Untargeted Metabolomics to Explore the Bacteria Exo-Metabolome Related to Plant Biostimulants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Solid Bacterial Culture Concentrate
2.3. Sample Preparation
2.4. Agronomic Field Experiment to Evaluate the Biostimulant Activity of Different Bacteria
2.4.1. Soil
2.4.2. Plant
2.4.3. Statistical Study for the Agronomic Experiment
2.5. UPLC-ESI-QTOF-MS Analysis
2.6. Metabolomics Data Treatment
3. Results
3.1. Biostimulant Effect of Different Bacteria on Lettuce Yield
3.2. Multivariate Study Results
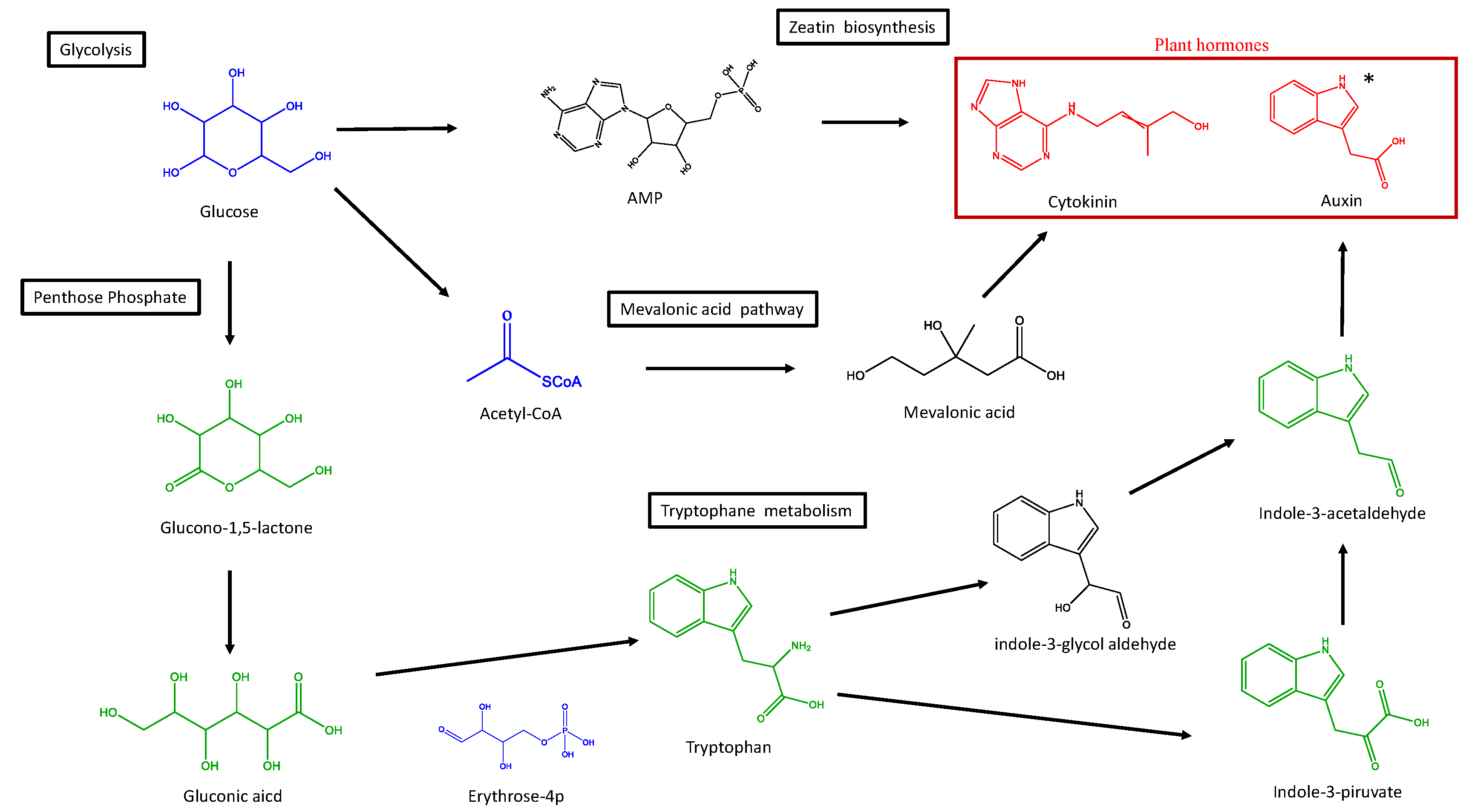
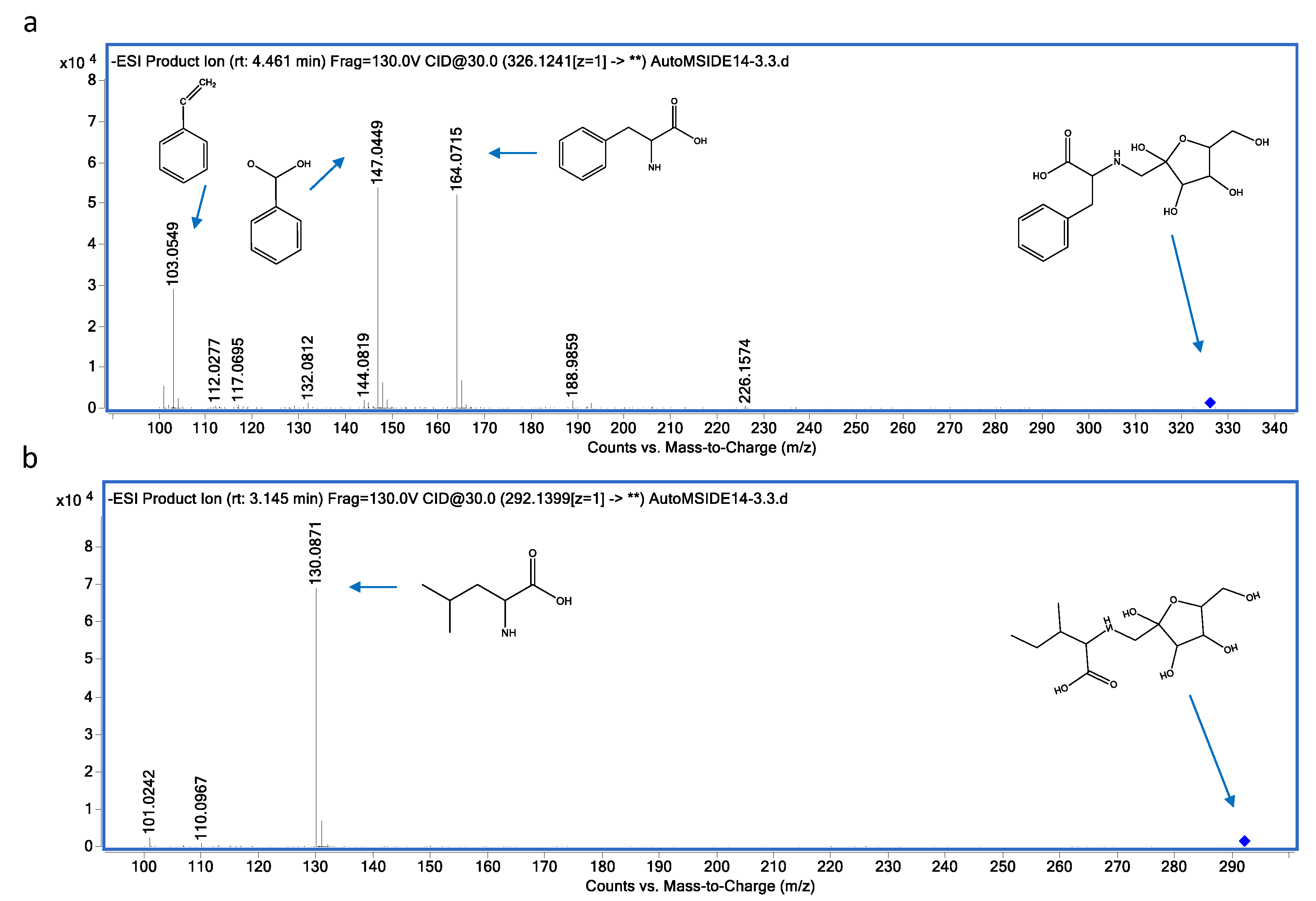
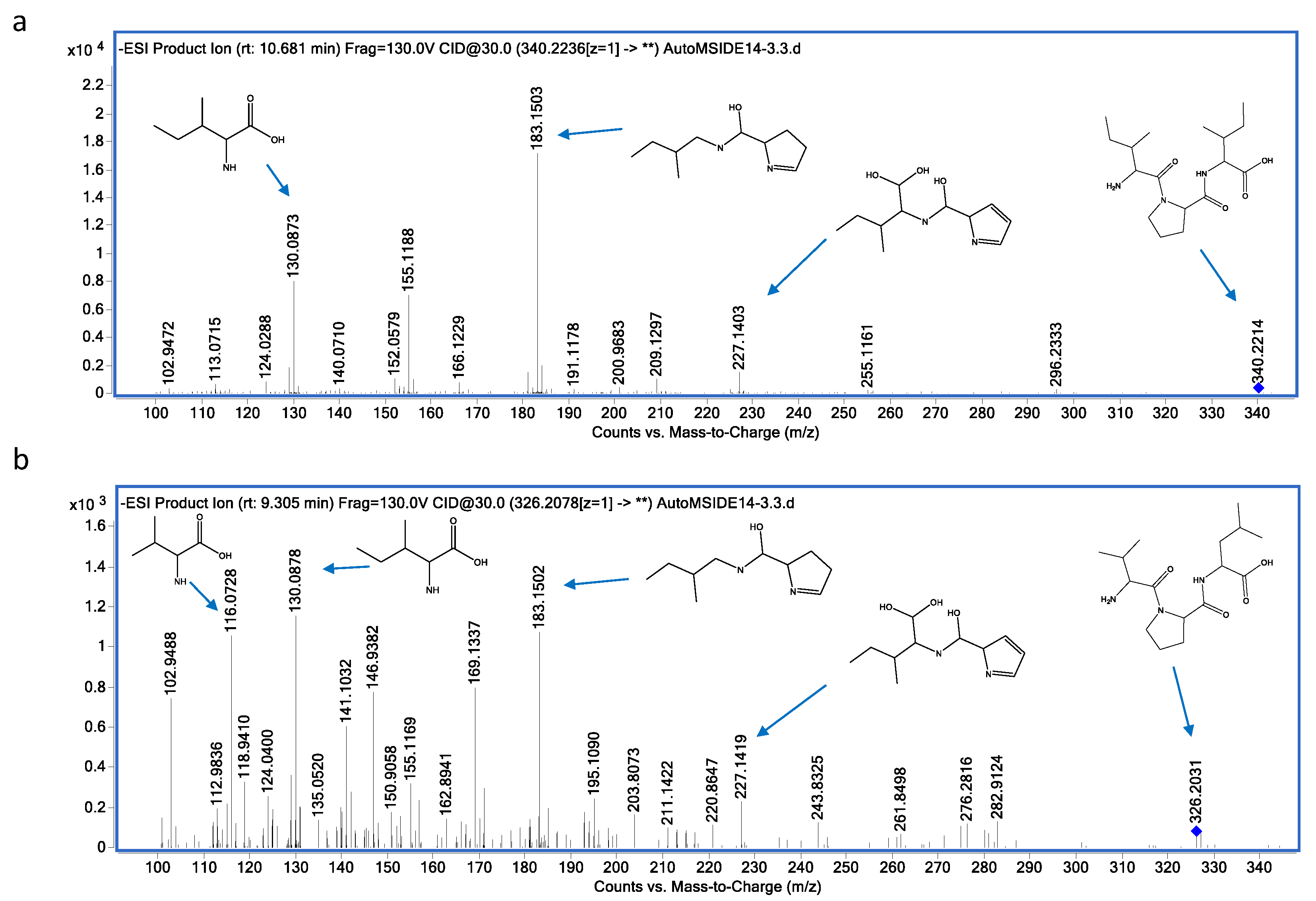
3.3. Metabolite Identification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed Extracts as Biostimulants of Plant Growth and Development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Ronga, D.; Biazzi, E.; Parati, K.; Carminati, D.; Carminati, E.; Tava, A. Microalgal Biostimulants and Biofertilisers in Crop Productions. Agronomy 2019, 9, 192. [Google Scholar] [CrossRef]
- Sible, C.; Seebauer, J.; Below, F. Plant Biostimulants: A Categorical Review, Their Implications for Row Crop Production, and Relation to Soil Health Indicators. Agronomy 2021, 11, 1297. [Google Scholar] [CrossRef]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. Chapter Two—The Use of Biostimulants for Enhancing Nutrient Uptake. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 130, pp. 141–174. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants Application in Horticultural Crops under Abiotic Stress Conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]
- Andreotti, C. Management of Abiotic Stress in Horticultural Crops: Spotlight on Biostimulants. Agronomy 2020, 10, 1514. [Google Scholar] [CrossRef]
- EU. Regulation of the European Parliament and of the Council Laying down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003. 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ:L:2019:170:TOC (accessed on 25 July 2022).
- Rouphael, Y.; Colla, G. Editorial: Biostimulants in Agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant Action of Protein Hydrolysates: Unraveling Their Effects on Plant Physiology and Microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef]
- Ashour, M.; Hassan, S.; Elshobary, M.; Ammar, G.; Gaber, A.; Alsanie, W.; Mansour, A.; El-Shenody, R. Impact of Commercial Seaweed Liquid Extract (TAM®) Biostimulant and Its Bioactive Molecules on Growth and Antioxidant Activities of Hot Pepper (Capsicum annuum). Plants 2021, 10, 1045. [Google Scholar] [CrossRef]
- Hassan, S.; Ashour, M.; Soliman, A.; Hassanien, H.; Alsanie, W.; Gaber, A.; Elshobary, M. The Potential of a New Commercial Seaweed Extract in Stimulating Morpho-Agronomic and Bioactive Properties of Eruca vesicaria (L.) Cav. Sustainability 2021, 13, 4485. [Google Scholar] [CrossRef]
- Ashour, M.; El-Shafei, A.A.; Khairy, H.M.; Abd-Elkader, D.Y.; Mattar, M.A.; Alataway, A.; Hassan, S.M. Effect of Pterocladia capillacea Seaweed Extracts on Growth Parameters and Biochemical Constituents of Jew’s Mallow. Agronomy 2020, 10, 420. [Google Scholar] [CrossRef]
- Spaepen, S. Plant hormones produced by microbes. In Principles of Plant-Microbe Interaction; Lugthenberg, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Davies, P.J. Plant Hormones: Biosynthesis, Signal Transduction, Action; Springer: Berlin, Germany, 2010. [Google Scholar]
- He, Y.; Pantigoso, H.A.; Wu, Z.; Vivanco, J.; Zhansheng, W. Co-inoculation of Bacillus sp. and Pseudomonas putida at different development stages acts as a biostimulant to promote growth, yield and nutrient uptake of tomato. J. Appl. Microbiol. 2019, 127, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Novello, G.; Cesaro, P.; Bona, E.; Massa, N.; Gosetti, F.; Scarafoni, A.; Todeschini, V.; Berta, G.; Lingua, G.; Gamalero, E. The Effects of Plant Growth-Promoting Bacteria with Biostimulant Features on the Growth of a Local Onion Cultivar and a Commercial Zucchini Variety. Agronomy 2021, 11, 888. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Oliveira, C.E.D.S.; Zoz, T.; Vendruscolo, E.; Andrade, A.D.F.; Seron, C.D.C.; Witt, T. Does Azospirillum brasilense and biostimulant improve the initial growth of rice sown at greater depths? J. Crop Sci. Biotechnol. 2020, 23, 461–468. [Google Scholar] [CrossRef]
- Ritter, G.; Villa, F.; da Silva, D.F.; Alberton, O.; Menegusso, F.J.; Eberling, T.; Dória, J. Microbiological biostimulant promotes rooting of olive cuttings. Int. J. Agric. Biol. Eng. 2021, 14, 207–212. [Google Scholar] [CrossRef]
- Fasciglione, G.; Casanovas, E.M.; Quillehauquy, V.; Yommi, A.K.; Goñi, M.G.; Roura, S.I.; Barassi, C.A. Azospirillum inoculation effects on growth, product quality and storage life of lettuce plants grown under salt stress. Sci. Hortic. 2015, 195, 154–162. [Google Scholar] [CrossRef]
- Del Amor, F.M.; Cuadra-Crespo, P. Plant growth-promoting bacteria as a tool to improve salinity tolerance in sweet pepper. Funct. Plant Biol. 2012, 39, 82–90. [Google Scholar] [CrossRef]
- Nguyen, M.L.; Spaepen, S.; Du Jardin, P.; Delaplace, P. Biostimulant effects of rhizobacteria on wheat growth and nutrient uptake depend on nitrogen application and plant development. Arch. Agron. Soil Sci. 2018, 65, 58–73. [Google Scholar] [CrossRef]
- Płaza, A.; Rzążewska, E.; Gąsiorowska, B. Effect of Bacillus megaterium var. phosphaticum Bacteria and L-Alpha Proline Amino Acid on Iron Content in Soil and Triticum aestivum L. Plants in Sustainable Agriculture System. Agronomy 2021, 11, 511. [Google Scholar] [CrossRef]
- Nascimento, F.X.; Hernández, A.G.; Glick, B.R.; Rossi, M.J. Plant growth-promoting activities and genomic analysis of the stress-resistant Bacillus megaterium STB1, a bacterium of agricultural and biotechnological interest. Biotechnol. Rep. 2019, 25, e00406. [Google Scholar] [CrossRef] [PubMed]
- Paul, K.; Sorrentino, M.; Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Miras Moreno, M.B.; Reynaud, H.; Canaguier, R.; Trtílek, M.; et al. A Combined Phenotypic and Metabolomic Approach for Elucidating the Biostimulant Action of a Plant-Derived Protein Hydrolysate on Tomato Grown Under Limited Water Availability. Front. Plant Sci. 2019, 10, 493. [Google Scholar] [CrossRef] [PubMed]
- Tugizimana, F.; Djami-Tchatchou, A.T.; Steenkamp, P.A.; Piater, L.A.; Dubery, I.A. Metabolomic Analysis of Defense-Related Reprogramming in Sorghum bicolor in Response to Colletotrichum sublineolum Infection Reveals a Functional Metabolic Web of Phenylpropanoid and Flavonoid Pathways. Front. Plant Sci. 2019, 9, 1840. [Google Scholar] [CrossRef] [PubMed]
- Nephali, L.; Piater, L.A.; Dubery, I.A.; Patterson, V.; Huyser, J.; Burgess, K.; Tugizimana, F. Biostimulants for Plant Growth and Mitigation of Abiotic Stresses: A Metabolomics Perspective. Metabolites 2020, 10, 505. [Google Scholar] [CrossRef]
- Barrajón-Catalán, E.; Álvarez-Martínez, F.J.; Borrás, F.; Pérez, D.; Herrero, N.; Ruiz, J.J.; Micol, V. Metabolomic analysis of the effects of a commercial complex biostimulant on pepper crops. Food Chem. 2019, 310, 125818. [Google Scholar] [CrossRef]
- van den Berg, R.A.; Hoefsloot, H.C.J.; Westerhuis, J.A.; Smilde, A.K.; Van Der Werf, M.J. Centering, scaling, and transformations: Improving the biological information content of metabolomics data. BMC Genomic. 2006, 7, 142. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis. Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Alqarawi, A.; Abd_Allah, E.; Hashem, A. Phytohormones and Beneficial Microbes: Essential Components for Plants to Balance Stress and Fitness. Front. Microbiol. 2017, 8, 2104. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Behrendt, U.; Ahmad, P.; Berg, G. Antimicrobial Activity of Medicinal Plants Correlates with the Proportion of Antagonistic Endophytes. Front. Microbiol. 2017, 8, 199. [Google Scholar] [CrossRef]
- Asgher, M.; Khan, M.I.R.; Anjum, N.A.; Khan, N.A. Minimising toxicity of cadmium in plants—role of plant growth regulators. Protoplasma 2014, 252, 399–413. [Google Scholar] [CrossRef]
- Spaepen, S.; Vanderleyden, J. Auxin and Plant-Microbe Interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, a001438. [Google Scholar] [CrossRef] [PubMed]
- Merino, E.; Jensen, R.A.; Yanofsky, C. Evolution of bacterial trp operons and their regulation. Curr. Opin. Microbiol. 2008, 11, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Baca, B.E.; Soto-Urzua, L.; Xochinua-Corona, Y.G.; CuervoGarcia, A. Charaterization of two aromatic amino acid aminotransferases and production of indoleacetic acid in Azospirillum strains. Soil. Biol. Biochem. 1994, 26, 57–63. [Google Scholar] [CrossRef]
- Zakharova, E.A.; Shcherbakov, A.A.; Brudnik, V.V.; Skripko, N.G.; Bulkhin, N.S.; Ignatov, V.V. Biosynthesis of indole-3-acetic acid in Azospirillum brasilense. JBIC J. Biol. Inorg. Chem. 2001, 259, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Patten, C.L.; Glick, B.R. Role of Pseudomonas putida Indoleacetic Acid in Development of the Host Plant Root System. Appl. Environ. Microbiol. 2002, 68, 3795–3801. [Google Scholar] [CrossRef]
- Garcia, C.; Hernandez, T.; Costa, F. Potential use of dehydrogenase activity as an index of microbial activity in degraded soils. Commun. Soil Sci. Plant Anal. 1997, 28, 123–134. [Google Scholar] [CrossRef]
- Schmülling, T. New Insights into the Functions of Cytokinins in Plant Development. J. Plant Growth Regul. 2002, 21, 40–49. [Google Scholar] [CrossRef]
- Kieber, J.J.; Schaller, G.E. Cytokinin signaling in plant development. Development 2018, 145, dev149344. [Google Scholar] [CrossRef]
- Gibb, M.; Kisiala, A.B.; Morrison, E.N.; Emery, R.J.N. The Origins and Roles of Methylthiolated Cytokinins: Evidence From Among Life Kingdoms. Front. Cell Dev. Biol. 2020, 8, 605672. [Google Scholar] [CrossRef]
- Spichal, L. Cytokinins—Recent news and views of evolutionally old molecules. Funct. Plant Biol. 2012, 39, 267–284. [Google Scholar] [CrossRef]
- Mayaka, J.B.; Huang, Q.; Xiao, Y.; Zhong, Q.; Ni, J.; Shen, Y. The Lonely Guy (LOG) Homologue SiRe_0427 from the Thermophilic Archaeon Sulfolobus islandicus REY15A Is a Phosphoribohydrolase Representing a Novel Group. Appl. Environ. Microbiol. 2019, 85, e01739-19. [Google Scholar] [CrossRef] [PubMed]
- Naz, I.; Bano, A.; Ul-Hassan, T. Isolation of phytohormones producing plant growth promoting rhizobacteria from weeds growing in Khewra salt range, Pakistan and their implication in providing salt tolerance to Glycine max L. Afr. J. Biotechnol. 2009, 8, 5762–5768. [Google Scholar]
- Havaux, M.; Ksas, B.; Szewczyk, A.; Rumeau, D.; Franck, F.; Caffarri, S.; Triantaphylidès, C. Vitamin B6 deficient plants display increased sensitivity to high light and photo-oxidative stress. BMC Plant Biol. 2009, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Ferri, S.; Kim, S.; Tsugawa, W.; Sode, K. Review of Fructosyl Amino Acid Oxidase Engineering Research: A Glimpse into the Future of Hemoglobin A1c Biosensing. J. Diabetes Sci. Technol. 2009, 3, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Pischetsrieder, M.; Monnier, V.M. Isolation, Purification, and Characterization of Amadoriase Isoenzymes (Fructosyl Amine-oxygen Oxidoreductase EC 1.5.3) from Aspergillus sp. J. Biol. Chem. 1997, 272, 3437–3443. [Google Scholar] [CrossRef]
- Deppe, V.M.; Bongaerts, J.; O’Connell, T.; Maurer, K.-H.; Meinhardt, F. Enzymatic deglycation of Amadori products in bacteria: Mechanisms, occurrence and physiological functions. Appl. Microbiol. Biotechnol. 2011, 90, 399–406. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, D.; Liu, Q. Connections Between Amino Acid Metabolisms in Plants: Lysine as an Example. Front. Plant Sci. 2020, 11, 928. [Google Scholar] [CrossRef]
- Umezawa, H.; Aoyagi, T.; Ogawa, K.; Naganawa, H.; Hamada, M.; Takeuchi, T. Diprotins A and B, inhibitors of dipeptidyl aminopeptidase IV, produced by bacteria. J. Antibiot. 1984, 37, 422–425. [Google Scholar] [CrossRef]
- Turdu, G.; Gao, H.; Jiang, Y.; Kabas, M. Plant dipeptidyl peptidase-IV inhibitors as antidiabetic agents: A brief review. Futur. Med. Chem. 2018, 10, 1229–1239. [Google Scholar] [CrossRef]
- Tavormina, P.; De Coninck, B.; Nikonorova, N.; De Smet, I.; Cammue, B.P. The Plant Peptidome: An Expanding Repertoire of Structural Features and Biological Functions. Plant Cell 2015, 27, 2095–2118. [Google Scholar] [CrossRef]


| Parameter | Unit | Result |
|---|---|---|
| Sand | % | 13.3 |
| Loam | % | 62.5 |
| Clay | % | 24.2 |
| Texture | (U.S.D.A.) | Clay loam |
| pH | Extract 1:2.5 (soil/wáter) | 8.47 |
| EC | mmhos/cm | 0.29 |
| Available Na | meq/100 g | 0.63 |
| Available K | meq/100 g | 1.05 |
| Available Ca | meq/100 g | 13.74 |
| Available Mg | meq/100 g | 4.64 |
| Total carbonates | % | 58.00 |
| Nitrate | mg/Kg | 62.56 |
| Clhoride | meq/100 g | 0.24 |
| Sulphate | meq/100 g | 0.42 |
| Available P | mg/Kg | 83.80 |
| Available Fe | mg/Kg | 8.34 |
| Available Mn | mg/Kg | 3.88 |
| Available Cu | mg/Kg | 6.65 |
| Available Zn | mg/Kg | 1.93 |
| Total N | % | 0.143 |
| Total organic carbon | % | 1.326 |
| Relación C/N | - | 9.273 |
| Yield/Plant (Fresh Weight, g) | Yield/Plant (Dry Weight, g) | Root Dry Weight/Plant (g) | |
|---|---|---|---|
| Control | 486 | 21.5 | 4.3 |
| IDE-01 (Pseudomona putida) | 536 | 23.7 | 4.8 |
| IDE-06 (Azospirillum brasilense) | 515 | 21.9 | 4.5 |
| IDE-14 (Bacillus megaterium) | 542 | 24.8 | 5.0 |
| ID | m/z | Name | Formula | Rt | Specie | MS/MS Fragments |
|---|---|---|---|---|---|---|
| 1 | 195.0510 | Gluconic acid | C6H12O7 | 1.18 | (M-H)- | 129.0191; 101.0235; 195.0532 |
| 2 | 177.0405 | Glucono-1,5-lactone | C6H10O6 | 1.25 | (M-H)- | 159.0297; 59.0150 |
| 3 | 346.0580 | Adenosine5′-monophosphate | C10H14N5O7P | 3.08 | (M-H)- | N/D |
| 4 | 147.0654 | Mevalonic acid | C6H12O4 | 3.92 | (M-H)- | N/D |
| 5 | 205.0972 | Tryptophan | C11H12N2O2 | 6.6 | (M+H)+ | 146.0601; 143.0728; 118.0655 |
| 6 | 202.0560 | Indole-3-piruvate | C11H9NO3 | 2.85 | (M-H)- | 130.0077; 84.9999; 151.0884 |
| 7 | 160.0760 | Indole-3-acetaldehyde | C10H9NO | 6.7 | (M+H)+ | 130.0560; 160.0750; 118.0652 |
| 8 | 174.0561 | 3-Indoleglycolaldehyde | C10H9NO2 | 11.9 | (M-H)- | N/D |
| 9 | 182.0459 | 4-Pyridoxic acid | C8H9NO4 | 5.54 | (M-H)- | 80.0502; 108.0457 |
| 10 | 326.1245 | N-(1-Deoxy-1-fructosyl)phenylalanine | C15H21NO7 | 4.74 | (M-H)- | 164.0715; 147.0449; 103.0549 |
| 11 | 292.1399 | N-(1-Deoxy-1-fructosyl)isoleucine | C12H23NO7 | 3.14 | (M-H)- | 130.0871; 131.0904 |
| 12 | 340.2236 | Diprotin A (Ile-Pro-Ile) | C17H31N3O4 | 10.6 | (M-H)- | 183.1503; 130.0873; 155.1188; 227.1403; 113.0715 |
| 13 | 326.2078 | Diprotin B (Val-Pro-Leu) | C16H29N3O4 | 9.3 | (M-H)- | 183.1502; 130.0878; 116.0728; 227.1419 |
| 14 | 164.0715 | Phenylalanine | C9H11NO2 | 4.4 | (M-H)- | 147.8924; 103.0553 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García, C.J.; Alacid, V.; Tomás-Barberán, F.A.; García, C.; Palazón, P. Untargeted Metabolomics to Explore the Bacteria Exo-Metabolome Related to Plant Biostimulants. Agronomy 2022, 12, 1926. https://doi.org/10.3390/agronomy12081926
García CJ, Alacid V, Tomás-Barberán FA, García C, Palazón P. Untargeted Metabolomics to Explore the Bacteria Exo-Metabolome Related to Plant Biostimulants. Agronomy. 2022; 12(8):1926. https://doi.org/10.3390/agronomy12081926
Chicago/Turabian StyleGarcía, Carlos J., Verónica Alacid, Francisco A. Tomás-Barberán, Carlos García, and Pedro Palazón. 2022. "Untargeted Metabolomics to Explore the Bacteria Exo-Metabolome Related to Plant Biostimulants" Agronomy 12, no. 8: 1926. https://doi.org/10.3390/agronomy12081926
APA StyleGarcía, C. J., Alacid, V., Tomás-Barberán, F. A., García, C., & Palazón, P. (2022). Untargeted Metabolomics to Explore the Bacteria Exo-Metabolome Related to Plant Biostimulants. Agronomy, 12(8), 1926. https://doi.org/10.3390/agronomy12081926