1. Introduction
Proline has many different functions in the plant. It is an essential component of protein biosynthesis, plays a role in root elongation, flowering, and embryo development, and increases tolerance to abiotic and biotic stresses [
1,
2,
3,
4,
5,
6,
7,
8,
9]. Proline plays a significant role in the scavenging of hydroxyl radicals, osmotic regulation, interactions with stress tolerance enzymes, the protection of the protein structure, enzymatic and photosynthetic activities, the maintenance of the pH balance, and the supplementation of carbon, nitrogen, and energy [
1,
6,
7,
8,
10,
11,
12]. For this reason, research is currently being conducted on the use of exogenous proline in plant production [
8,
10].
Exogenous proline has been shown to increase plant tolerance to abiotic stresses [
2,
5,
13,
14,
15,
16,
17,
18,
19,
20]. It has also been noted that the use of proline may affect the quantity and quality of the yield, as well as the biometric features of plants [
21,
22,
23]. The positive effects of proline application were observed even in plants that were not exposed to stress factors [
13,
24,
25]. However, the time and the number of proline applications are important too [
2,
26,
27], and the effectiveness of the treatments may also depend on the plant species or even the cultivar [
2,
7,
24,
28].
There have been many studies on the use of proline, but little information is available about its effects on the yield of root vegetables [
29], especially in field conditions, and the influence of exogenous proline on the structure of marketable and non-marketable yields has not been investigated thus far.
Increasingly, bio-stimulants are used in crops, including those based on amino acids. One of the amino acids included in the composition of the bio-stimulants is proline. However, for a better understanding of the usefulness of such preparations in modern agriculture, it is necessary to understand the effects of their individual components. The aim of our study was to determine the effects of the time of the proline application on the quantity and structure of root parsley yields. It was assumed in the study that the growth stage of the parsley plants and the number of applications may affect the yield quantity and quality of the roots and leaves, as well as the content and composition of the essential oil. Two cultivars were also taken into account in order to assess the impact of plant genetic diversity on the effectiveness of this amino acid.
2. Materials and Methods
2.1. Description of the Station’s Location
The field experiment was conducted in the years 2016, 2018, and 2019 at the Felin research station (51°13′37″ N 22°37′58″ E, 214 m a.s.l.) of the University of Life Sciences in Lublin, located in central-eastern Poland.
2.2. Details of the Field Experiment
The experimental material comprised of two parsley cultivars (Petroselinum crispum (Mill.) Nyman ex A.W. Hill), ‘Halblange’ (PNOS Ożarów Mazowiecki Sp. z o.o., Poland) and ‘Sonata’ (PlantiCo, Hodowla i Nasiennictwo Ogrodnicze Zielonki Sp. z o.o., Poland). The cultivars were selected on the basis of previous research, as they differed significantly in their responses to the use of a preparation containing amino acids. The ‘Halblange’ is classified as an aromatic, very late-fertile cultivar of parsley. It is characterized by a long growing season and high storage stability. The ‘Sonata’ is a very fertile variety with large roots, classified as medium–late parsley.
The experiment had a two-factorial (treatment and cultivar, 7x2), randomized complete block design with four replications. The experimental factors were the parsley cultivars (‘Halblange’ and ‘Sonata’) and the time of the L-proline application (the stage of plant development was determined using the Biologische Bundesanstalt, Bundessortenamt i Chemical Industry, or BBCH, scale). The L-proline (C5H9NO2, molar mass 115.13 g mol−1, CAS number: 147-85-3, purity 99.0%, Hadron Scientific, Kielce, Poland) was sprayed (1000 mg L−1, 265 L ha−1 water) early in the morning at the scales of BBCH 15–16 (I: 5–6 leaf phase), BBCH 41 (II: roots start to widen, diameter >0.5 cm), and BBCH 42–43 (III: roots are 20–30% of the typical diameter), including I + II, II + III, and I + II + III. The control plants were sprayed with distilled water at the 5–6-leaf stage (BBCH 15-16).
The weather conditions during the study years are given in
Table 1.
The area of one plot was 2.025 m2 (0.675 m × 3.00 m). The seeds were sown at a rate of 1.5 kg·ha−1 in the last third of April (27 April 2016, 23 April 2018, and 25 April 2019) on a ridge (0.675 m apart, the 0.23 m high and 0.20 m wide on top ridges), with two rows on each ridge at a spacing of 0.12 m.
The field experiment was carried out on grey-brown podzolic soil (1.8% of organic matter, pH in KCl 6.7) developed from loess parent materials. The soil texture was loam with 39.0 % silt, 35.2 % sand, and 25.8 % clay.
Based on the soil chemical analysis, the content of the nutrients in the soil was supplemented to the level of 120 mg N·dm−3 (urea fertilizer), 80 mg P·dm−3 (triple superphosphate), and 250 mg K·dm−3 (potassium sulfate). The agricultural practices as usually recommended for parsley production were applied.
2.3. Data Collection
The parsley was harvested in the second half of October. After harvest, the roots were divided according to their diameter into the following groups: <20, 21–30, 31–40, 41–50, 51–60, 61–70, and >70 mm. They were further categorized into the following conditions: bifurcated, split, rotted, and damaged by pests. The bifurcated, split, rotted and pest-damaged < 20 mm and >70 mm roots were classified among the non-marketable yield, while the straight, healthy roots of diameters of 20–70 mm were included in the marketable yield. The average root weight was calculated for all plants, and the average root length was calculated for 40 randomly selected plants.
2.4. Essential Oil Isolation and Analysis
For this analysis, we used roots that were fully developed, healthy, and without discoloration and damage caused by pests. In order to obtain the essential oil from the roots of a given variety of parsley, samples weighing 250 g were prepared, which were then hydrodistilled for 3 h. Samples were stored in the dark and at less than 4 °C. The analysis of the composition of the essential oils was determined by GC/MS. The essential oils were analyzed by gas chromatography using a Varian 4000 GC/MS/MS instrument. Helium with a flow rate of 0.5 mL min−1 was used as the carrier gas. Automatic dosing using a sample division (1 µL) with a division ratio of 1: 100 was used in the analysis. The analysis was performed using a VF-5ms column, and a temperature increase was applied during the analysis, where the starting temperature was 50 °C, which was maintained for 1 min, then increased by 4 °C per min−1 up to 250 °C. The compounds were detected with a Vatran 4000 MS/MS detector. The mass spectrometer worked in the mass scanning range of 40–1000 m z−1, while the scanning speed was 0.8 s scan−1.
The retention indices (Kovats’a) were calculated using series of n-alkanes, C6–C40. The qualitative analysis was based on MS spectrums, which were compared with the spectrums of the NIST library (62,000 spectrums) and LIBR terpene library (TR), provided by the Finnigan MAT company. The identification of the compounds was confirmed by the literature data. The essential oil components are reported here as the relative percent of the total oil by the peaks area, with values of ≥0.01% being taken into account.
2.5. Statistical Analysis
The data obtained are presented as means and were statistically analyzed using ANOVA, according to a completely randomized design, and the averages were compared using HSD Tukey’s test at a 0.05 probability level. All statistical analyses were carried out using Statistica 10.0 PL software (StatSof Inc., Tulsa, OK, USA).
3. Results
The proline application time did not affect the mean number of parsley plants at harvest. However, compared to the control, the highest number of plants was observed after the use of this amino acid in the ‘Halblange’ variety, and a smaller number was observed in ‘Sonata’. In ‘Sonata’, this situation occurred even in 2019, when the plant density was low. Spraying with amino acids, in most of the times of application, increased the number of plants during harvest in ‘Halblange’ but decreased the number in ‘Sonata’. However, in the case of ‘Halblange’ in 2019, the II application of the proline did not increase the number of plants compared to the control. However, in the case of Sonata, the same application in 2018 increased the number of plants, as in 2019, in treatments II + III. The cultivar and weather conditions during plant growth influenced the number of plants at harvest (
Table 2).
Compared to the control, the spraying of the proline did not cause significant changes in the fresh leaf weight. On the other hand, the fresh leaf weight depended on the time of the proline application. It was the highest in the plants sprayed twice in treatment II + III and the lowest when the plants were sprayed only once at BBCH 15–16. It was noticed that proline decreased (apart from in treatment II + III) the fresh leaf weight of ‘Sonata’ (
Table 3).
The highest total yield of the roots was obtained from the plants sprayed at time II + III and the lowest yield was obtained when proline was applied once at BBCH 15–16. The use of this amino acid in the examined times did not significantly affect the total yield compared to the control. The time of the proline application influenced the yield of ‘Sonata’ but not ‘Halblange’ (
Table 4).
The marketable yield depended on the time of the proline application. On average, the highest marketable yield was harvested from the plants sprayed at time II + III, and a lower yield was observed in the plants subjected to spraying at BBCH 42-43. The response of the cultivars was different. In ‘Sonata’, the marketable yield decreased (except for time II + III), whereas in ‘Halblange’ it increased (except for III), but in the ‘Halblange’ the differences were statistically insignificant. In all the tested growth stages, the use of proline did not significantly affect the marketable yield compared to the control (
Table 5).
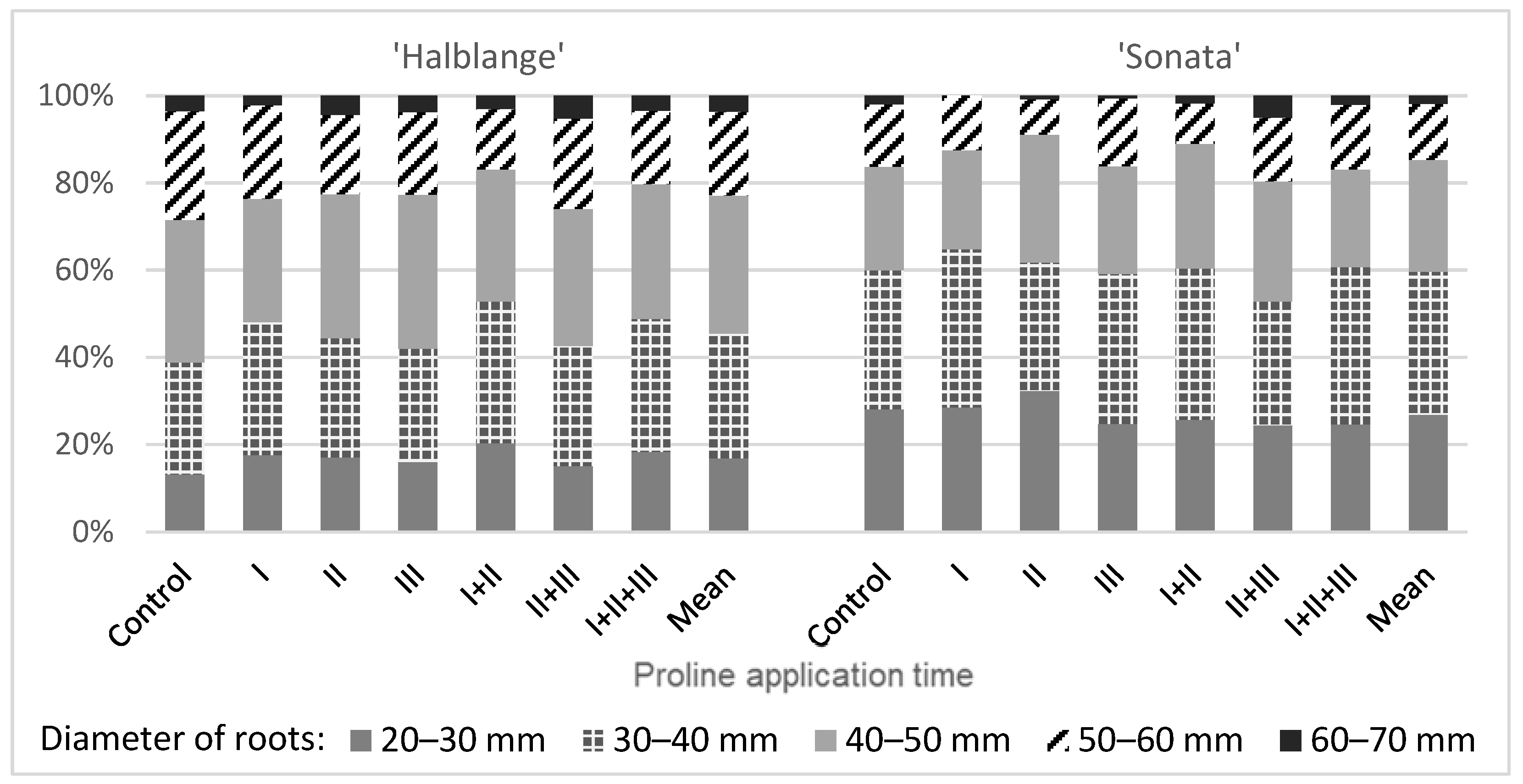
The use of proline changed the structure of the marketable yield. In the ‘Halblange’ plants, an increase in the share of the roots with diameters of 20–30 mm and 30–40 mm, and a decrease in the roots with a diameter of 50–60 mm, were observed. In ‘Sonata’, after using proline, the share of the roots with diameters of 30–40 and 40–50 mm most often increased, while the share of the roots with diameters of 20–30 and 50–60 mm decreased (
Figure 1).
The share of the forked roots ranged from 44.4% (‘Halblange’, treatment I + II) to 60.8% (‘Halblange’, treatment III) in the non-marketable yield. In both tested cultivars, depending on the time of the proline application, both an increase and a decrease in the share of the forked roots were observed, but the differences were greater in the ‘Halblange’ group (
Figure 2).
The use of proline had little effect on the share of the small roots (<20 mm) in the non-marketable yield of ‘Sonata’, which was characterized by a high share of such roots. In ‘Halblange’, the use of proline increased the share of the small roots, especially when application was carried out at BBCH 41 (
Figure 2).
A single application of proline at BBCH 15–16, and especially at BBCH 41, resulted in a reduction in the share of the split roots, but after the use of this amino acid at BBCH 42–43, a higher share of split roots was observed compared to the earlier application times (in ‘Sonata’ and also in comparison to the control). The high share of split roots in both cultivars was observed in plants sprayed twice at time I + II, while in plants sprayed twice, at time II + III, and three times (I + II + III), the share of the split roots was lower than in the control (
Figure 2 and
Figure 3a).
In both cultivars, an increase in the share of the roots damaged by pests, compared to the control, was observed after the application of proline at time I, and a decrease was observed at times II, III, and I + II. In the plants sprayed at times II + III and I + II + III, the share of the roots damaged by the pests was similar (‘Halblange’) or greater (‘Sonata’) as compared to the control (
Figure 2 and
Figure 3b).
Very large roots (>70 mm in diameter) were found only in the ‘Halblange’ yield, and the use of proline contributed to a reduction in the share of such roots, excepting the application at times II + III (
Figure 2).
The share of rotted roots in the non-marketable yield after the application of proline was most often lower than that in the control plants (4.1%), and it was larger only in the ‘Halblange’ plants sprayed with proline at times III (5.6%) and II + III (5.4%). The share of rotted roots was greater in ‘Halblange’ compared to ‘Sonata’ (
Figure 2).
The exogenous proline did not affect the root length of the parsley, but this depended on the growing season. ‘Sonata’ plants had longer storage roots with a higher mean weight than the ‘Halblange’ group (
Table 6).
The foliar application of proline did not affect the mean root weight compared to the control, but differences between the times of the proline application were observed. The storage roots of the plants sprayed twice at time II + III had the highest weight, whereas those sprayed at time I + II had the smallest weight (
Table 6).
A one-time spraying of the parsley plants with proline did not change the content of the essential oil in the roots, while a two- or three-time application significantly reduced its content. The use of proline also changed the composition of the essential oil in the parsley roots. Spraying with proline increased the share of apiol as the number of applications increased. A similar relationship was observed in the case of β-pinene and β-phellandrene, and the opposite scenario was observed in the case of myristicin. A single application of proline had little effect on the content of elemycin and z-ligustilide, while the two- or three-time application reduced the contents of these compounds (
Table 7).
4. Discussion
The optimal plant density is one of the basic factors determining crop productivity. Stawiarz and Gruszecki [
30] found that the use of bio-stimulants containing amino acids could have a positive effect on the number of parsley plants yielded, but they also noted differences between cultivars. The results of the present study indicate that different reactions of cultivars may also occur after the use of a single amino acid: proline. Different cultivar reactions may result from their different accumulations of the proline [
29,
31,
32,
33], while in some cultivars, additional amounts of this amino acid may have a toxic effect [
5,
8,
10,
34].
El-Sherbeny and da Silva [
35] found that proline increases the fresh weight of red beet leaves, but the effect depends on the level of this amino acid. In the present experiment, exogenous proline did not affect the leaf weight of the parsley compared to the control, but the time of application did. The highest leaf weight was obtained when proline was applied twice, in stages BBCH 41 and BBCH 42–43. The cultivars responded to proline to different extents, and the mean leaf weight increased in ‘Halblange’ and decreased in ‘Sonata’.
Proline application often increases the yields of crops [
21,
23,
27,
28,
36,
37,
38,
39,
40,
41]. However, there are also reports of the absence of such an effect or its variation with the level of proline [
21,
42]. In this experiment, the application of proline did not affect the total and marketable yield of the parsley roots compared to the control, but the time of application did affect the yield. The highest total and marketable yields were gathered from the plants sprayed twice, in the stages BBCH 41 and BBCH 42–43. Dhar et al. [
27] also obtained the highest yield after using proline twice at the seedling and vegetative stages. However, based on results from a preliminary pot trial, Abdelhamid et al. [
26] considered a triple proline application as the most promising method. In the present experiment, increasing the number of applications to three resulted in lower yields. In most of the investigated treatments, the use of proline decreased the total root yield in ‘Sonata’ and increased it in ‘Halblange’, which indicates the different effects of exogenous proline on the studied cultivars of parsley. Other authors also reported differences in the yields of cultivars resulting from application of proline [
28,
29,
39,
43,
44].
The spraying of parsley plants with proline caused changes in the structure of the marketable yield, contributing to an increase in the share of the storage roots with a diameter of 20–40 mm in ‘Halblange’ and roots with a diameter of 30–50 mm in ‘Sonata’. Changes to the yield structure in onion after applications of proline were also reported by Semida et al. [
41].
Gouda et al. [
38] reported a decrease of the non-marketable yield after proline application. This was finding observed in this experiment only for ‘Halblange’ and not for ‘Sonata’.
The use of proline did not affect the share of the forked roots, but it increased the share of the small roots (<20 mm) in ‘Halblange’, probably as a result of the higher plant density. Spraying with proline reduced the share of rotted and large roots (>70 mm in diameter). As regards the share of the split and pest-damaged roots, depending on the time of proline application, both an increase and a decrease were observed. However, Haglund [
45] showed that spraying plants with proline stimulated insect feeding.
Exogenous proline may influence plant biometric features [
21,
22,
27,
37,
38,
39,
46]. Qirat et al. [
29] showed that proline can increase the length and weight of carrot roots. In the present study, the use of proline did not affect the length and weight of parsley roots, but the mean root weight depended on the time of the proline application. El-Sherbeny and da Silva [
35] reported that proline increases the fresh weight and length of red beet roots, but this effect depends on the level of the proline.
Many studies have shown that the use of proline contributes to an increase in the content of essential oils [
44,
47,
48]. In the case of parsley, a single application of this amino acid did not change the content of the essential oils in the roots, but a two- or three-time application reduced the content. Spraying with proline also caused changes in the composition of the essential oil.
5. Conclusions
The time of the application may affect the effectiveness of proline and be more important than the number of sprays administered. Proline spraying, when properly selected according to the plant growth stage, can increase the quantity and quality of the crop, improve the uniformity of the marketable yield, and reduce the susceptibility of the roots to split and damage by pests. The use of proline can reduce the content of essential oils and change their composition. The obtained results indicate that it is also important to determine the mechanism of action of proline in plant growth and development, but this requires further research. In the present study, two cultivars of parsley, which were fertile and very fertile, and which differed in their response to the use of proline, were used. In root parsley, the most favorable effect was observed after the applications of proline in two stages, BBCH 41 and BBCH 42–43. However, it is very important to take the cultivar into account, because this largely determines the effectiveness of this amino acid. However, this aspect requires further research.