Biophysical Characterization of Autochthonous and New Apple Cultivar Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Preparation of Apple Surfaces
2.2. Optical Profilometry
2.3. Apparent Static and Dynamic Contact Angle Measurements
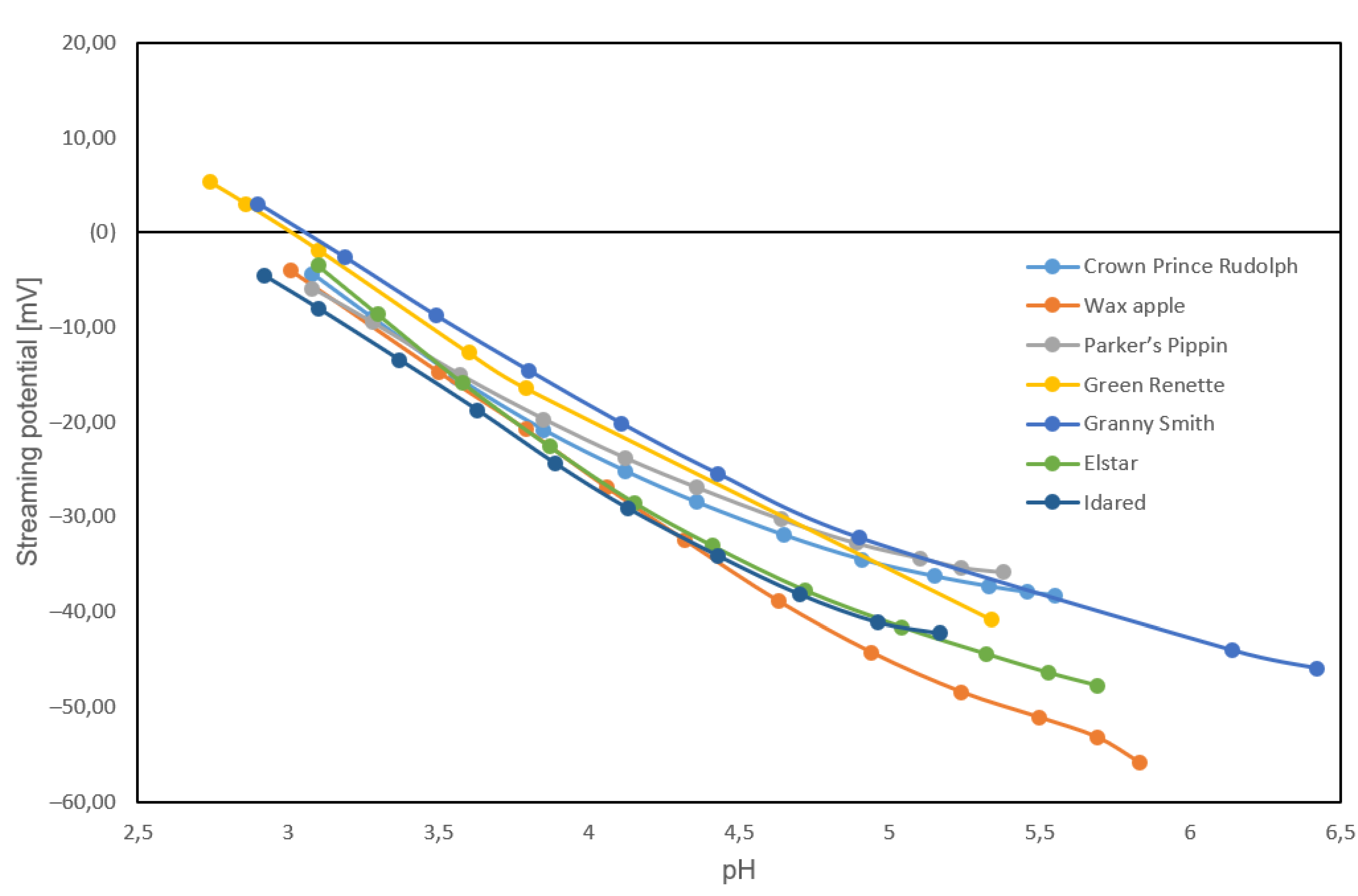
2.4. Streaming Potential Measurements
2.5. Color Measurements
2.6. Statistical Analyses
3. Results
3.1. Apple Photos
3.2. Optical Profilometry
3.3. Contact Angle
3.4. Streaming Potential
3.5. Color Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bondonno, N.P.; Bondonno, C.P.; Ward, N.C.; Hodgson, J.M.; Croft, K.D. The cardiovascular health benefits of apples: Whole fruit vs. isolated compounds. Trends Food Sci. 2017, 69, 243–256. [Google Scholar] [CrossRef]
- Tu, S.-H.; Chen, L.-C.; Ho, Y.-S. An apple a day to prevent cancer formation: Reducing cancer risk with flavonoids. J. Food Drug Anal. 2017, 25, 119–124. [Google Scholar] [CrossRef]
- Ogura, K.; Ogura, M.; Shoji, T.; Sato, Y.; Tahara, Y.; Yamano, G.; Sato, H.; Sugizaki, K.; Fujita, N.; Tatsuoka, H. Oral administration of apple procyanidins ameliorates insulin resistance via suppression of pro-inflammatory cytokine expression in liver of diabetic ob/ob mice. J. Agric. Food Chem. 2016, 64, 8857–8865. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Yu, R.; Geng, S.; Xiong, L.; Yan, Q.; Kumar, V.; Wen, C.; Peng, M. Apple polyphenols modulates the antioxidant defense response and attenuates inflammatory response concurrent with hepatoprotective effect on grass carp (Ctenopharyngodon idellus) fed low fish meal diet. Aquaculture 2021, 534, 736284. [Google Scholar] [CrossRef]
- Kunst, L.; Samuels, A.L. Biosynthesis and secretion of plant cuticular wax. Prog. Lipid Res. 2003, 42, 51–80. [Google Scholar] [CrossRef]
- Schuster, A.-C.; Burghardt, M.; Alfarhan, A.; Bueno, A.; Hedrich, R.; Leide, J.; Thomas, J.; Riederer, M. Effectiveness of cuticular transpiration barriers in a desert plant at controlling water loss at high temperatures. AoB Plants 2016, 8, plw027. [Google Scholar] [CrossRef]
- Tafolla-Arellano, J.C.; Báez-Sañudo, R.; Tiznado-Hernández, M.E. The cuticle as a key factor in the quality of horticultural crops. Sci. Hortic. 2018, 232, 145–152. [Google Scholar] [CrossRef]
- Kschonsek, J.; Wolfram, T.; Stöckl, A.; Böhm, V. Polyphenolic compounds analysis of old and new apple cultivars and contribution of polyphenolic profile to the in vitro antioxidant capacity. Antioxidants 2018, 7, 20. [Google Scholar] [CrossRef]
- Wandjou, J.G.N.; Lancioni, L.; Barbalace, M.C.; Hrelia, S.; Papa, F.; Sagratini, G.; Vittori, S.; Dall’Acqua, S.; Caprioli, G.; Beghelli, D. Comprehensive characterization of phytochemicals and biological activities of the Italian ancient apple ‘Mela Rosa dei Monti Sibillini’. Food Res. Int. 2020, 137, 109422. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Laskowski, P. Polyphenolic compounds and antioxidant activity of new and old apple varieties. J. Agric. Food Chem. 2008, 56, 6520–6530. [Google Scholar] [CrossRef]
- Leide, J.; de Souza, A.X.; Papp, I.; Riederer, M. Specific characteristics of the apple fruit cuticle: Investigation of early and late season cultivars ‘Prima’and ‘Florina’ (Malus domestica Borkh.). Sci. Hortic. 2018, 229, 137–147. [Google Scholar] [CrossRef]
- Pietrysiak, E.; Ganjyal, G.M. Apple peel morphology and attachment of Listeria innocua through aqueous environment as shown by scanning electron microscopy. Food Control. 2018, 92, 362–369. [Google Scholar] [CrossRef]
- Fallik, E.; Grinberg, S.; Alkalai, S.; Yekutieli, O.; Wiseblum, A.; Regev, R.; Beres, H.; Bar-Lev, E. A unique rapid hot water treatment to improve storage quality of sweet pepper. Postharvest Biol. Technol. 1999, 15, 25–32. [Google Scholar] [CrossRef]
- Bohinc, K.; Dražić, G.; Oder, M.; Jevšnik, M.; Nipič, D.; Godič-Torkar, K.; Raspor, P. Available surface dictates microbial adhesion capacity. Int. J. Adhes. Adhes. 2014, 50, 265–272. [Google Scholar] [CrossRef]
- Good, R.J. Contact angle, wetting, and adhesion: A critical review. J. Adhes. Sci. Technol. 1992, 6, 1269–1302. [Google Scholar] [CrossRef]
- Lorenzetti, M.; Luxbacher, T.; Kobe, S.; Novak, S. Electrokinetic behaviour of porous TiO2-coated implants. J. Mater. Sci. Mater. Med. 2015, 26, 1–4. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, B.; Zhang, J.; Wang, C.; Liu, C.; Liu, Y.; Zhu, X.; Ren, X. Relationships between cuticular waxes and skin greasiness of apples during storage. Postharvest Biol. Technol. 2017, 131, 55–67. [Google Scholar] [CrossRef]
- Cajuste, J.F.; González-Candelas, L.; Veyrat, A.; García-Breijo, F.J.; Reig-Armiñana, J.; Lafuente, M.T. Epicuticular wax content and morphology as related to ethylene and storage performance of ‘Navelate’orange fruit. J. Postharvest Biol. Technol. 2010, 55, 29–35. [Google Scholar] [CrossRef]
- Bohinc, K.; Kukić, L.; Štukelj, R.; Zore, A.; Abram, A.; Klačić, T.; Kovačević, D. Bacterial adhesion capacity of uropathogenic Escherichia coli to polyelectrolyte multilayer coated urinary catheter surface. Coatings 2021, 11, 630. [Google Scholar] [CrossRef]
- Martin, L.B.; Rose, J.K.J.J.o.E.B. There’s more than one way to skin a fruit: Formation and functions of fruit cuticles. J. Exp. Bot. 2014, 65, 4639–4651. [Google Scholar] [CrossRef] [Green Version]
- Konarska, A.J.P. The structure of the fruit peel in two varieties of Malus domestica Borkh. (Rosaceae) before and after storage. Protoplasma 2013, 250, 701–714. [Google Scholar] [CrossRef]
- Dobrowolska-Iwanek, J.; Gąstoł, M.; Adamska, A.; Krośniak, M.; Zagrodzki, P. Traditional versus modern apple cultivars—A comparison of juice composition. Folia Hortic. 2015, 27, 33–41. [Google Scholar] [CrossRef]
- Bhide, S.; Salvi, D.; Schaffner, D.W.; Karwe, M.V.J. Effect of surface roughness in model and fresh fruit systems on microbial inactivation efficacy of cold atmospheric pressure plasma. J. Food Prot. 2017, 80, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ortega, I.; García-Almendárez, B.E.; Santos-López, E.M.; Reyes-González, L.R.; Regalado, C. Characterization and antimicrobial effect of starch-based edible coating suspensions. Food Hydrocoll. 2016, 52, 906–913. [Google Scholar] [CrossRef]
- Wang, H.; Feng, H.; Liang, W.; Luo, Y.; Malyarchuk, V. Effect of surface roughness on retention and removal of Escherichia coli O157: H7 on surfaces of selected fruits. J. Food Sci. 2009, 74, E8–E15. [Google Scholar] [CrossRef]
- Palma-Salgado, S.; Ku, K.-M.; Dong, M.; Nguyen, T.H.; Juvik, J.A.; Feng, H. Adhesion and removal of E. coli K12 as affected by leafy green produce epicuticular wax composition, surface roughness, produce and bacterial surface hydrophobicity, and sanitizers. Int. J. Food Microbiol. 2020, 334, 108834. [Google Scholar] [CrossRef] [PubMed]
- Pathak, P.D.; Mandavgane, S.A.; Kulkarni, B.D. Characterizing fruit and vegetable peels as bioadsorbents. Curr. Sci. 2016, 2114–2123. [Google Scholar] [CrossRef]
- Maillard, A.P.F.; Espeche, J.C.; Maturana, P.; Cutro, A.C.; Hollmann, A. Zeta potential beyond materials science: Applications to bacterial systems and to the development of novel antimicrobials. Biochim. Et Biophys. Acta -Biomembr. 2021, 1863, 183597. [Google Scholar] [CrossRef]
- He, Q.; Zhang, L.; Yang, Z.; Ding, T.; Ye, X.; Liu, D.; Guo, M. Antibacterial mechanisms of thyme essential oil nanoemulsions against Escherichia coli O157: H7 and Staphylococcus aureus: Alterations in membrane compositions and characteristics. Innov. Food Sci. Emerg. Technol. 2022, 75, 102902. [Google Scholar] [CrossRef]
- Kovačević, D.; Pratnekar, R.; Godič Torkar, K.; Salopek, J.; Dražić, G.; Abram, A.; Bohinc, K. Influence of polyelectrolyte multilayer properties on bacterial adhesion capacity. Polymers 2016, 8, 345. [Google Scholar] [CrossRef]

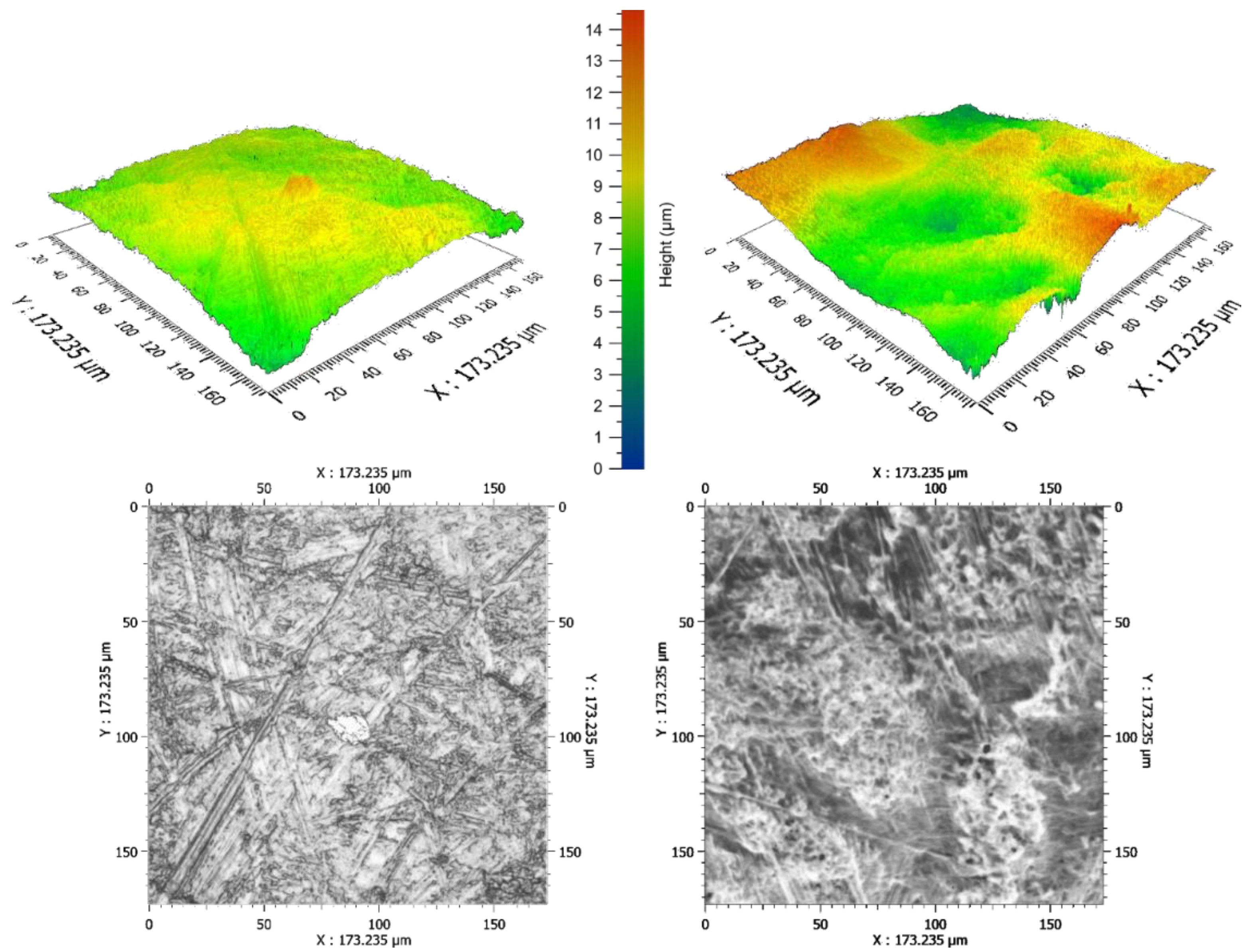

| Cultivar | Average RMS [μm] | Mean RMS [μm] | I [μm] | N |
|---|---|---|---|---|
| Crown Prince Rudolph | 0.462 ± 0.431 | 0.303 | 0.217 ± 0.480 | 25 |
| Wax apple | 0.754 ± 0.450 | 0.689 | 0.443 ± 1.039 | 22 |
| Parker’s Pippin | 0.531 ± 0.431 | 0.344 | 0.224 ± 0.609 | 10 |
| Green Renette | 0.598 ± 0.313 | 0.607 | 0.297 ± 0.748 | 35 |
| Granny Smith | 0.693 ± 0.587 | 0.562 | 0.355 ± 0.721 | 33 |
| Elstar | 1.402 ± 1.949 | 0.684 | 0.432 ± 1.461 | 72 |
| Idared | 0.538 ± 0.441 | 0.365 | 0.282 ± 0.597 | 65 |
| Golden Delicious | 0.964 ± 0.355 | 0.958 | 0.688 ± 1.231 | 10 |
| Cultivar | |
|---|---|
| Crown Prince Rudolph | 78.31 ± 4.12 |
| Wax apple | 76.87 ± 2.96 |
| Parker’s Pippin | 86.99 ± 4.77 |
| Green Renette | 79.36 ± 1.53 |
| Granny Smith | 85.97 ± 6.39 |
| Elstar | 100.88 ± 16.38 |
| Idared | 91.05 ± 8.61 |
| Golden Delicious | 87.45 ± 7.60 |
| CIE Color Parameters | |||
|---|---|---|---|
| Cultivar | L* | a* | b* |
| Crown Prince Rudolph | 76.43 ± 2.06 | −10.97 ± 0.98 | 45.28 ± 3.03 |
| Wax apple | 80.86 ± 0.90 | −7.32 ± 1.21 | 47.24 ± 2.24 |
| Parker’s Pippin | 53.09 ± 0.35 | 7.15 ± 1.70 | 38.71 ± 3.82 |
| Green Renette | 64.76 ± 0.88 | −16.80 ± 0.21 | 51.34 ± 2.72 |
| Granny Smith | 64.21 ± 2.03 | −18.59 ± 0.31 | 48.13 ± 1.20 |
| Elstar | 50.43 ± 7.95 | 27.13 ± 2.92 | 28.19 ± 7.68 |
| Idared | 46.47 ± 2.17 | 33.34 ± 1.24 | 24.20 ± 0.72 |
| Golden Delicious | 58.32 ± 2.00 | 29.86 ± 3.60 | 37.71 ± 0.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bohinc, K.; Štukelj, R.; Abram, A.; Jerman, I.; Van de Velde, N.; Vidrih, R. Biophysical Characterization of Autochthonous and New Apple Cultivar Surfaces. Agronomy 2022, 12, 2051. https://doi.org/10.3390/agronomy12092051
Bohinc K, Štukelj R, Abram A, Jerman I, Van de Velde N, Vidrih R. Biophysical Characterization of Autochthonous and New Apple Cultivar Surfaces. Agronomy. 2022; 12(9):2051. https://doi.org/10.3390/agronomy12092051
Chicago/Turabian StyleBohinc, Klemen, Roman Štukelj, Anže Abram, Ivan Jerman, Nigel Van de Velde, and Rajko Vidrih. 2022. "Biophysical Characterization of Autochthonous and New Apple Cultivar Surfaces" Agronomy 12, no. 9: 2051. https://doi.org/10.3390/agronomy12092051
APA StyleBohinc, K., Štukelj, R., Abram, A., Jerman, I., Van de Velde, N., & Vidrih, R. (2022). Biophysical Characterization of Autochthonous and New Apple Cultivar Surfaces. Agronomy, 12(9), 2051. https://doi.org/10.3390/agronomy12092051









