Two Allelopathic Substances from Plumbago rosea Stem Extracts and Their Allelopathic Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Plant Materials
2.2. Extraction and Growth Assay
2.3. Separation and Isolation of the Allelopathic Substances from the Plumbago Rosea Extracts
2.4. Germination Test
2.5. Statistical Analysis
3. Results
3.1. Allelopathic Property of Plumbago rosea Extract
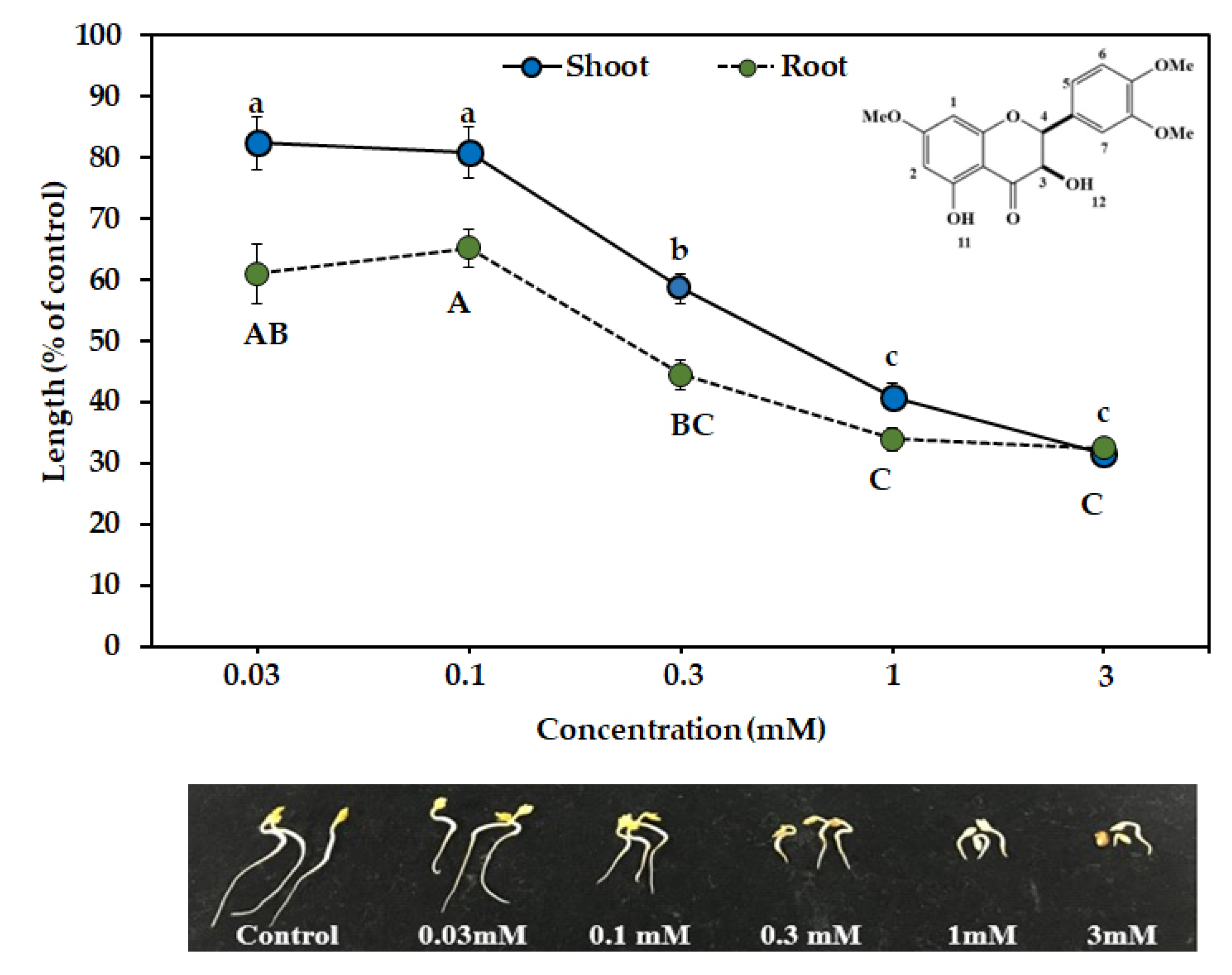
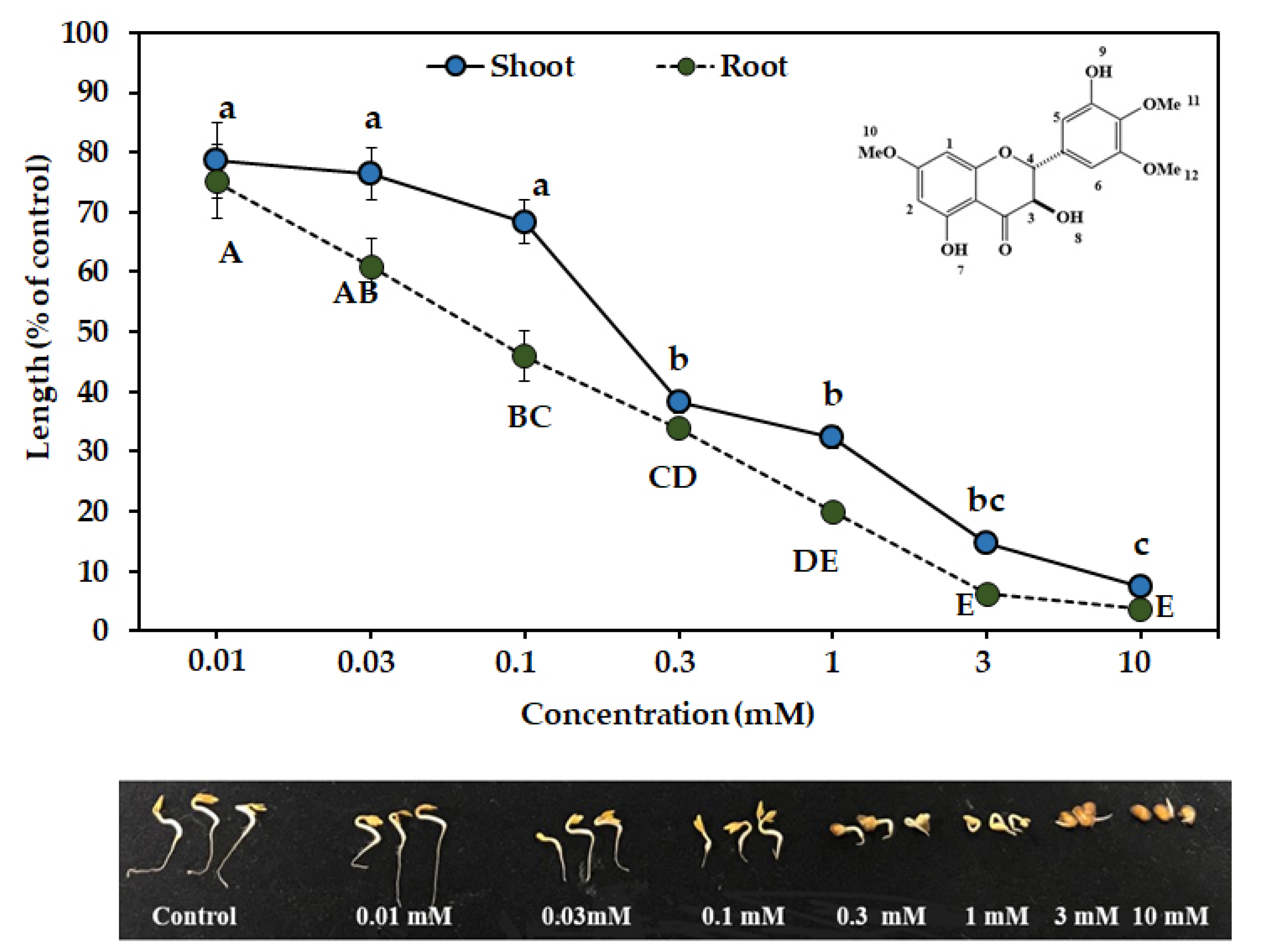
3.2. Isolation and Identification of the Growth Inhibitory Substances
3.3. Biological Activities of the Two Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Varshney, S.; Hayat, S.; Alyemeni, M.N.; Ahmad, A. Effects of herbicide applications in wheat fields: Is phytohormones application a remedy? Plant Signal Behav. 2012, 7, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Al-Samarai, G.F.; Mahdi, W.M.; Al-Hilali, B.M. Reducing environmental pollution by chemical herbicides using natural plant derivatives—Allelopathy effect. Ann. Agric. Environ. Med. 2018, 25, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Gasnier, C.; Dumont, C.; Benachour, N.; Clair, E.; Chagnon, M.C. Glyphosate-based herbicides are toxic and endocrine disruptors in human cell lines. Toxicology 2009, 262, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, I.; Imadi, S.R.; Shazadi, K.; Gul, A.; Hakeem, K.R. Effect of pesticides on environments. In Plant, Soil and Microbes; Hakeem, K., Akhtar, M., Abdullah, S., Eds.; Springer: Cham, Switzerland, 2016; pp. 253–269. [Google Scholar]
- Bhadoria, P. Allelopathy: A natural way towards weed management. Am. J. Exp. Agric. 2011, 1, 7–20. [Google Scholar] [CrossRef]
- Zeng, R.S.; Mallik, A.U.; Luo, S.M. Allelopathy in Sustainable Agriculture and Forestry; Springer: Berlin, Germany, 2008; p. 412. [Google Scholar]
- Alsharekh, A.; El-Sheikh, M.A.; Alatar, A.A.; Abdel-Salam, E.M. Natural Control of Weed Invasions in Hyper-Arid Arable Farms: Allelopathic Potential Effect of Conocarpus erectus against Common Weeds and Vegetables. Agronomy 2022, 12, 703. [Google Scholar] [CrossRef]
- Bari, I.N.; Kato-Noguchi, H. Phytotoxic effects of Cerbera manghas L. leaf extracts on seedling elongation of four monocot and four dicot test species. Acta Agrobot. 2017, 70, 1720. [Google Scholar] [CrossRef]
- Bachheti, A.; Sharma, A.; Bachheti, R.K.; Husen, A.; Pandey, D.P. Plant allelochemicals and their various applications. In Co-Evolution of Secondary Metabolites; Springer: Berlin/Heidelberg, Germany, 2020; pp. 441–465. [Google Scholar] [CrossRef]
- Fuji, Y.; Hiradate, S. Allelopathy: New Concepts and Methodology; Science Publishers Inc.: Enfield, NH, USA, 2007; p. 5. [Google Scholar]
- Hussain, M.I.; Reigosa, M.J. Secondary metabolites, ferulic acid and p-hydroxybenzoic acid-induced toxic effects on photosyntheticprocess in Rumex acetosa L. Biomolecules 2021, 11, 233. [Google Scholar] [CrossRef]
- Duke, S.O.; Dayan, E.F.; Romagni, J.G.; Rimando, A.M. Natural products as sources of herbicides, current status and future trends. Weed Res. 2000, 40, 99–111. [Google Scholar] [CrossRef]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Ann. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef] [Green Version]
- Kato-Noguchi, H.; Fushimi, Y.; Shigemori, H. An allelopathic substance in red pine needles (Pinus densiflora). J. Plant Physiol. 2009, 166, 442–446. [Google Scholar] [CrossRef]
- Akhtar, N.; Javaid, A.; Bajwa, R. Herbicidal activity of aqueous extracts of Cirsium arvense and Ageratum conyzoides against weeds of wheat. Pak. J. Biol. Sci. 2001, 4, 1364–1367. [Google Scholar]
- Khan, P.A.; Quisar, K.N.; Khan, M.A. Effect of leaf extract of Populus deltoides on seed germination and seedling growth of radish, French bean and mustard. Ind. J. For. 2006, 29, 403–406. [Google Scholar]
- Okeyo, J.M. Medicinal Plants; Schmelzer, G.H., Gurib-Fakim, A., Eds.; Foundation PROTA: Leiden, The Netherlands; Backhuys Publishers: Leiden, The Netherlands, 2008; p. 473. [Google Scholar]
- Schmelzer, G.H.; Gurib-Fakim, A. Plant resources of tropical Africa. In Medicinal Plants; Foundation PROTA: Leiden, The Netherlands; Backhuys Publishers: Leiden, The Netherlands, 2008; p. 474. [Google Scholar]
- Dorni, A.I.C.; Vidyalakshmi, K.S.; Hannah, R.V.; Rajamanickam, G.V.; Dubey, G.P. Anti-inflammatory activity of Plumbago capensis. Pharmacogn. Mag. 2006, 2, 239–243. [Google Scholar]
- Misra, M.K.; Behera, S.K.; Panda, A.; Behera, S.K. Medicinal plants used by the Kandhas of Kandhamal district of Orissa. Ind. J. Trad. Knowl. 2006, 5, 519–528. [Google Scholar]
- Nath, S.C.; Purkayastha, J. Biological activity of ethnomedical claim of some plant species of Assam. Ind. J. Trad. Knowl. 2004, 5, 229–236. [Google Scholar]
- Sarma, H.N.; Mahanta, H.C. Effects of composite root extract on rat granulosa cells: A transmission electron microscopic observations. J. Exp. Zool. 2000, 3, 217–221. [Google Scholar]
- Devi, P.U.; Solomon, F.E.; Sharada, A.C. In vivo tumor inhibitory and radiosensitizing effects of an Indian medicinal plant, Plumbago rosea on experimental mouse tumors. Ind. J. Exp. Biol. 1994, 32, 523–528. [Google Scholar]
- Mary, N.K.; Babu, B.H.; Padikkala, J. Antiatherogenic effect of Caps HT2, an herbal Ayurvedic medicine formulation. Phytomedicine 2003, 10, 474–482. [Google Scholar] [CrossRef]
- Sattar, M.A.A.; Khan, M.A.; Dewa, A.; Samshia, D. Uterotrophic, fetotoxic and abortifacient effect of a Malaysian variety of Plumbago rosea L. on isolated rat uterus and pregnant mice. Pak. J. Biol. Sci. 2007, 10, 763–767. [Google Scholar]
- Poosarla, A.; Athota, R.R. Immunosuppressive properties of aqueous extract of Plumbago zeylanica in Balb/c mice. J. Med. Plants 2010, 4, 2138–2143. [Google Scholar]
- Parimala, R.; Sachdanandam, P. Effect of plumbagin on some glucose metabolising enzymes studied in rats in experimental hepatoma. Mol. Cell Biochem. 1993, 12, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Sugie, S.; Okamoto, K.; Rahman, K.M.W.; Tanaka, T. Inhibitory effects of plumbagin and juglone on azoxymethane-induced intestinal carcinogenesis in rats. Cancer Lett. 1998, 127, 177–183. [Google Scholar] [CrossRef]
- Aziz, M.H.; Dreckschmidt, N.E.; Verma, A.K. Plumbagin a medicinal plant−derived naphthoquinone is a novel inhibitor of the growth and invasion of hormone-refractory prostate cancer. Cancer Res. 2008, 68, 9024–9032. [Google Scholar] [CrossRef] [PubMed]
- Santhakumari, G.; Rathinam, K.; Seshadri, C. Anticoagulant activity of plumbagin. Ind. J. Exp. Biol. 1978, 16, 485. [Google Scholar]
- Shin, K.; Lee, S.; Cha, B. Antifungal activity of plumbagin purified from leaves of Nepenthes ventricosa x maxima against phytopathogenic fungi. Plant. Pathol. J. 2007, 23, 113. [Google Scholar] [CrossRef]
- Tokunaga, T.; Takada, N.; Ueda, M. Mechanism of antifeedant activity of plumbagin, a compound concerning the chemical defense in carnivorous plant. Tetrahedron Lett. 2004, 45, 7115–7119. [Google Scholar] [CrossRef]
- Devi, P.U.; Rao, B.S.; Solomon, F.E. Effect of plumbagin on the radiation-induced cytogenetic and cell cycle changes in mouse Ehrlich ascites carcinoma in vivo. Ind. J. Exp. Biol. 1998, 36, 891–895. [Google Scholar]
- Anonymous. The Wealth of India; Council for Scientific and Industrial Research: New Delhi, India, 1985; Volume 8. [Google Scholar]
- Kannan, E.; Palayian, L. Allelopathic potential of Annona muricata (L.) on physiological and biochemical changes of Vigna radiata (L.) and Eleusine coracana (L.) Gaertn. J. Appl. Biol. Biotechnol. 2022, 10, 145–153. [Google Scholar] [CrossRef]
- Krumsri, R.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Assessment of allelopathic potential of Senna garrettiana leaves and identification of potent phytotoxic substances. Agronomy 2022, 12, 139. [Google Scholar] [CrossRef]
- Lun, T.L.; Kato-Noguchi, H. Assessment of the allelopathic potential of Leucas cephalotes (Roth) Spreng. extracts on the seedling growth of six test plants. Plant Omics J. 2021, 14, 72–77. [Google Scholar] [CrossRef]
- Hossen, K.; Das, K.R.; Asato, Y.; Teruya, T.; Kato-Noguchi, H. Allelopathic activity and characterization of allelopathic substances from Elaeocarpus floribundus Blume leaves for the development of bioherbicides. Agronomy 2022, 12, 57. [Google Scholar] [CrossRef]
- Kyaw, E.H.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Allelopathy of the medicinal plant Dregea volubilis (L.f.) Benth. ex Hook.f. and its phytotoxic substances with allelopathic activity. Agronomy 2022, 12, 303. [Google Scholar] [CrossRef]
- Krumsri, R.; Boonmee, S.; Kato-Noguchi, H. Evaluation of the Allelopathic Potential of Leaf Extracts from Dischidia imbricata (Blume) Steud. on the Seedling Growth of Six Test Plants. Not. Bot. Horti Agrobot. 2019, 47, 1019–1024. [Google Scholar] [CrossRef]
- Appiah, K.S.; Mardani, H.K.; Osivand, A.; Kpabitey, S.; Amoatey, C.A.; Oikawa, Y.; Fujii, Y. Exploring alternative use of medicinal plants for sustainable weed management. Sustainability 2017, 9, 1468. [Google Scholar] [CrossRef]
- Aniya; Nomura, Y.; Fuerdeng; Appiah, K.S.; Fujii, Y. Evaluation of Allelopathic Activity of Chinese Medicinal Plants and Identification of Shikimic Acid as an Allelochemical from Illicium verum Hook. f. Plants 2020, 9, 684. [Google Scholar] [CrossRef]
- Dayan, F.E.; Romagni, J.G.; Duke, S.O. Investigating the mode of action of natural phytotoxins. J. Chem. Ecol. 2000, 26, 2079–2094. [Google Scholar] [CrossRef]
- Cheng, F.; Cheng, Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant. Sci. 2015, 6, 1020. [Google Scholar] [CrossRef]
- Hussein, R.A.; El-Anssary, A.A. Plants secondary metabolites: The key drivers of the pharmacological actions of medicinal plants. In Herbal Medicine; Chapter 2; Builders, P., Ed.; Intech Open: London, UK, 2018. [Google Scholar]
- Xiong, H.P.; Ji, H.W.; Yang, W.L.; Zhang, Y.S. Antimicrobial activity of extracts from Ampelopsis grossedentata on common respiratory tract pathogens. J. Guangxi Agric. Biol. Sci. 2007, 26, 150–153. [Google Scholar]
- Tuckmantel, W.; Kozikowski, A.P.; Romanczyk, L.J., Jr. Studies in polyphenol chemistry and bioactivity. 1. Preparation of building blocks from (+)-catechin. Procyanidin formation. Synthesis of the cancer cell growth inhibitor, 3-O-galloyl-(2R,3R)-epicatechin-4β,8-[3-O-galloyl-(2R,3R)-epicatechin]. J. Am. Chem. Soc. 1999, 121, 12073–12081. [Google Scholar] [CrossRef]
- Oyama, Y.; Fuchs, P.A.; Katayama, N.; Noda, K. Myricetin and quercetin, the flavonoid constituents of Ginkgo biloba extract, greatly reduce oxidative metabolism in both resting and Ca(2+)-loaded brain neurons. Brain Res. 1994, 635, 125–129. [Google Scholar] [CrossRef]
- Gordon, M.H.; Roedig-Penmanm, A. Antioxidant activity of quercetin and myricetin in liposomes. Chem. Phys. Lipids 1998, 97, 79–85. [Google Scholar] [CrossRef]

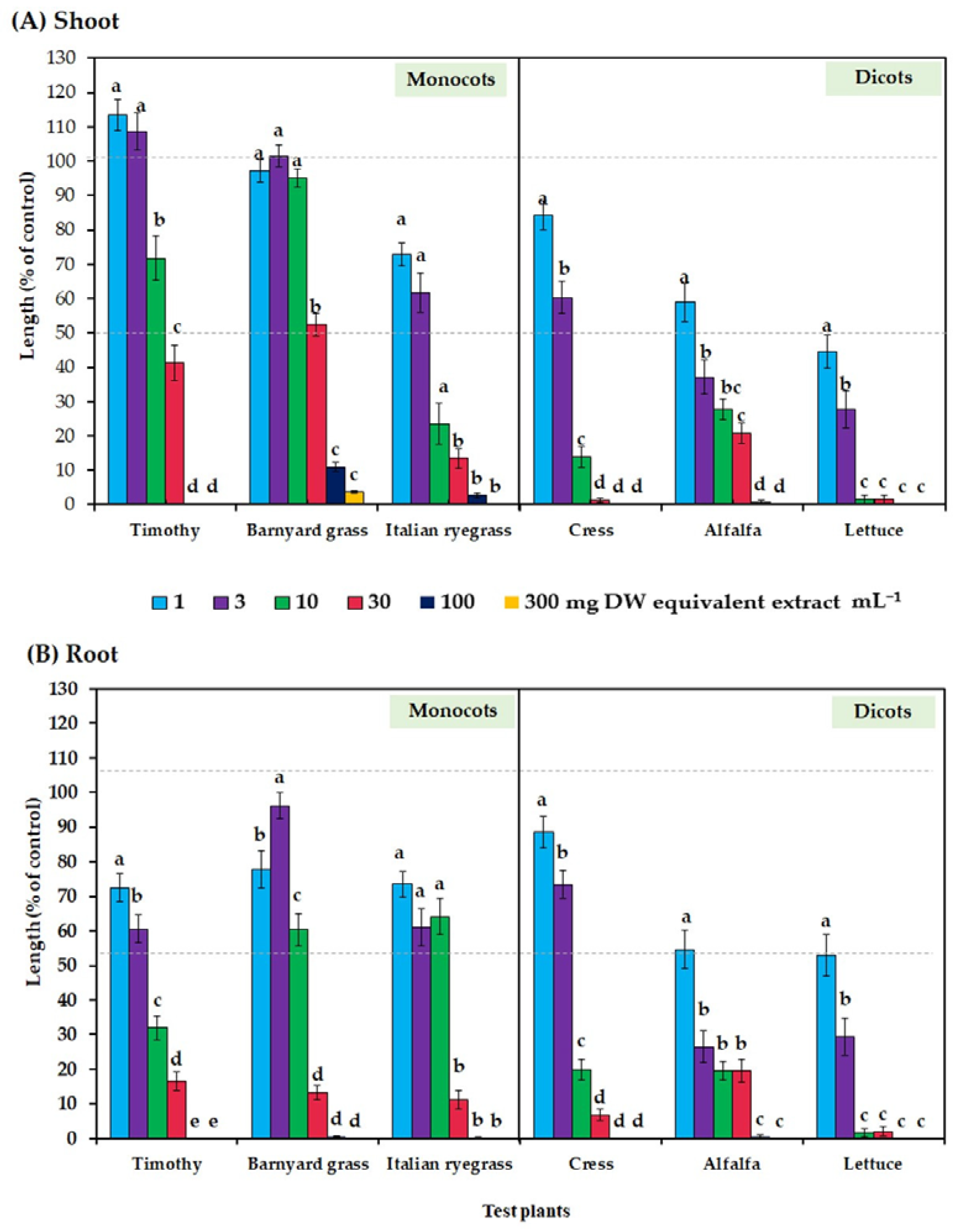

| Test Plant | Monocot | Dicot | ||||
|---|---|---|---|---|---|---|
| Timothy | Barnyard Grass | Italian Ryegrass | Cress | Alfalfa | Lettuce | |
| Shoot | 21.87 | 33.50 | 3.71 | 3.47 | 1.83 | 0.87 |
| Root | 4.16 | 11.68 | 7.02 | 4.83 | 1.18 | 1.13 |
| Compound Name | EC50 (mM) | |
|---|---|---|
| Shoot | Root | |
| 7,4′,5′-tri-O-methyl dihydroquercetin | 0.59 | 0.24 |
| 7,4′,5′-tri-O-methylampelopsin | 0.21 | 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lun, T.L.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Two Allelopathic Substances from Plumbago rosea Stem Extracts and Their Allelopathic Effects. Agronomy 2022, 12, 2020. https://doi.org/10.3390/agronomy12092020
Lun TL, Iwasaki A, Suenaga K, Kato-Noguchi H. Two Allelopathic Substances from Plumbago rosea Stem Extracts and Their Allelopathic Effects. Agronomy. 2022; 12(9):2020. https://doi.org/10.3390/agronomy12092020
Chicago/Turabian StyleLun, Thang Lam, Arihiro Iwasaki, Kiyotake Suenaga, and Hisashi Kato-Noguchi. 2022. "Two Allelopathic Substances from Plumbago rosea Stem Extracts and Their Allelopathic Effects" Agronomy 12, no. 9: 2020. https://doi.org/10.3390/agronomy12092020
APA StyleLun, T. L., Iwasaki, A., Suenaga, K., & Kato-Noguchi, H. (2022). Two Allelopathic Substances from Plumbago rosea Stem Extracts and Their Allelopathic Effects. Agronomy, 12(9), 2020. https://doi.org/10.3390/agronomy12092020





