Allelopathic Activity of a Novel Compound, 5,6-Dihydrogen-11α-O-acetyl-12β-O-tigloyl-17β-marsdenin, and a Known Steroidal Glycoside from the Leaves of Marsdenia tenacissima (Roxb.) Moon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction and Growth Bioassay
2.3. Purification of the Active Substances
2.4. Biological Activity of Compounds 1 and 2
2.5. Statistical Analysis
3. Results
3.1. Plant Growth Inhibitory Activity of the Marsdenia tenacissima Leaf Extracts
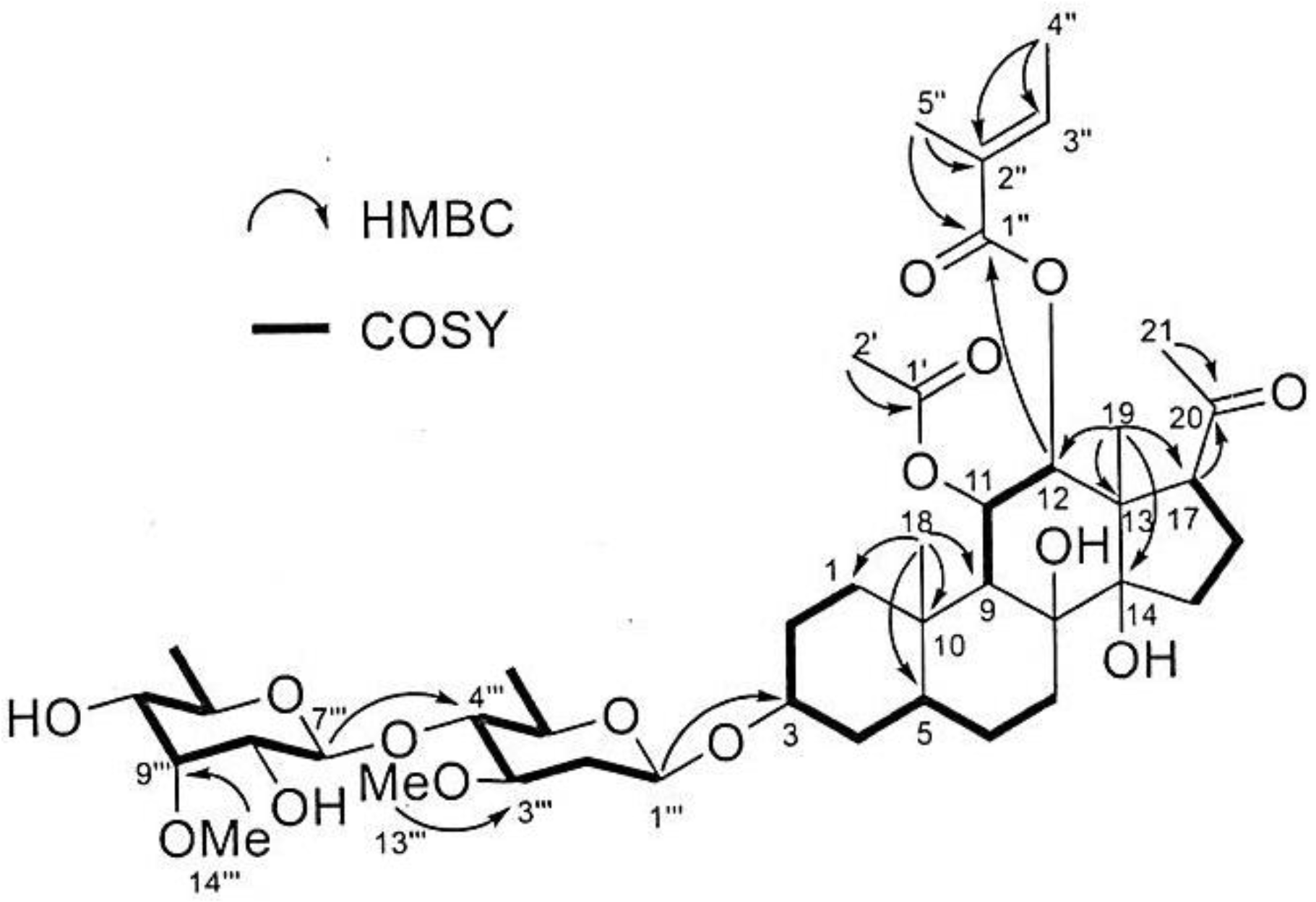
3.2. Isolation and Investigation of the Allelopathic Substances from the Leaf Extracts of M. tenacissima
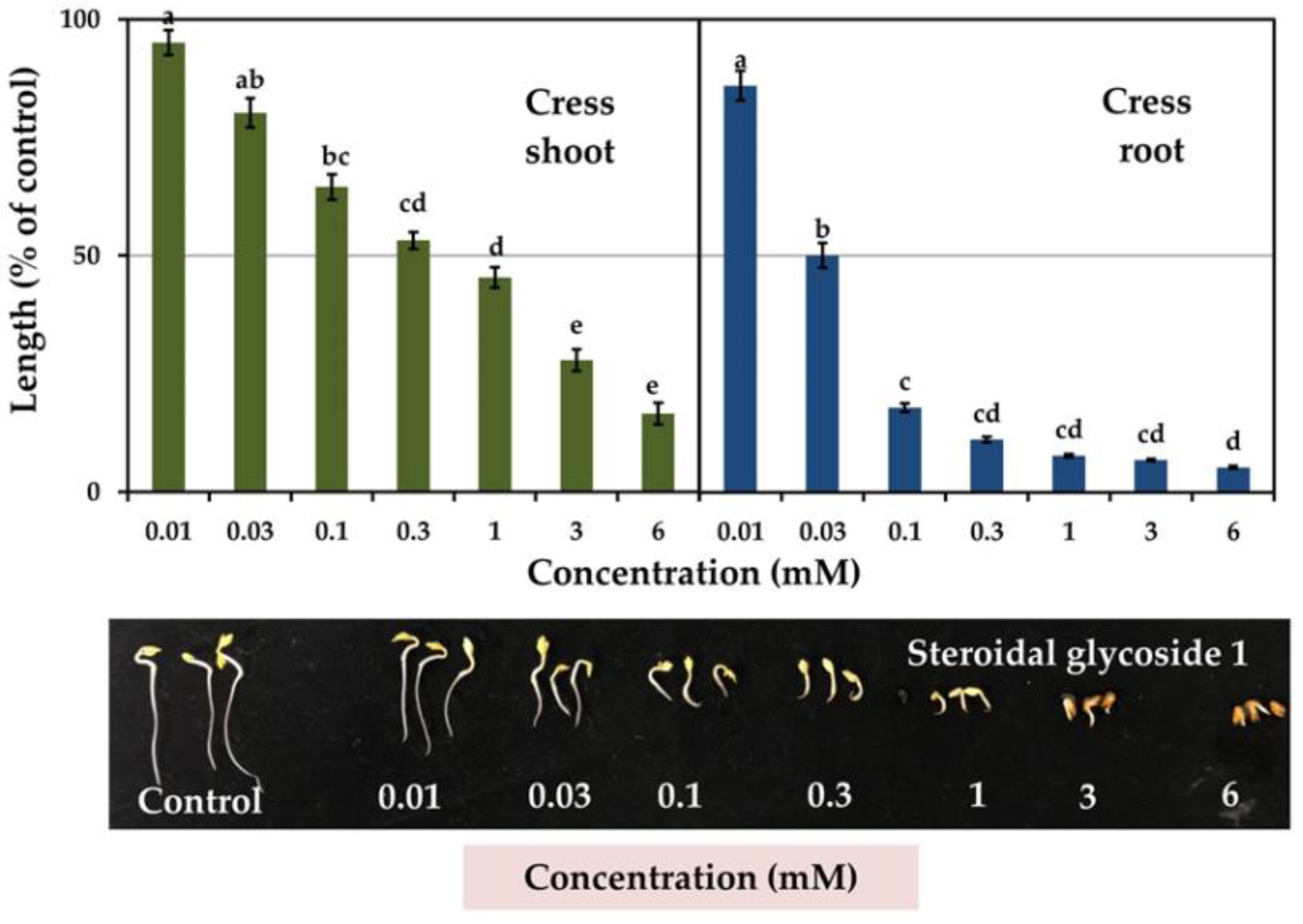
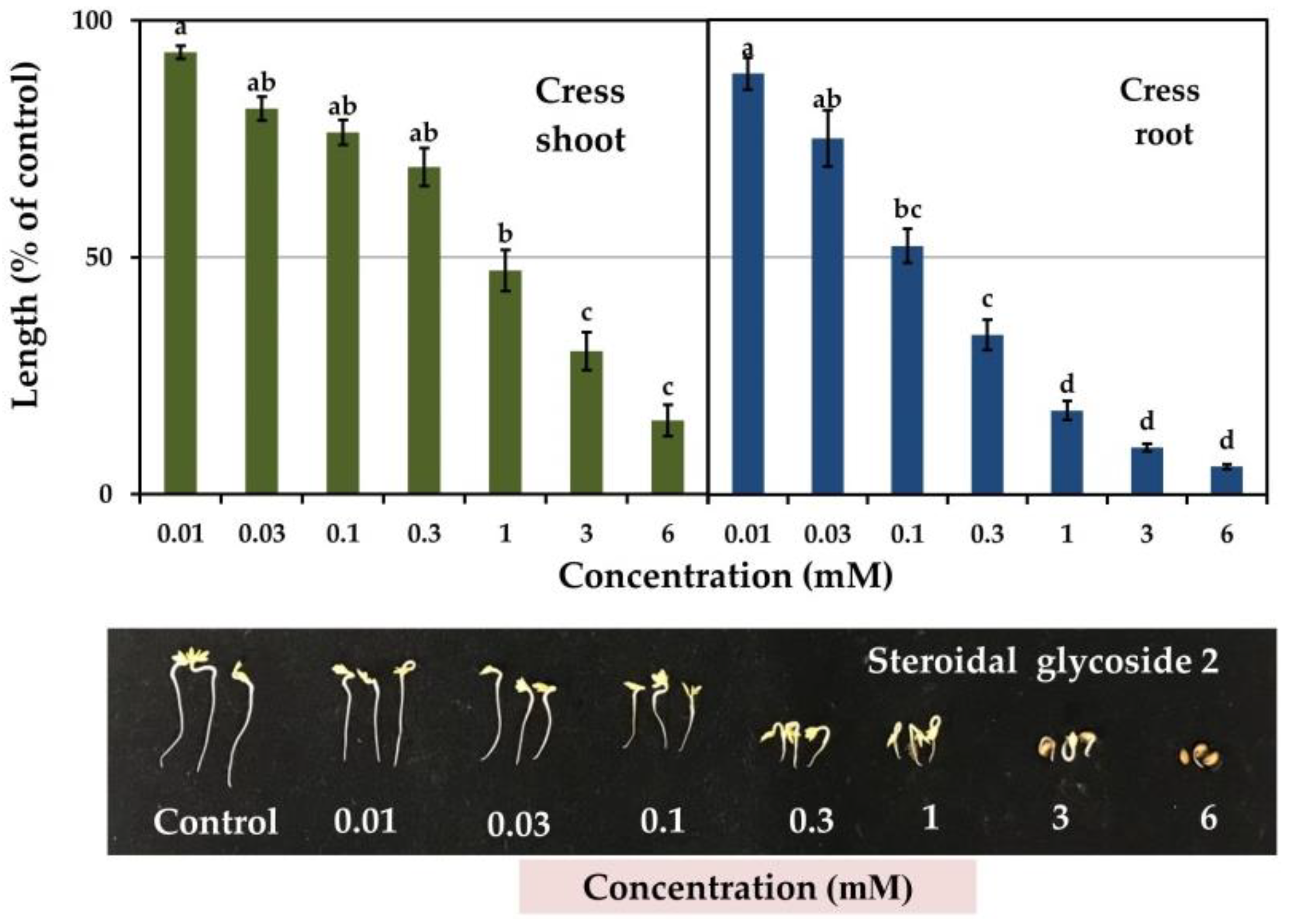
3.3. Allelopathic Activity of Compounds 1 and 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Fried, G.; Chauvel, B.; Reynaud, P.; Sache, I. Decreases in crop production by non-native weeds, pests, and pathogens. In Impact of Biological Invasions on Ecosystem Services; Springer: New York, NY, USA, 2017; pp. 83–101. [Google Scholar]
- Kniss, A.R. Long-term trends in the intensity and relative toxicity of herbicide use. Nat. Commun. 2016, 8, 14865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scavo, A.; Mauromicale, G. Crop allelopathy for sustainable weed management in agroecosystems: Knowing the present with a view to the future. Agronomy 2021, 11, 2104. [Google Scholar] [CrossRef]
- Anwar, S.; Naseem, S.; Karimi, S.; Asi, M.R.; Akrem, A.; Ali, Z. Bioherbicidal activity and metabolic profiling of potent allelopathic plant fractions against major weeds of wheat—Way forward to lower the risk of synthetic herbicides. Front. Plant Sci. 2021, 12, 632390. [Google Scholar] [CrossRef]
- Hussain, M.I.; Reigosa, M.J. Secondary metabolites, ferulic acid and p-hydroxybenzoic acid induced toxic effects on photosynthetic process in Rumex acetosa L. Biomolecules 2021, 11, 233. [Google Scholar] [CrossRef]
- Singh, A.A.; Rajeswari, G.; Nirmal, L.A.; Jacob, S. Synthesis and extraction routes of allelochemicals from plants and microbes: A review. Rev. Anal. Chem. 2021, 40, 293–311. [Google Scholar] [CrossRef]
- Soltys, D.; Krasuska, U.; Bogatek, R.; Gniazdow, A. Allelochemicals as bio-herbicides -present and perspectives. In Herbicides—Currxent Research and Case Studies in Use; Price, A.J., Kelton, J.A., Eds.; InTech: Rijeka, Croatia, 2013; pp. 517–542. [Google Scholar]
- Cheng, F.; Cheng, Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar] [CrossRef]
- Hasan, M.; Ahmad-Hamdani, M.S.; Rosli, A.M.; Hamdan, H. Bioherbicides: An eco-friendly tool for sustainable weed management. Plants 2021, 10, 1212. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Salam, M.A.; Ohno, O.; Suenaga, K. Nimbolide B and nimbic acid B, phytotoxic substances in neem leaves with allelopathic activity. Molecules 2014, 19, 6929–6940. [Google Scholar] [CrossRef] [Green Version]
- Kyaw, E.H.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Allelopathy of the medicinal plant Dregea volubilis (L.f.) Benth. ex Hook.f. and its phytotoxic substances with allelopathic activity. Agronomy 2022, 12, 303. [Google Scholar] [CrossRef]
- Krumsri, R.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Assessment of allelopathic potential of Senna garrettiana leaves and identification of potent phytotoxic substances. Agronomy 2022, 12, 139. [Google Scholar] [CrossRef]
- Kumar, G.; Banu, G.S.; Murugesan, A.G.; Pandian, M.R. Effect of Helicteres isora bark extract on protein metabolism and marker enzymes in streptozotocin induced diabetic rats. Iran. J. Pharm. Sci. 2007, 6, 123–129. [Google Scholar]
- Cheng, G.L.; Kong, L.Y.; Zang, C. Progress in chemical and pharmacological studies on Marsdenia tenacissima. Pharm. Clin. Res. 2009, 17, 135–138. [Google Scholar]
- Kaur, A.; Batish, D.R.; Kaur, S.; Chauhan, B.S. An overview of the characteristics and potential of Calotropis procera from botanical, ecological, and economic perspectives. Front. Plant Sci. 2021, 12, 690806. [Google Scholar] [CrossRef] [PubMed]
- Moronkola, D.O.; Ogukwe, C.; Awokoya, K.N. Chemical compositions of leaf and stem essential oils of Calotropis procera Ait R.Br (Asclepiadaceae). Chem. Sin. 2011, 2, 255–260. [Google Scholar]
- Karale, P.A.; Karale, M.A. A review on phytochemistry and pharmacological properties of milkweed family herbs (Asclepiadaceae). Asian J. Pharm. Clin. Res. 2017, 10, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Bari, I.N.; Kato-Noguchi, H. Phytotoxic effects of Cerbera manghas L. leaf extracts on seedling elongation of four monocot and four dicot test species. Acta Agrobot. 2017, 70, 1–7. [Google Scholar] [CrossRef]
- Gulzar, A.; Siddiqui, M.B.; Bi, S. Phenolic acid allelochemicals induced morphological, ultrastructural, and cytological modification on Cassia sophera L. and Allium cepa L. Protoplasma 2016, 253, 1211–1221. [Google Scholar] [CrossRef]
- Krumsri, R.; Boonmee, S.; Kato-Noguchi, H. Evaluation of the allelopathic potential of leaf extracts from Dischidia imbricata (Blume) Steud. on the seedling growth of six test plants. Not. Bot. Horti Agrobot. 2019, 47, 1019–1024. [Google Scholar] [CrossRef] [Green Version]
- Al-Harbi, N.A. Allelopathic effect of Calotropis procera, Hyoscyamus muticus and Pulicaria undulata extracts on seed germination of Portulaca oleracea and Chenopodium murale. Pak. J. Biol. Sci. 2020, 23, 1260–1266. [Google Scholar]
- Yoganarasimhan, S.N. Medicinal Plants of India: Tamil Nadu; Cyber Media: Bangalore, India, 2000; Volume 2, p. 263. [Google Scholar]
- Wang, P.; Yang, J.; Zhu, Z.; Zhang, X. Marsdenia tenacissima: A review of traditional uses, phytochemistry and pharmacology. Am. J. Chin. Med. 2018, 46, 1449–1480. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.; Singh, A.; Tiwari, A. Phytopharmacological overview on controversial drug: Murva. Tradit. Folk. Herb. Med. Recent Res. 2018, 2, 475–526. [Google Scholar]
- Kumar, P.; Gairola, S. Ethno medicinal uses of roots of fourteen species of family Apocynaceae by indigenous communities of India. Res. Rev. Biotechnol. Biosci. 2020, 7, 104–119. [Google Scholar]
- Li, H.T.; Kang, L.P.; Guo, B.L.; Zhang, Z.L.; Guan, Y.H. Original plant identification of Dai nationality herb. China J. Chin. Mater. Med. 2014, 39, 1525–1529. [Google Scholar]
- Zhang, W.; Wang, Z.F.; Wang, J.; Yang, J.; Min, Y. Chemical constituents from Marsdenia tenacissima and their anti-tumor activities. Chin. Tradit. Patent Med. 2017, 39, 334–338. [Google Scholar]
- Tripathi, M.; Shivhare, D.; Tiwari, A.; Ahirwar, P.; Pathak, S. Pharmacognostical evaluation of Marsdenia tenacissima Wight. & Arn. Root. Int. J. Recent Biotechnol. 2014, 2, 18–23. [Google Scholar]
- Mondal, M.; Saha, S.; Hossain, M.; Foyjul, I.A.; Sarkar, C.; Hossain, M.S.; Khalipha, A.B.R.; Kundu, S.; Bimbima, K. Phytochemical profiling and evaluation of bioactivities of methanolic and ethyl acetate extracts of Marsdenia tenacissima leaves. J. Herbs Spices Med. Plants 2020, 26, 405–422. [Google Scholar] [CrossRef]
- Rob, M.M.; Iwasaki, A.; Suzuki, R.; Suenaga, K.; Kato-Noguchi, H. Garcienone, a novel compound involved in allelopathic activity of Garcinia Xanthochymus Hook. Plants 2019, 8, 301. [Google Scholar] [CrossRef] [Green Version]
- Boonmee, S.; Suwitchayanon, P.; Krumsri, R.; Kato-Noguchi, H. Investigation of the allelopathic potential of Nephrolepis cordifolia (L.) C. Presl against dicotyledonous and monocotyledonous plant species. Environ. Control Biol. 2020, 58, 71–78. [Google Scholar] [CrossRef]
- Hossen, K.; Ozaki, K.; Teruya, T.; Kato-Noguchi, H. Three active phytotoxic compounds from the leaves of Albizia richardiana (Voigt.) King and Prain for the development of bioherbicides to control weeds. Cells 2021, 10, 2385. [Google Scholar] [CrossRef]
- Kyaw, E.H.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic activity of Clerodendrum indicum (L.) Kuntze and its potential phytotoxic substance. Emir. J. Food Agric. 2021, 33, 884–892. [Google Scholar]
- Krumsri, R.; Ozaki, K.; Teruya, T.; Kato-Noguchi, H. Isolation and identification of two potent phytotoxic substances from Afzelia xylocarpa for controlling weeds. Appl. Sci. 2021, 11, 3542. [Google Scholar] [CrossRef]
- Huang, X.D.; Liu, T.; Wang, S. Two new polyoxypregnane glycosides from Marsdenia tenacissima. Helv. Chim. Acta 2009, 92, 2111. [Google Scholar] [CrossRef]
- Wang, S.; Lai, Y.H.; Tian, B.; Yang, L. Two new C21 steroidal glycosides from Marsdenia tenacissima (ROXB.) WIGHT et ARN. Chem. Pharm. Bull. 2006, 54, 696–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warashina, T.; Noro, T. Steroidal glycosides from the aerial part of Asclepias incarnata. Phytochemistry 2000, 53, 485–498. [Google Scholar] [CrossRef]
- Pawar, R.S.; Shukla, Y.J.; Khan, S.I.; Avula, B.; Khan, I.A. New oxypregnane glycosides from appetite suppressant herbal supplement Hoodia gordonii. Steroids 2007, 72, 524–534. [Google Scholar] [CrossRef]
- Liu, X.J.; Shi, Y.; Jia, S.H.; Deng, Y.L.; Lv, F.; Dai, R.J. Six new C-21 steroidal glycosides from Dregea sinensis Hemsl. J. Asian Nat. Prod. Res. 2017, 19, 745–753. [Google Scholar] [CrossRef]
- Araya, J.J.; Binns, F.; Kindscher, K.; Timmermann, B.N. Verticillosides A–M: Polyoxygenated pregnane glycosides from Asclepias verticillata L. Phytochemistry 2012, 78, 179–189. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, A.M.; Zhang, A.Y.; Chen, R.; Yang, S.B.; Huang, X. Five new C21 steroidal glycosides from the stems of Marsdenia tenacissima. Steroids 2010, 75, 176–183. [Google Scholar] [CrossRef]
- Yu, J.Q.; Zhao, L. Seco-pregnane steroidal glycosides from the roots of Cynanchum stauntonii. Phytochem. Lett. 2016, 16, 34–37. [Google Scholar] [CrossRef]
- Bailly, C. Anticancer properties of caudatin and related C-21 steroidal glycosides from Cynanchum plants. Steroids 2021, 172, 108855. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Shinoda, D.; Nakamaru, A.; Kamohara, K.; Sakagami, H.; Mimaki, Y. Steroidal glycosides from Convallaria majalis whole plants and their cytotoxic activity. Int. J. Mol. Sci. 2017, 18, 2358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, W.; Zhang, R.; Wang, J.; Ma, Y.; Li, W.; Jiang, R.W.; Cai, S. Asclepiasterol, a novel C21 steroidal glycoside derived from Asclepias curassavica, reverses tumor multidrug resistance by down-regulating P-glycoprotein expression. Oncotarget 2016, 7, 31466–31483. [Google Scholar] [CrossRef]
- Timité, G.; Mitaine-Offer, A.C.; Miyamoto, T.; Tanaka, C.; Mirjolet, J.F.; Duchamp, O.; Lacaille-Dubois, M.A. Structure and cytotoxicity of steroidal glycosides from Allium schoenoprasum. Phytochemistry 2012, 88, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Xu, D.W.; Li, R.T.; Wang, S.H.; Hu, Y.L.; Shi, S.Y.; Li, J.Y.; Huang, Y.H.; Kang, L.W.; Liu, T.X. A combined phytochemistry and network pharmacology approach to reveal potential anti-NSCLC effective substances and mechanisms in Marsdenia tenacissima (Roxb.) Moon (Stem). Front. Pharmacol. 2021, 12, 518406. [Google Scholar] [CrossRef] [PubMed]

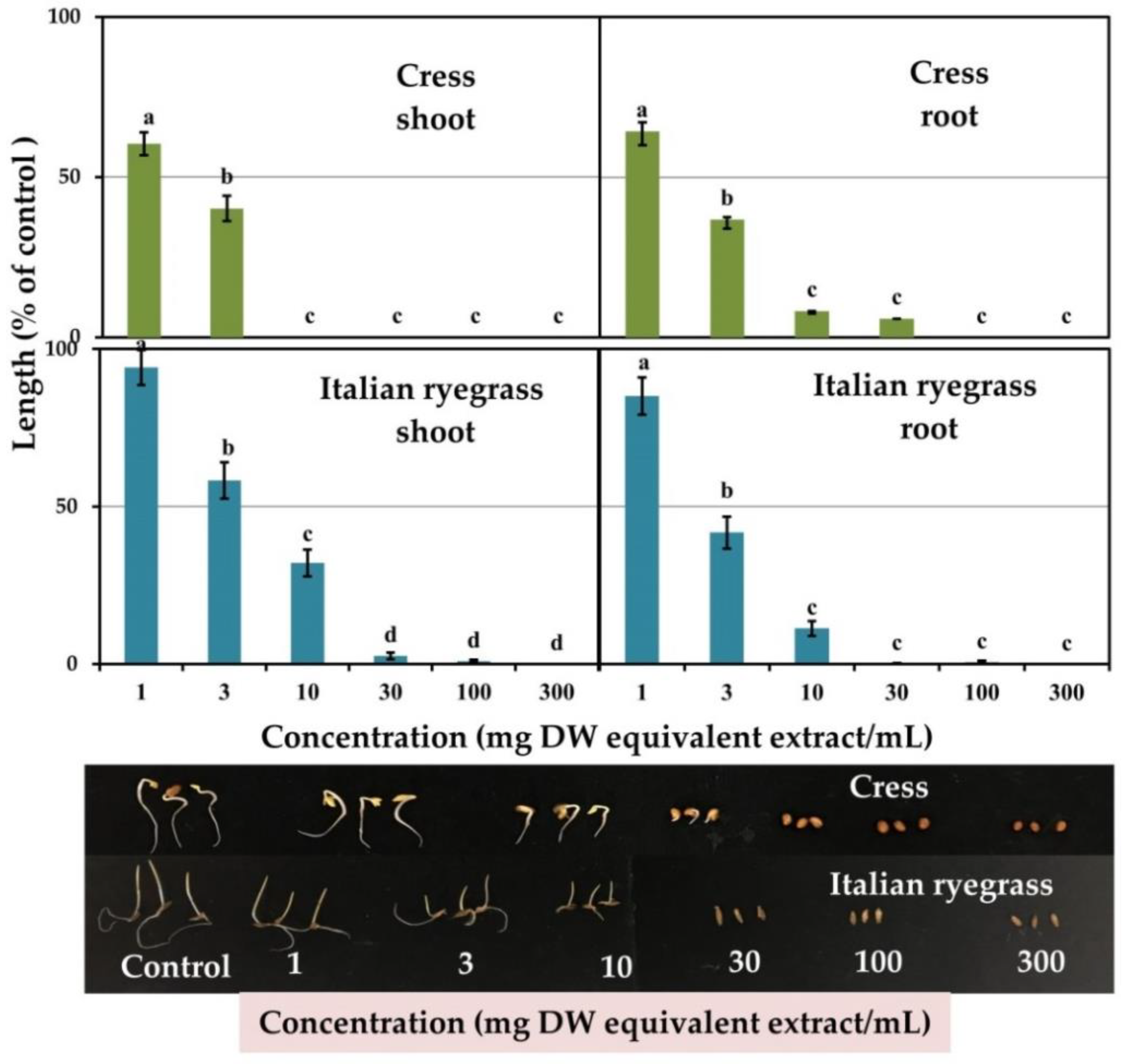
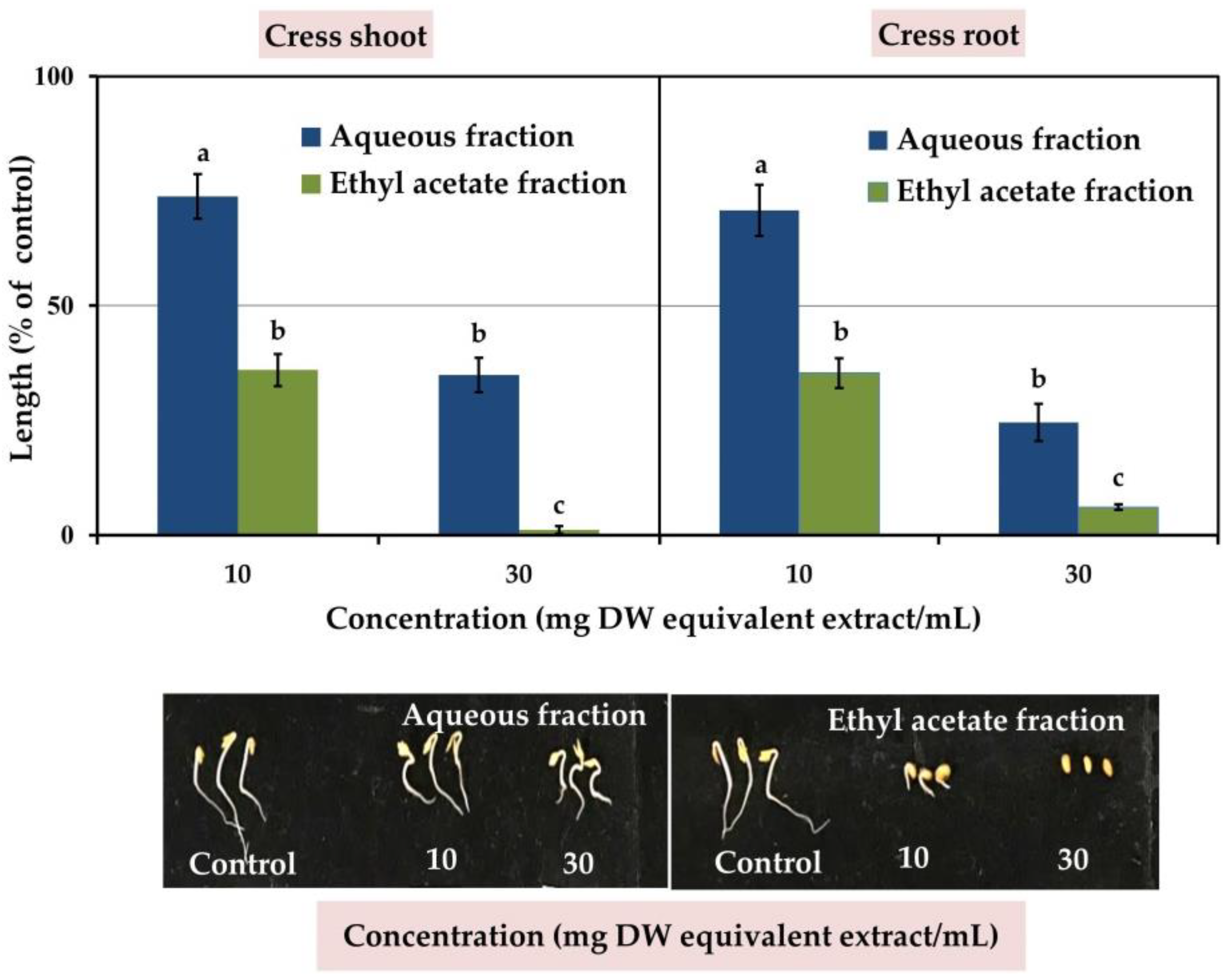
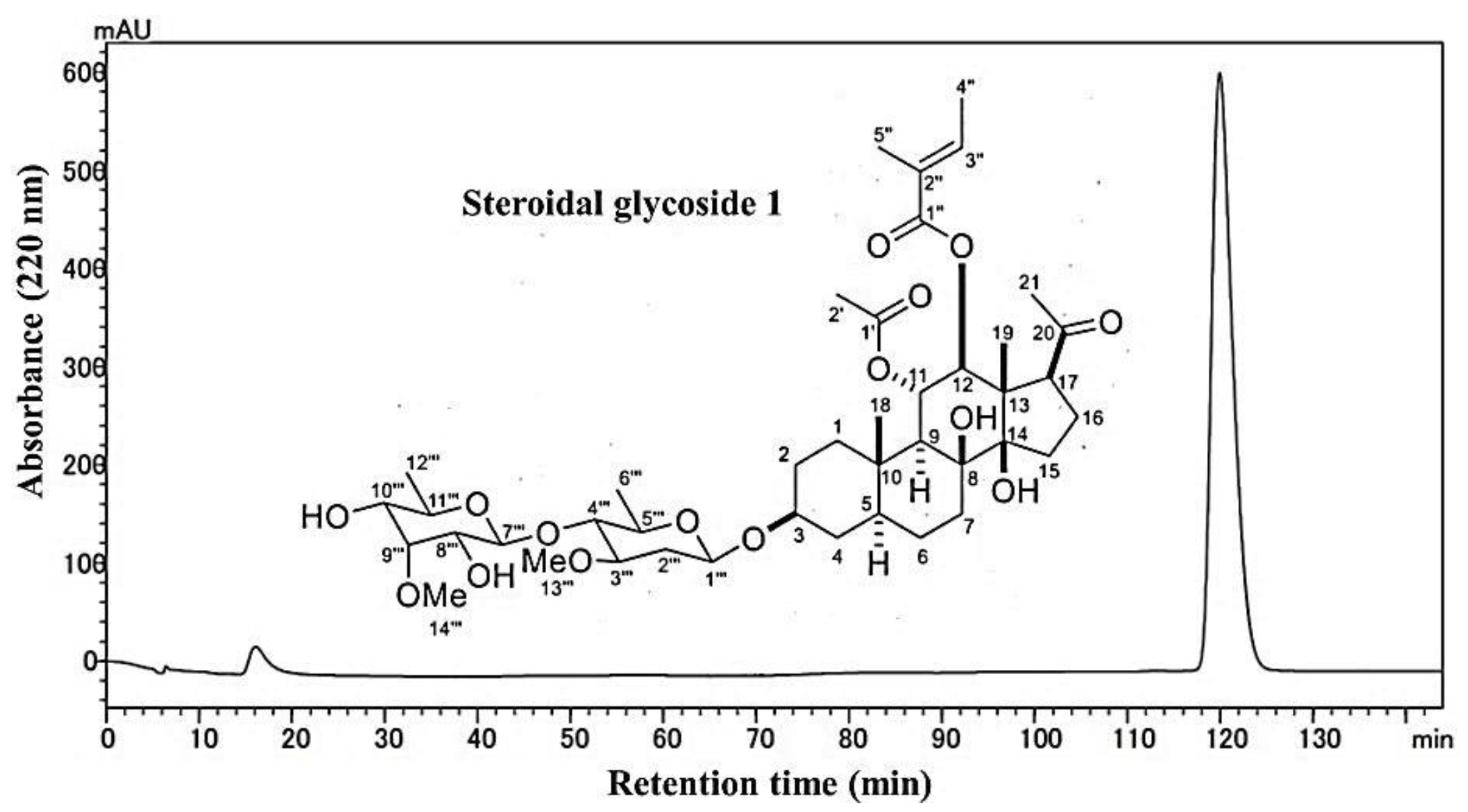
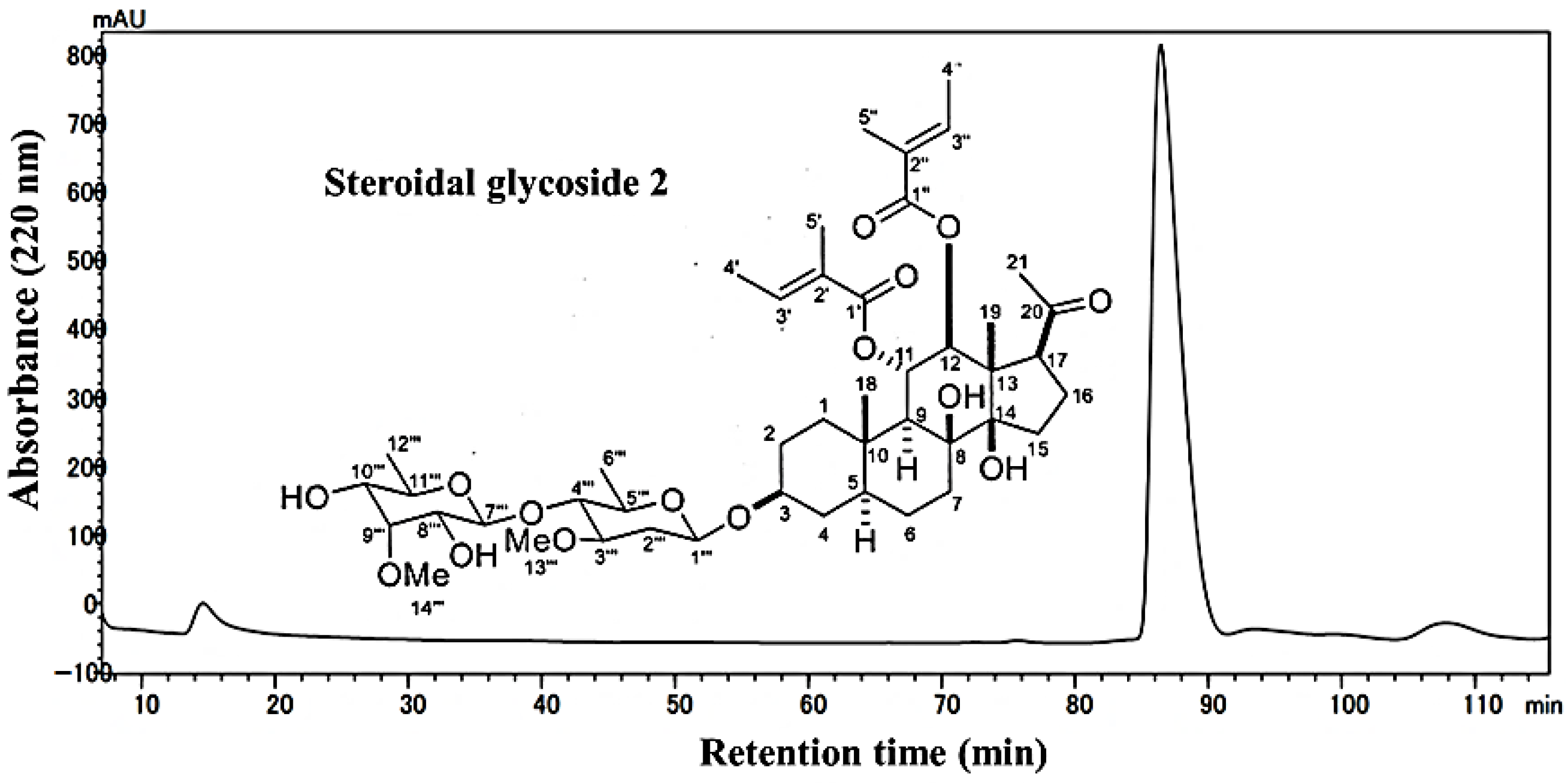
| Test Plant Species | I50 Value (mg DW Equivalent Extract/mL) | Correlation Coefficient (r) | ||
|---|---|---|---|---|
| Shoot | Root | Shoot | Root | |
| Cress | 1.5 c | 1.7 c | −0.80 *** | −0.81 *** |
| Italian ryegrass | 4.6 a | 2.6 b | −0.74 *** | −0.76 *** |
| Position | δC a | δH (J in Hz) b | Selected COZY | Selected HMBC |
|---|---|---|---|---|
| 1a | 40.4 | 1.20, m | 2a | |
| 1b | 1.14, m | 2b | ||
| 2a | 30.3 | 1.78, m | 1a, 1b, 3 | |
| 2b | 1.24, m | 3 | ||
| 3 | 78.1 | 3.64, m | 2a, 2b, 4a, 4b | |
| 4a | 35.7 | 1.67, m | 3, 5 | |
| 4b | 1.38, m | 3, 5 | ||
| 5 | 46.5 | 1.16, m | 4a, 4b, 6a | |
| 6a | 25.9 | 1.67, m | 5, 7a | |
| 6b | 1.12, m | 7b | ||
| 7a | 3.61 | 1.98, m | 6a | |
| 7b | 1.35, m | 6b | ||
| 8 | 85.2 | |||
| 9 | 51.3 | 1.65, m | 11 | |
| 10 | 38.9 | |||
| 11 | 72.5 | 5.82, t (10.8) | 9, 12 | |
| 12 | 79.4 | 4.95, d (10.8) | 11 | 1″ |
| 13 | 56.4 | |||
| 14 | 86.3 | |||
| 15a | 32.0 | 2.22, m | 16 | |
| 15b | 1.89, m | |||
| 16 | 25.4 | 2.04, m | 15a, 17 | |
| 17 | 60.1 | 2.95, dd (8.3, 6.0) | 16 | 20 |
| 18 | 13.3 | 1.06, s | 1, 5, 9, 10 | |
| 19 | 14.0 | 1.23, s | 12, 13, 14, 17 | |
| 20 | 216.7 | |||
| 21 | 32.0 | 2.13, s | 20 | |
| 1′ | 171.8 | |||
| 2′ | 21.7 | 1.83, s | ||
| 1″ | 169.3 | |||
| 2″ | 129.2 | |||
| 3″ | 140.4 | 6.93, m | 4″, 5″ | |
| 4″ | 14.6 | 1.83, brs | 3″ | 2″, 3″ |
| 5″ | 12.1 | 1.85, brs | 3″ | 1″, 2″ |
| 1‴ | 98.6 | 4.64, m | 2‴ a, 2‴b | 3 |
| 2‴a | 37.9 | 2.22, m | 1‴, 3‴ | |
| 2‴b | 1.34, m | 1‴, 3‴ | ||
| 3‴ | 80.6 | 3.37, m | 2‴ a, 2‴ b, 4 | |
| 4‴ | 84.0 | 3.17, m | 3‴, 5‴ | |
| 5‴ | 72.5 | 3.38, m | 4‴, 6‴ | |
| 6‴ | 18.8 | 1.35, d (6.1) | 5‴ | |
| 7‴ | 102.2 | 4.72, d (8.1) | 8‴ | 4‴ |
| 8‴ | 73.6 | 3.30, m | 7‴, 9‴ | |
| 9‴ | 84.0 | 3.62, m | 8‴, 10‴ | |
| 10‴ | 75.0 | 3.18, m | 9‴, 11‴ | |
| 11‴ | 71.2 | 3.65, m | 10‴, 12‴ | |
| 12‴ | 18.2 | 1.23, d (6.3) | 11‴ | |
| 13‴ | 57.4 | 3.40, s | 3‴ | |
| 14‴ | 62.5 | 3.60, s | 9‴ |
| Test Plant | I50 Value (mM) | Correlation Coefficient (r) | |||
|---|---|---|---|---|---|
| Compound 1 | Compound 2 | Compound 1 | Compound 2 | ||
| Cress | Shoot | 0.46 b | 0.74 a | −0.83 *** | −0.69 *** |
| Root | 0.03 c | 0.12 c | −0.82 *** | −0.79 *** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moh, S.M.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Allelopathic Activity of a Novel Compound, 5,6-Dihydrogen-11α-O-acetyl-12β-O-tigloyl-17β-marsdenin, and a Known Steroidal Glycoside from the Leaves of Marsdenia tenacissima (Roxb.) Moon. Agronomy 2022, 12, 1536. https://doi.org/10.3390/agronomy12071536
Moh SM, Iwasaki A, Suenaga K, Kato-Noguchi H. Allelopathic Activity of a Novel Compound, 5,6-Dihydrogen-11α-O-acetyl-12β-O-tigloyl-17β-marsdenin, and a Known Steroidal Glycoside from the Leaves of Marsdenia tenacissima (Roxb.) Moon. Agronomy. 2022; 12(7):1536. https://doi.org/10.3390/agronomy12071536
Chicago/Turabian StyleMoh, Seinn Moh, Arihiro Iwasaki, Kiyotake Suenaga, and Hisashi Kato-Noguchi. 2022. "Allelopathic Activity of a Novel Compound, 5,6-Dihydrogen-11α-O-acetyl-12β-O-tigloyl-17β-marsdenin, and a Known Steroidal Glycoside from the Leaves of Marsdenia tenacissima (Roxb.) Moon" Agronomy 12, no. 7: 1536. https://doi.org/10.3390/agronomy12071536
APA StyleMoh, S. M., Iwasaki, A., Suenaga, K., & Kato-Noguchi, H. (2022). Allelopathic Activity of a Novel Compound, 5,6-Dihydrogen-11α-O-acetyl-12β-O-tigloyl-17β-marsdenin, and a Known Steroidal Glycoside from the Leaves of Marsdenia tenacissima (Roxb.) Moon. Agronomy, 12(7), 1536. https://doi.org/10.3390/agronomy12071536






