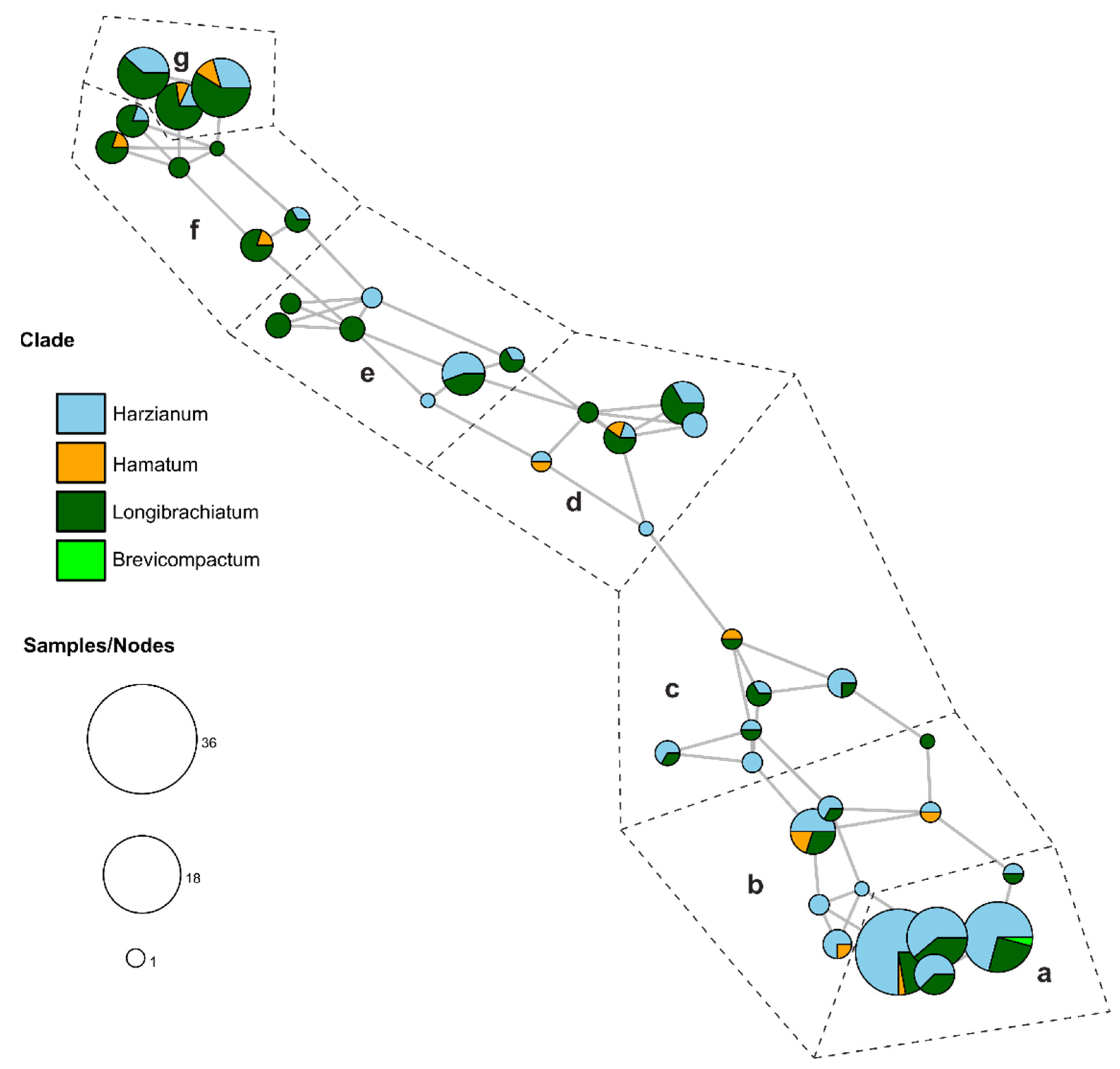
3.4. Association Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Díaz-Valderrama, J.R.; Leiva-Espinoza, S.T.; Catherine Aime, M. The history of cacao and its diseases in the Americas. Phytopathology 2020, 110, 1604–1619. [Google Scholar] [CrossRef] [PubMed]
- Bailey, B.A.; Bae, H.; Strem, M.D.; Crozier, J.; Thomas, S.E.; Samuels, G.J.; Vinyard, B.T.; Holmes, K.A. Antibiosis, mycoparasitism, and colonization success for endophytic Trichoderma isolates with biological control potential in Theobroma cacao. Biol. Control 2008, 46, 24–35. [Google Scholar] [CrossRef]
- Phillips-Mora, W.; Aime, M.C.; Wilkinson, M.J. Biodiversity and biogeography of the cacao (Theobroma cacao) pathogen Moniliophthora roreri in tropical America. Plant Pathol. 2007, 56, 911–922. [Google Scholar] [CrossRef]
- Zin, N.A.; Badaluddin, N.A. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 2020, 65, 168–178. [Google Scholar] [CrossRef]
- Brotman, Y.; Gupta, J. Trichoderma. Curr. Biol. 2017, 20, 390–391. [Google Scholar] [CrossRef]
- Torres de la Cruz, M.; Ortiz García, C.F.; Téliz Ortiz, D.; Mora Aguilera, A.; Nava Díaz, C. Temporal progress and integrated management of frosty pod rot (Moniliophthora roreri) of cocoa in Tabasco, Mexico. J. Plant Pathol. 2011, 93, 31–36. [Google Scholar] [CrossRef]
- Krauss, U.; Soberanis, W. Rehabilitation of diseased cacao fields in Peru through shade regulation and timing of biocontrol measures. Agrofor. Syst. 2001, 53, 179–184. [Google Scholar] [CrossRef]
- Soberanis, W.; Ríos, R.; Arévalo, E.; Zúñiga, L.; Cabezas, O.; Krauss, U. Increased frequency of phytosanitary pod removal in cacao (Theobroma cacao) increases yield economically in eastern Peru. Crop Prot. 1999, 18, 677–685. [Google Scholar] [CrossRef]
- Bateman, R.P.; Hidalgo, E.; García, J.; Arroyo, C.; Ten Hoopen, G.M.; Adonijah, V.; Krauss, U. Application of chemical and biological agents for the management of frosty pod rot (Moniliophthora roreri) in Costa Rican cocoa (Theobroma cacao). Ann. Appl. Biol. 2005, 147, 129–138. [Google Scholar] [CrossRef]
- Gidoin, C.; Avelino, J.; Deheuvels, O.; Cilas, C.; Bieng, M.A.N. Shade tree spatial structure and pod production explain frosty pod rot intensity in cacao agroforests, Costa Rica. Phytopathology 2014, 104, 275–281. [Google Scholar] [CrossRef][Green Version]
- Ortíz-García, C.F.; Torres-de la Cruz, M.; Hernández-Mateo, S. del C. Comparación de dos sistemas de manejo del cultivo del cacao, en presencia de Moniliophthora roreri, en México. Rev. Fitotec. Mex. 2015, 38, 191–196. [Google Scholar] [CrossRef]
- Leandro-Muñoz, M.E.; Tixier, P.; Germon, A.; Rakotobe, V.; Phillips-Mora, W.; Maximova, S.; Avelino, J. Effects of microclimatic variables on the symptoms and signs onset of Moniliophthora roreri, causal agent of moniliophthora pod rot in cacao. PLoS ONE 2017, 12, e0184638. [Google Scholar] [CrossRef]
- Leiva, S.; Oliva, M.; Hernández, E.; Chuquibala, B.; Rubio, K.; García, F.; Torres de la Cruz, M. Assessment of the potential of Trichoderma spp. strains native to Bagua (Amazonas, Peru) in the biocontrol of frosty pod rot (Moniliophthora roreri). Agronomy 2020, 10, 1376. [Google Scholar] [CrossRef]
- Reyes-Figueroa, O.; Ortiz-García, C.F.; Torres de la Cruz, M.; Lagunes-Espinoza, L.D.C.; Valdovinos-Ponce, G. Especies de Trichoderma del agroecosistema cacao con potencial de biocontrol sobre Moniliophthora roreri. Rev. Chapingo Ser. Cienc. Y Del Ambient. 2016, 22, 149–163. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Woo, S.L.; Lorito, M. Trichoderma–Plant–Pathogen interactions. Soil Biol. Biochem. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Poveda, J. Trichoderma as biocontrol agent against pests: New uses for a mycoparasite. Biol. Control 2021, 159, 104634. [Google Scholar] [CrossRef]
- De Palma, M.; Docimo, T.; Guida, G.; Salzano, M.; Albrizio, R.; Giorio, P.; Ruocco, M.; Tucci, M. Transcriptome modulation by the beneficial fungus Trichoderma longibrachiatum drives water stress response and recovery in tomato. Environ. Exp. Bot. 2021, 190, 104588. [Google Scholar] [CrossRef]
- Hanada, R.E.; Pomella, A.W.V.; Costa, H.S.; Bezerra, J.L.; Loguercio, L.L.; Pereira, J.O. Endophytic fungal diversity in Theobroma cacao (cacao) and T. grandiflorum (cupuaçu) trees and their potential for growth promotion and biocontrol of black-pod disease. Fungal Biol. 2010, 114, 901–910. [Google Scholar] [CrossRef]
- Yassin, M.T.; Mostafa, A.A.-F.; Al-Askar, A.A. In vitro antagonistic activity of Trichoderma harzianum and T. viride strains compared to carbendazim fungicide against the fungal phytopathogens of Sorghum bicolor L. moench. Egypt. J. Biol. Pest Control 2021, 31, 118. [Google Scholar] [CrossRef]
- Begum, M.F.; Rahman, M.A.; Alam, M.F. Biological control of alternaria fruit rot of chili by Trichoderma species under field conditions. Mycobiology 2010, 38, 113. [Google Scholar] [CrossRef] [PubMed]
- Soytong, K.; Srinon, W.; Rattanacherdchai, K.; Kanokmedhakul, S.; Kanokmedhakul, K. Application of antagonistic fungi to control anthracnose disease of grape. J. Agric. Technol. 2005, 1, 33–41. [Google Scholar]
- Mbarga, J.B.; Ten Hoopen, G.M.; Kuaté, J.; Adiobo, A.; Ngonkeu, M.E.L.; Ambang, Z.; Akoa, A.; Tondje, P.R.; Begoude, B.A.D. Trichoderma ssperellum: A potential biocontrol agent for Pythium myriotylum, causal agent of cocoyam (Xanthosoma sagittifolium) root rot disease in Cameroon. Crop Prot. 2012, 36, 18–22. [Google Scholar] [CrossRef]
- El-Komy, M.H.; Saleh, A.A.; Eranthodi, A.; Molan, Y.Y. Characterization of novel Trichoderma asperellum isolates to select effective biocontrol agents against tomato fusarium wilt. Plant Pathol. J. 2015, 31, 50–60. [Google Scholar] [CrossRef]
- Manzar, N.; Singh, Y.; Kashyap, A.S.; Sahu, P.K.; Rajawat, M.V.S.; Bhowmik, A.; Sharma, P.K.; Saxena, A.K. Biocontrol potential of native Trichoderma spp. against anthracnose of great millet (Sorghum bicolour L.) from Tarai and Hill regions of India. Biol. Control 2021, 152, 104474. [Google Scholar] [CrossRef]
- Carvajal, V.; Enrique, J.; Valbuena, B.; Orlando, J.; Rosero, V.; Edgar, S.; Facultad, R.; Agronomía, N. Evaluación in vitro de microorganismos nativos por su antagonismo contra Moniliophthora roreri Cif & Par en cacao (Theobroma cacao L.). Rev. Fac. Nac. Agron. Medellín 2012, 65, 6305–6315. [Google Scholar]
- Galarza, L.; Akagi, Y.; Takao, K.; Kim, C.S.; Maekawa, N.; Itai, A.; Peralta, E.; Santos, E.; Kodama, M. Characterization of Trichoderma species isolated in Ecuador and their antagonistic activities against phytopathogenic fungi from Ecuador and Japan. J. Gen. Plant Pathol. 2015, 81, 201–210. [Google Scholar] [CrossRef]
- Crozier, J.; Arroyo, C.; Morales, H.; Melnick, R.L.; Strem, M.D.; Vinyard, B.T.; Collins, R.; Holmes, K.A.; Bailey, B.A. The influence of formulation on Trichoderma biological activity and frosty pod rot management in Theobroma cacao. Plant Pathol. 2015, 64, 1385–1395. [Google Scholar] [CrossRef]
- Zeilinger, S.; Gruber, S.; Bansal, R.; Mukherjee, P.K. Secondary metabolism in Trichoderma—Chemistry meets genomics. Fungal Biol. Rev. 2016, 30, 74–90. [Google Scholar] [CrossRef]
- Monte, E. Understanding Trichoderma: Between biotechnology and microbial Ecology. Int. Microbiol. 2001, 4, 1–4. [Google Scholar] [CrossRef]
- Garcia Simoes, M.L.; Tauk-Tornisielo, S.M.; Niella, G.R.; Tapia, D.M.T. Evaluation of Trichoderma spp. for the biocontrol of Moniliophthora perniciosa subgroup 1441. J. Biol. Life Sci. 2011, 3, 18–36. [Google Scholar] [CrossRef]
- Nelson, P.E.; Marasas, W.F.O.; Toussoun, T.A. Fusarium Species an Illustrates Manual for Identification; The Pennsylvania State University Press: University Park, PK, USA, 1983. [Google Scholar]
- Estrada, M.N.; Vélez, P.E.; López, J.C. Estandarizacion de una metodologia para obtener cultivos monoesporicos del hongo Beauveria bassiana. Cenicafé 1997, 48, 59–65. [Google Scholar]
- Leiva, S.; Aguilar, V.; Rubio, K.; Díaz-Valderrama, J.R.; Granda-Santos, M.; Mattos, L. Instituto de Investigación para el Desarrollo Sustentable de Ceja de Selva, Universidad Nacional Toribio Rodríguez de Mendoza de Amazonas, Chachapoyas 01001, Peru. Manuscript in preparation.
- Cai, F.; Druzhinina, I.S. In honor of John Bissett: Authoritative guidelines on molecular identification of Trichoderma. Fungal Divers. 2021, 107, 1–69. [Google Scholar] [CrossRef]
- de Respinis, S.; Vogel, G.; Benagli, C.; Tonolla, M.; Petrini, O.; Samuels, G.J. MALDI-TOF MS of Trichoderma: A Model system for the identification of microfungi. Mycol. Prog. 2010, 9, 79–100. [Google Scholar] [CrossRef]
- Evans, H.C.; Holmes, K.A.; Thomas, S.E. Endophytes and mycoparasites associated with an indigenous forest tree, Theobroma gileri, in Ecuador and a preliminary assessment of their potential as biocontrol agents of cocoa diseases. Mycol. Prog. 2003, 2, 149–160. [Google Scholar] [CrossRef]
- Holmes, K.A.; Schroers, H.; Thomas, S.E.; Evans, H.C.; Samuels, G.J.; Samuels, J. Taxonomy and biocontrol potential of a new species of Trichoderma from the Amazon basin of South America. Mycol. Prog. 2004, 3, 199–210. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat Version 2020. Available online: https://www.infostat.com (accessed on 1 June 2022).
- De Mendiburu, F.; Simon, R. Agricolae—Ten years of an open source statistical tool for experiments in breeding, agriculture and biology. PeerJ PrePrints 2015, 3, e1404v1. [Google Scholar]
- Kamvar, Z.N.; Tabima, J.F.; Gr̈unwald, N.J. Poppr: An R Package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ 2014, 2014, e281. [Google Scholar] [CrossRef]
- Hoyos-Carvajal, L.; Duque, G.; Orduz, S. Antagonismo in vitro de Trichoderma spp. sobre aislamientos de Sclerotinia spp. y Rhizoctonia spp. Rev. Colomb. Ciencias Horticolas 2011, 2, 76–86. [Google Scholar] [CrossRef]
- Sarria, G.; Garcia, A.; Mestizo, Y.; Medina, C.; Varón, F.; Mesa, E.; Hernandez, S. Antagonistic interactions betwwen Trichoderma spp. and Phytophthora palmivora (Butler) from oil palm in Colombia. Eur. J. Plant Pathol. 2021, 161, 751–768. [Google Scholar] [CrossRef]
- Martínez-Salgado, S.J.; Andrade-Hoyos, P.; Parraguirre Lezama, C.; Rivera-Tapia, A.; Luna-Cruz, A.; Romero-Arenas, O. Biological Control of charcoal rot in peanut crop through strains of Trichoderma spp., in Puebla, Mexico. Plants 2021, 10, 2630. [Google Scholar] [CrossRef] [PubMed]
- Infante, D.; Martínez, B.; González, N.; Reyes, Y. Mecanismos de acción de Trichoderma frente a hongos fitopatogenos. Rev. Protección Veg. 2009, 24, 14–21. [Google Scholar]
- Filizola, P.R.B.; Luna, M.A.C.; De Souza, A.F.; Coelho, I.L.; Laranjeira, D.; Campos-Takaki, G.M. Biodiversity and phylogeny of novel Trichoderma isolates from mangrove sediments and potential of biocontrol against Fusarium strains. Microb. Cell Fact. 2019, 18, 89. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Denis, M.; Sutton, D.A.; Cano-Lira, J.F.; Gené, J.; Fothergill, A.W.; Wiederhold, N.P.; Guarro, J. Phylogeny of the clinically relevant species of the emerging fungus Trichoderma and their antifungal susceptibilities. J. Clin. Microbiol. 2014, 52, 2112–2125. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leiva, S.; Rubio, K.; Díaz-Valderrama, J.R.; Granda-Santos, M.; Mattos, L. Phylogenetic Affinity in the Potential Antagonism of Trichoderma spp. against Moniliophthora roreri. Agronomy 2022, 12, 2052. https://doi.org/10.3390/agronomy12092052
Leiva S, Rubio K, Díaz-Valderrama JR, Granda-Santos M, Mattos L. Phylogenetic Affinity in the Potential Antagonism of Trichoderma spp. against Moniliophthora roreri. Agronomy. 2022; 12(9):2052. https://doi.org/10.3390/agronomy12092052
Chicago/Turabian StyleLeiva, Santos, Karol Rubio, Jorge R. Díaz-Valderrama, Milagros Granda-Santos, and Leonor Mattos. 2022. "Phylogenetic Affinity in the Potential Antagonism of Trichoderma spp. against Moniliophthora roreri" Agronomy 12, no. 9: 2052. https://doi.org/10.3390/agronomy12092052
APA StyleLeiva, S., Rubio, K., Díaz-Valderrama, J. R., Granda-Santos, M., & Mattos, L. (2022). Phylogenetic Affinity in the Potential Antagonism of Trichoderma spp. against Moniliophthora roreri. Agronomy, 12(9), 2052. https://doi.org/10.3390/agronomy12092052







