Abstract
Drought is one of the major limitations to rice productivity worldwide. The present study compared variation in seventeen rice genotypes of Egyptian origin for morpho-physiological traits to identify the best genotypes with combination of adaptive traits under water-limited condition (DS). The DS reduced days to heading (DTH), plant height (PH), flag leaf angle (FLA), flag leaf area (FLAR), chlorophyll content (CHC), relative water content (RWC), grain yield (GY), and its components. Among genotypes, Hybrid 2 expressed the highest GY, panicle length (PL), number of tillers (NT), panicles per plant (NPP), and harvest index (HI) with maximum spikelet sterility (SS) under non-stress condition (NS), while the same genotype expressed ≈ 41% yield reduction under DS. The genotype Giza 179 had earlier DTH, higher and stable GY, FLAR, and yield component traits such as NPP, PW, and HI across the water regimes with least yield reduction (30.5%) under DS. The GY and FLAR, RWC, PL, NT, NPP, PW, and HI were positively correlated under DS. The cluster analysis showed a similarity index of 25% among genotypes. The high yielding genotypes Giza 179, IET 1444, and IRAT 170 had also increased yield components (PL, NT, NPP, PW, TGW and HI) under DS that were attributed to highest FLAR, RWC, and PH, while having reduced LR, FLA, TR, and SS; therefore, these genotypes were categorized as drought-tolerant. The Hybrid 2 and Giza 179 genotypes can perform well under NS; however, the cultivation of Giza 179, Sakha 107, IET 1444, and IRAT 170 would give an advantage in DS-prone areas, hence, these can be used as a donor parental line in future rice breeding programs.
1. Introduction
Drought stress exerts several negative effects on growth and productivity of crops [1,2,3], and high drought risk often occurs with huge economic losses [4,5,6]. Even in the most productive agricultural regions, exposure to short periods of drought showed great yield losses. Growing world population and decline of water resources for crop production prioritize the development of high-yielding drought-tolerant cultivars with better adaptability to water-limited condition [7]. Drought is one of the major limitations to rice production, causing huge economic losses and having large effects on rice yield and its components, particularly during the reproductive stage [8]. If drought period matches with panicles initiation stage in rice, the number of spikelets and grains per panicle, including grain yield, are reduced [9,10]. Drought also delays the panicle emergence and flowering stages in rice. Nonetheless, drought affects morphological, physiological, biochemical, and molecular responses in rice plant [11,12,13]. Therefore, varietal adaptability for better crop performance and yield stability are required to reduce the yield gaps and sustain rice productivity under water-limited conditions [14].
Nonetheless, rice plant adapts several mechanisms to water-limited conditions through drought escape, avoidance, and tolerance [15]. Escape strategies rely on successful reproduction before the onset of severe stress, by shortened lifecycle associated with early flowering and maturity, a higher growth and photosynthesis rate, or the efficient storage and remobilization of reserves for seed production. Dehydration avoidance mechanisms in rice involve the maintenance of a tissue water status associated with minimized water loss caused by stomata closure, leaf rolling, stay green, deep roots, and high transpiration efficiency for maximized water uptake [15,16]. Nevertheless, developing rice for tolerance to drought stress through breeding is a sustainable and cost-effective approach in improving its productivity [17]. Several efforts have been made to improve drought tolerance by identifying donor genotypes, but success rate for their translation into drought-tolerant varieties is very low [18]. For example, screening a large set of genotypes at International Rice Research Institutes (IRRI) of different origins showed that most of the drought-tolerant accessions identified were of aus and indica types that belong to India, Bangladesh, and Sri Lanka [19,20]. Further, earlier genotypes being grown under drought-prone areas have been bred for irrigated environments rather than selected for drought tolerance [21].
As morphological, physiological, and genetic responses in rice to drought are of complex nature [11,12,13], characterizing genotypes based on these bases for drought tolerance is of prime importance for using them in future breeding programs. Nevertheless, identification of genotypes directly based on grain yield or development of cultivars with combination of putative traits which resist and produce economical yield under target environment is the effective strategy for improving drought tolerance in rice [21,22]. Moreover, identifying new donors’ genotypes for drought tolerance may be helpful to overcome yield constraints under water-limited conditions. For example, evaluation of rice germplasm under irrigated and water-limited conditions may be useful to identify rice types with wide adaptability simultaneously under drought in some years and sufficient rainfall in others [21]. The stable cultivars usually express better yields and have high adaptability across a wide range of environments. Therefore, evaluating different genotypes across different environments or growing seasons helps to identify donor germplasm with adaptive traits for drought resistance, high yields, and grain quality in target environments [23,24,25]. No such comprehensive studies have been carried out to identify donor genotypes with adaptive traits in both irrigated and drought environments in cultivars of Egyptian origin [26]. The present study therefore evaluated the genotypic variation for morphological and physiological characteristics of drought tolerance, and to determine the most desirable ideotype with combination of traits, cluster analysis was performed. The genotypes identified may be used in future breeding programs of rice for both environments in particular drought.
2. Materials and Methods
2.1. Experimental Site
The field experiment was conducted at Sakha Research Station of Rice Research Department, Field Crops Research Institute, Agriculture Research Center, Egypt, during 2019 and 2020 growing seasons. The experimental soil was clay type with 13.5 g kg−1 organic matter contents, 43.8 mg kg−1 of available N, 11.4 mg kg−1 of available P, and 8.5 soil pH. Average soil moisture contents of the experimental site for both years are given in Table 1.

Table 1.
Soil moisture contents of the experimental site on average in the 2019 and 2020 rice-growing season.
2.2. Experimental Design and Treatments
The 17 rice genotypes used for experimental material with pedigree and origin are presented in Table 2.

Table 2.
The pedigree and characteristics of rice genotypes.
These rice genotypes were grown under well-irrigated conditions with continuous submergence (NS), and water stress (DS) conditions exposed to irrigation with twelve days interval started fifteen days after transplanting of nursery seedlings. The experimental design used was a split arrangement with irrigation in main plots and rice genotypes randomized in sub-plots using three replications. The average weather conditions for both growing seasons are given in Table 3. The rice nursery seedlings raised on 28 and 29 April during 2019 and 2020, respectively, were transplanted after 30 days in both seasons. The nursery seedlings of each genotype were transplanted in 20 cm apart rows and at similar distance between the hills with a net plot area of 6 m2.

Table 3.
Average weather conditions for experimental period during growing seasons.
2.3. Crop Husbandry Practices
Whole phosphatic and 50% nitrogen fertilizers were applied at 36 kg P2O5 ha–1 and 144 kg N ha−1 using superphosphate (15.5% P2O5) and urea (46% N) as base during soil preparation. Remaining nitrogen was applied in two splits with 30% at initial tillering and 20% at panicle initiation stages.
2.4. Measurements of Morpho-Physiological and Agronomic Traits
Data on agronomical traits were recorded from ten randomly selected plants for each genotype of each replicate. Among morphological traits, days to heading (DTH) was determined from sowing to date for first panicle exertion, plant height (PH) from the soil surface to the tip of the main panicle of each plant, and leaf rolling (LR) recorded by a visual estimation [27]. The flag leaf angle (FLA) and its area (FLAR) were measured at heading stage following Yoshida et al. (1976):
where K (0.75) is a rectification factor used for the whole growth period, except for the seedling and maturity periods. The leaf temperature (LT) was calculated by the thermocouple of the steady-state porometer pressed against the adaxial and abaxial surfaces of the leaf, and the leaf-to-air temperature gradient (TL–TA) was measured by using the atmospheric temperature [28]. The chlorophyll content (CC) was determined using SPAD chlorophyll meter (Minnolta, Japan). Relative water content (RWC) of flag leaf was calculated using the following formula:
where FW is flag leaf fresh weight, DW is flag leaf dry weight, and TW is flag leaf turgid weight.
A portable steady-state porometer, LICOR, (LI-1600, Lincoln, NE, USA) was used for assessing the steady-state CO2 and H2O exchange degrees of leaves. Stomatal conductance (SC) and transpiration rate (TR) were measured in the fully expanded flag leaf.
At harvesting, ten panicles were selected randomly from each replicate to measure panicle length (PL) and weight (PW), number of tillers (NTP) and panicles per plant (NPP), 1000-grain weight (TGW), and spikelet sterility (SS). Spikelet sterility was determined by dividing the unfilled spikelets from a panicle to the total spikelets. The rice grain yield (GY) was estimated from unit area and adjusted to 14% moisture content and converted to t ha−1. Harvest index (HI) was calculated by dividing grain yield by biological yield and expressing into percentage.
2.5. Statistical Analysis
Analysis of variance was conducted for all the traits using combined analysis according to Steel et al. [29]. Year was also considered as a factor, and where it was found to be nonsignificant, the data were pooled. The analysis of covariance analysis (ANCOVA), mean comparisons, correlation, and cluster analysis were performed using Minitab version 17. Days to heading was taken as covariable for all data analysis, and then adjusted means were used for further statistical analysis.
3. Results
3.1. Effect of Studied Factor on Morpho-Physiological Traits
The analysis of covariance showed that year (Y) effect was nonsignificant for all morpho-physiological traits except for LT, SC, and TR. The irrigation (I) and genotypic (G) difference were highly significant for all traits, while, only I × G interaction was significant for all traits except LT (Table 4).

Table 4.
Analysis of covariance for different morpho-physiological traits.
3.2. Morpho-Physiological Variation among Genotypes
The drought stress (DS) reduced the morpho-physiological traits compared to NS, except for LT, while leaf rolling score (LR) significantly increased under DS. Among genotypes, Giza 177, Giza 177, and Sakha 103 observed earlier heading under both NS and DS condition, respectively. Egyptian Yasmine was the latest heading genotype under both NS (120.50 days) and DS (110.50 days). The Sakha 101 and Sakha 108 genotypes were shortest, while IRAT I70 had the tallest plants under both NS and DS. Among genotypes, IET 1444 and IRAT 170 had minimum, while Sakha 103 had the maximum LR score under both NS and DS conditions. Maximum FLA was obtained for Sakha 105 and Sakha 106 genotypes while the lowest was obtained for GZ 1368-S-5-4 under both NS and DS (Table 5). Nonetheless, maximum FLAR was expressed by Egyptian Yasmine and Giza 179 genotypes while minimum values in Giza-103 under both NS and DS, respectively. Maximum CHC was observed for Sakha 102 and minimum for Egyptian Yasmine, while highest RWC was for IET 1444 under both NS and DS (Table 2). Maximum SC was found in IRAT 170 and Sakha-103 under irrigated, while it was similar in IET 1444, Egyptian Yasmine, and Giza 177 under both NS and DS conditions. Minimum TR was found in IET 1444 under both NS and DS, while maximum was found in Giza 179 under NS condition (Table 5).

Table 5.
Morpho-physiological traits performance of rice genotypes under water-deficit (DS) conditions.
3.3. Agronomic Performance of Rice Genotypes
The analysis of covariance showed that year (Y) effect was nonsignificant for all agronomic traits except for NPP and PW. The irrigation (I) and genotypic (G) difference were highly significant for all traits, while among interactions, Y × I and Y × G interaction was only significant for NPP and NT, and NPP and TGW, respectively. Likely, I × G interaction was significant for all studied traits. Interestingly, Y × I × G interaction was significant only for PL, NPP, and TGW traits (Table 6).

Table 6.
Analysis of covariance for agronomical traits.
3.4. Agronomic Performance of Rice Genotypes
The DS significantly reduced the yield traits compared to NS except spikelet sterility, which increased under DS. Among genotypes, maximum PL was recorded in Egyptian Yasmine, Hybrid 2, and IRAT 170 under NS, while genotypes IRAT 170, IET 1444, including Sakha 107, Sakha 101, and Giza 179, had maximum PL under DS. Minimum PL was found in Sakha 103. Highest GY, NT, NPP, PW, and HI were observed in Hybrid 2 and Giza 179 under NS, followed by Giza 178 and IET 1444. Under DS, genotypes Giza 179 and Sakha 107 expressed higher GY and HI, as well as NT, NPP, PW, and reduced SS. These genotypes both also produced GY > 8.0 t ha−1 under DS (Table 7).

Table 7.
Agronomic and yield traits of rice genotypes under water-deficit (DS) treatments.
3.5. Cluster Analysis and Pearson Correlation
Seventeen rice genotypes were clustered into four clusters with a similarity index of 25% (Figure 1).
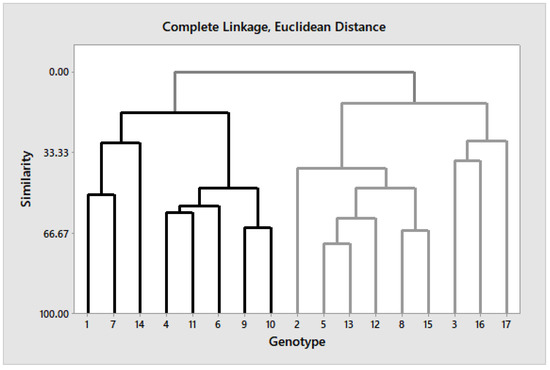
Figure 1.
Clustering of 17 rice genotypes with response to water-deficit treatment (DS).
Both cluster I and IV shared the same number of members (three) and were the smallest groups. Cluster III was the biggest group with six members, while cluster II was represented by five members. Cluster IV consisted of genotypes Giza 179, IET 1444, and IRAT 170. This cluster showed the highest values of GY and all of its components (PL, NT, NPP, PW, TGW, and HI) under DS. This cluster also showed highest values of FLAR, RWC, and PH, but lowest values of LR, FLA, TR, and SS. Therefore, the members of this group can be categorized as drought-tolerant rice genotypes. Meanwhile, the lowest values of most of the agronomical traits (GY, PL, NT, NPP, TGW, and HI) under DS were observed in cluster I. Cluster I also recorded the lowest values of FLAR, CHC, and RWC, but highest values of SS, LR, and LT. Under DS, high positive correlations were observed between GY and FLAR, RWC, PL, NT, NPP, PW, and HI. Though positive correlations were also observed between GY and PH, CHC, TR, and TGW, the results were not significant. Meanwhile, SS showed high negative correlation with GY (Table 8).

Table 8.
Pearson correlations showing the strength of relationships of all observed parameters under water-deficit treatment (DS).
4. Discussion
The present study evaluated the morpho-physiological and agronomical responses of rice genotypes to identify the plant types with combination of traits adaptive to water-limited conditions. The significant differences observed for all studied traits among rice genotypes provide wide genetic variability and an opportunity for yield improvement under both NS and DS conditions (Table 1 and Table 2). A little variation in environment may affect wide adaptability and potential of rice genotypes. The variation in GY and secondary traits in rice genotypes of the present study shows that genotypes with combinations of traits adaptive to drought can be identified and complexity of their mechanisms can be characterized [30]. High GY and its contributing traits, including reduced SS, was observed under NS condition with little reduction under DS. The genotypes Giza 179 and Sakha 107 produced >8.0 t ha−1 GY under DS with reduction of 30.49% and 18.21%, respectively, compared to NS. Several studies report GY as the most appropriate and direct criteria for selection of genotypes for drought tolerance due to moderate to high heritability of this trait under DS [31,32]. The DS reduced PH, as observed with genotypic variation in the present study and also reported earlier [33,34], and could be attributed to decrease in turgor to impair the cell elongation and expansion under DS. Among genotypes, Giza 179 and Sakha 107 observed earlier heading under both NS and DS, respectively, while Egyptian Yasmine had delayed heading under DS. Under DS, earlier heading or flowering is advantageous to escape drought [35,36,37], which often incurs at the cost of yield penalty. In the present study, earlier heading genotypes Giza 179 and Sakha 107 observed small reduction in yield that might be associated with mild DS observed in the present study (Table 6); however, another plausibility may be the stable and relatively drought-tolerant character of these genotypes [38,39]. These effects of drought also depend on crop stage, duration, and intensity. For instance, drought before reproductive stage shortens plant cycle and plants escape from prolonged effects of stress by earlier flowering at later developmental stages and produce better GY than delayed flowering genotypes [36,37,40,41]. However, reduced yield in delayed flowering genotype Egyptian Yasmine explains that this trait is genotype-dependent and shows combined effect of slow crop development and reduced panicle elongation rate [40]. Flag leaf angle (FLA) and FLAR reduced under DS; however, genotypes Sakha 105 and Sakha 106 expressed maximum, while GZ 1368-S-5-4 expressed lowest FLA under both NS and DS conditions. Nonetheless, genotypes Sakha 104 and Giza 179 expressed maximum FLAR under both NS and DS, respectively.
The FLAR, leaf area index (LAI), leaf relative water contents (LRWC), and chlorophyll pigments are putative traits used as evaluation criteria for drought tolerance [8,41,42]. Decrease in leaf area under DS might be attributed to less leaf growth owing to decrease in cell division and expansion concomitantly with decreases in turgor potential and water potential, as evident from decrease in RWC in the present study [17,42,43]. Likely, genotypic variation for leaf traits, such as increased LR score and LT, decrease in CHC under DS show the plasticity of these traits in rice varietal selection for drought resistance [44,45]. Further, leaf rolling (LR) is a desirable trait to reduce dehydration, particularly when drought occurs suddenly, to maintain water status, and genotypic variation exists with intensity of water stress experienced by the plant [17,46,47]. On the other hand, some studies report that low LR score contributes to higher GY; however, no such evidence was found in the present study. Similar findings were reported earlier by Kadioglu and Terzi [48]. In the present study, the genotypes Sakha 105, Sakha 108, and Egyptian Yasmine had minimum LR score under NS, and IET 1444 under DS, while Sakha 103 had the maximum LR score under DS. The minimum LR score, by IET 1444 under DS, reflecting independence of this trait in this genotype and response, was also reflected by maintenance of higher RWC, reduced LT, and higher grain-filling rate. Therefore, these genotypes comparatively performed better for yield than other genotypes, also evident from cluster analysis (Figure 1).
Grain yield (GY) is highly associated with the chlorophyll contents, the most important component in photosynthesis, and had positive association with GY in the present study. However, decrease in CHC under DS shows the obvious effect of disruption in synthesis and degradation of photosynthetic pigments that might be associated with lipid-peroxidation-induced damages to chloroplast membrane [49,50]. Nonetheless, drought, nutrient, and light directly or indirectly affect CHC, which results in disruption of other physiological processes [17]. The significance of leaf traits such as CHC, LT, FLAR, and LR, including RWC during grain-filling, is well recognized. As higher FLAR is required for synthesis and transport of assimilates that is also evident from a positive relationship of flag leaf traits with GY under water-limited condition of present study [51]. Positive relationship of CHC, TGW, NPP, RWC, PW, and FLAR indicates the contribution of these traits in improving GY of rice genotypes, while LR, SS, and LT are negatively correlated with GY (Table 5). All genotypes showed increased SS under DS, indicating its association with limit of GY by affecting spikelet sterility and grain-filling [52,53,54,55,56], as evident from the negative association of SS with GY (Table 5). Maximum reduction in grain yield was recorded in Sakha 103 and Egyptian Jasmine genotypes, which was attributed to increased SS under DS in these genotypes. When drought occurs during flowering and grain-filling stages, both the photosynthesis and translocation processes are greatly affected, which ultimately reduces yield [52,53,54,55,56].
However, these traits seem to be of less heritability, as compared to heading time, plant height, and leaf rolling showing moderate to high heritability, and are considered favorable in selection of drought-tolerant genotypes [56,57,58,59]. Therefore, physiological traits such as CHC, TWG, RWC, and FLAR that had positive association with GY are used in the selection of Sakha 107 and Giza 179 as drought-tolerant genotypes. Hybrid 2 is highly recommended for irrigated rice areas; however, the cultivation of Sakha 107 and Giza 179 (GY under DS =>8.0 t ha−1) would give an advantage for water-stress-prone areas. Giza 179 can be considered as the most stable and promising rice genotype for both NS and DS areas (GY NS = 11.8 t ha−1; GY DS = 8.3 t ha−1). Nonetheless, the Sakha 107 and Giza 179, with the highest GY and its contributing traits under DS, can be utilized as donor parents in breeding programs for developing new high-yielding drought-tolerant rice genotypes.
5. Conclusions
Breeding for drought-tolerant rice is one of the most important approaches used to reduce detrimental effects of water stress. However, the selection of the parental lines is critical and must be emphasized to ensure the effectiveness of a breeding program. Rice subjected to water stress reduced DTH, PH, FLA, FLAR, CC, and RWC, and the effects were also reflected in yield and its components. The desirable mean values for NP, GW, ST, and GY were observed in Hybrid 2 and Giza 179. Giza 179, Sakha 107, IET 1444, and IRAT 170 also showed good performance under DS. The early heading exhibited by Giza 179 and Sakha 107 genotypes shows their drought escape strategy as an advantage under DS. The utilization of these genotypes as parental lines in a rice breeding program can help to develop promising breeding lines with earlier maturity, high yield, and drought characteristics.
Author Contributions
Conceptualization, M.M.G. and A.M.G.; methodology, H.U.R.; software, M.M.S.; validation, M.I.G., A.S.E.-I. and A.E.M.; formal analysis, M.W.; investigation, N.A.A.S.; resources, Y.C.; writing—original draft preparation, M.M.G.; writing—review and editing, A.M.G. and H.U.R.; visualization, H.U.R.; supervision, A.M.G. All authors have read and agreed to the published version of the manuscript.
Funding
The research is supported by the National Natural Science Foundation of China (52161145102). The authors wish to thank Rice Research and Training Center, Field Crops Research Institute, Agricultural Research Center, Egypt for supporting this research study.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
There were no conflicts of interests from the authors.
References
- Wu, H.; Hubbard, K.G.; Wilhite, D.A. An agricultural drought risk assessment model for corn and soybeans. Int. J. Climatol. 2004, 24, 723–741. [Google Scholar] [CrossRef]
- Sheffield, J.; Wood, E.F.; Chaney, N.; Guan, K.; Sadri, S.; Yuan, X.; Olang, L.; Amani, A.; Ali, A.; Demuth, S.; et al. A drought monitoring and forecasting system for Sub-Sahara African water resources and food security. Bull. Am. Meteorol. Soc. 2014, 95, 861–882. [Google Scholar] [CrossRef]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef]
- Li, X.; Kristiansen, K.; Rosenqvist, E.; Liu, F. Elevated CO2 modulates the effects of drought and heat stress on plant water relations and grain yield in wheat. J. Agron. Crop Sci. 2019, 205, 362–371. [Google Scholar] [CrossRef]
- Zhao, M.; Running, S.W. Drought-induced reduction in global terrestrial net primary production from 2000 through 2009. Science 2010, 329, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Dai, A. Increasing drought under global warming in observations and models. Nat. Clim. Chang. 2013, 3, 52–58. [Google Scholar] [CrossRef]
- Gaballah, M.M.; Af El-Ezz, A.F.; Ghoneim, A.M.; Yang, B.; Xiao, X. Exploiting heterosis and combining ability in two-line hybrid rice. Acta Agric. Slovenica 2021, 117, 1–16. [Google Scholar] [CrossRef]
- Gaballah, M.M.; Ghoneim, A.M.; Ghazy, M.I.; Mohammed, H.M.; Raghda, M.S.; Rehman, H.U.; Shamsudin, N.A. Root traits responses to irrigation intervals in rice (Oryza sativa L.). Inter. J. Agri. Biol. 2021, 26, 23–30. [Google Scholar] [CrossRef]
- Gewaily, E.E.; Ghoneim, A.M.; Osman, M.O. Effects of nitrogen levels on growth, yield and nitrogen use efficiency of some newly released Egyptian rice genotypes. Open Agric. 2018, 3, 310–318. [Google Scholar] [CrossRef]
- Ghoneim, A.M. Soil nutrients availability, rice productivity and water saving under deficit irrigation conditions. J. Plant Prod. 2020, 11, 7–16. [Google Scholar] [CrossRef]
- Upadhyaya, H.; Panda, S.K. Drought stress responses and its management in rice. In Advances in Rice Research for Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Nahar, K., Biswas, J.K., Eds.; Elsevier: London, UK, 2019; pp. 177–200. [Google Scholar]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Melandri, G.; AbdElgawad, H.; Riewe, D.; Hageman, J.A.; Asard, H.; Beemster, G.T.S.; Kadam, N.; Jagadish, K.; Altmann, T.; Ruyter-Spira, C.; et al. Biomarkers for grain yield stability in rice under drought stress. J. Exp. Bot. 2020, 71, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Ghazy, M.I. Genetic Studies on Components of Drought and Heat Stresses Tolerance in Rice. Ph.D. Thesis, Kafrelsheikh University, Kafr el-Sheikh, Egypt, 2017; p. 233. [Google Scholar]
- Krishnamurthy, S.L.; Sharma, P.C.; Sharma, D.K.; Singh, Y.P.; Mishra, V.K.; Burman, D.; Maji, B.; Mandal, S.; Sarangi, S.K.; Gautam, P.K.; et al. Additive main effects and multiplicative interaction analyses of yield performance in rice genotypes for general and specific adaptation to salt stress in locations in India. Euphytica 2021, 217, 20. [Google Scholar] [CrossRef]
- Ghazy, M.; Salem, S.; Sallam, A. Utilize of genetic diversity and marker-trait association to improve drought tolerance in rice (Oryza sativa L.). Mol. Biol. Rep. 2020, 1–18. [Google Scholar] [CrossRef]
- Pandey, V.; Shukla, A. Acclimation and tolerance strategies of rice under drought stress. Rice Sci. 2015, 22, 147–161. [Google Scholar] [CrossRef]
- Kumari, B.K.; Kumar, R.B.; Dpb, J.; Rao, R. Diversity analysis in rice breeding lines for yield and its components using principal component analysis. J. Pharmacogn. Phytochem. 2021, 10, 905–909. [Google Scholar] [CrossRef]
- Torres, R.O.; McNally, K.L.; Cruz, C.V.; Serraj, R.; Henry, A. Screening of rice Genebank germplasm for yield and selection of new drought tolerance donors. Field Crops Res. 2013, 147, 12–22. [Google Scholar] [CrossRef]
- Bin Rahman, A.N.M.R.; Zhang, J.H. Flood and drought tolerance in rice: Opposite but may coexist. Food Energy Secur. 2016, 5, 76–88. [Google Scholar] [CrossRef]
- Kumar, A.; Bernier, J.; Verulkar, R.; Lafitte, G.; Atlin, C. Breeding for drought tolerance: Direct selection for yield, response to selection and use of drought-tolerant donors in upland and lowland-adapted populations. Field Crops Res. 2008, 107, 221–231. [Google Scholar] [CrossRef]
- Venuprasad, R.; Cruz, M.T.S.; Amante, M.; Magbanua, R.; Kumar, A.; Atlin, G. Response to two cycles of divergent selection for grain yield under drought stress in four rice breeding populations. Field Crops Res. 2008, 107, 232–244. [Google Scholar] [CrossRef]
- Ruth, N.M.; Julia, S.; John, D.; John, M.K.; Pangirayi, T. Genotype environment interactions for grain yield in rice under no drought and drought conditions. Afr. J. Plant Sci. 2017, 11, 282–293. [Google Scholar] [CrossRef][Green Version]
- Sitaresmi, T.; Susanto, U.; Pramudyawardani, E.F.; Nafisah, E.; Nugraha, Y.; Sasmita, P. Genotype × environment interaction of rice genotype. IOP Conf. Ser. Earth Environ. Sci. 2020, 484, 012028. [Google Scholar] [CrossRef]
- Kumar, A.; Dhillon, T.S. Stability of French bean (Phaseolus vulgaris) genotypes under diverse environments. Indian J. Agric. Sci. 2020, 90, 157–162. [Google Scholar]
- Gaballah, M.M.; Metwally, A.M.; Skalicky, M.; Hassan, M.M.; Brestic, B.; Sabagh, A.; Fayed, A.M. Genetic diversity of selected rice genotypes under water stress conditions. Plants 2020, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- De Datta, S.K.; Malabuyoc, J.A.; Aragon, E.L. A field screening technique for evaluating rice germplasm for drought tolerance during vegetative stage. Field Crops Res. 1988, 19, 123–124. [Google Scholar] [CrossRef]
- Hall, A.E.; Lange, O.L.; Schulze, E.D.; Walz, H. LI-1600 Steady State Promoter Instruction Manual. October (8210–0030); LI-COR, Inc.: Lincoln, NE, USA, 1989; p. 16. [Google Scholar]
- Steel, R.D.; Torrie, G.; DA Dickey, J.H. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed.; McGraw Hill Book Co.: New York, NY, USA, 1997; pp. 400–408. [Google Scholar]
- Wang, B.; Zhong, Z.; Wang, X.; Han, X.; Yu, D.; Wang, C.; Song, W.W.; Zheng, X.; Chen, C.; Zhang, Y. Knockout of the OsNAC006 transcription factor causes drought and heat sensitivity in rice. Int. J. Mol. Sci. 2020, 21, 2288. [Google Scholar] [CrossRef] [PubMed]
- Zain, M.N.A.; Ismail, M.R.; Puteh, A.; Mahmood, M.; Islam, M.R. Drought tolerance and ion accumulation of rice following application of additional potassium fertilizer. Commun. Soil Sci. Plant Anal. 2014, 45, 2502–2514. [Google Scholar] [CrossRef]
- Bernier, J.; Kumar, A.; Spaner, D.; Verulkar, S.; Mandal, N.P.; Sinha, P.K.; Peeraju, P.; Dongre, P.R.; Mahto, R.N.; Atlin, G.N. Characterization of the effect of rice drought tolerance qtl12.1 over a range of environments in the Philippines and eastern India. Euphytica 2009, 166, 207–217. [Google Scholar]
- Bunnag, S.; Pongthai, P. Selection of Rice (Oryza sativa L.) cultivars tolerant to drought stress at the vegetative stage under field conditions. Am. J. Plant Sci. 2013, 4, 1701–1708. [Google Scholar] [CrossRef]
- Ashfaq, M.; Haider, M.S.; Khan, A.S.; Allah, S.U. Breeding potential of the basmati rice germplasm under water stress condition. Afr. J. Biotechnol. 2012, 11, 6647–6657. [Google Scholar]
- Naik, S.M.; Raman, A.K.; Nagamallika, M.; Venkateshwarlu, C.; Singh, S.P.; Kumar, S.; Singh, S.K.; Tomizuddin, A.; Das, S.P.; Prasad, T.; et al. Genotype × environment interactions for grain iron and zinc content in rice. J. Sci. Food Agric. 2020, 100, 4150–4164. [Google Scholar] [CrossRef] [PubMed]
- Fukai, S.; Pantuwan, G.; Jongdee, B.; Cooper, M. Screening for drought resistance in rainfed lowland rice. Field Crop Res. 1999, 64, 61–74. [Google Scholar] [CrossRef]
- Pantuwan, G.; Fukai, S.; Cooper, M.; Rajataserreekul, S.; O’Toole, J.C. Yield response of rice (Oryza sativa L.) genotypes to different types of drought under rainfed lowlands Part 1. Grain yield and yield components. Field Crop Res. 2002, 73, 153–168. [Google Scholar] [CrossRef]
- Shamsudin, N.A.A.; Swamy, B.P.M.; Ratnam, W.; Sta Cruz, M.T.; Raman, A.; Kumar, A. Marker assisted pyramiding of drought yield QTLs into a popular Malaysian rice cultivar, MR219. BMC Genet. 2016, 17, 30. [Google Scholar] [CrossRef] [PubMed]
- Romyen, P.; Hanviriyapant, P.; Rajatasereekul, S.; Khunthasuvon, S.; Fukai, S.; Basnayake, J.; Skulkhu, E. Lowland rice improvement in northern and northeast Thailand: 2. Cultivar differences. Field Crop Res. 1998, 59, 109–119. [Google Scholar] [CrossRef]
- Lafitte, H.R.; Price, A.H.; Courtois, B. Yield response to water deficit in an upland rice mapping population: Associations among traits and genetic markers. Theor. Appl. Genet. 2004, 109, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.S.; Panda, D. Leaf traits and antioxidant defense for drought tolerance during early growth stage in some popular traditional rice landraces from Koraput, India. Rice Sci. 2017, 24, 207–217. [Google Scholar] [CrossRef]
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, S.A.; Men, S.N.; Wang, L.C. Chilling and drought stresses in crop plants: Implications, cross talk, and potential management opportunities. Front. Plant Sci. 2018, 9, 393. [Google Scholar] [CrossRef]
- Tejaswini, K.L.; Manukonda, Y.; Kumar, S.; Rao, R.; Ahamed, M.L.; Raju, S.K. Application of principal component analysis for rice germplasm characterization and evaluation. Emergent Life Sci. Res. 2018, 4, 72–84. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, Y.; Hu, Z.; Wang, K.; Cao, L.; Wu, S. Association and principal component analyses of eating quality traits of 141 japonica rice cultivars in China. Am. J. Agric. For. 2021, 9, 37–41. [Google Scholar]
- Kumar, A.; Shalabh Dixit, T.; Ram, R.B.; Yadaw, K.K.; Mishra, N.P.; Mandal, P. Breeding high-yielding drought-tolerant rice: Genetic variations and conventional and molecular approaches. J. Exp. Bot. 2014, 65, 6265–6278. [Google Scholar] [CrossRef] [PubMed]
- Ha, P.T.T. Physiological responses of rice seedlings under drought stress. J. Sci. Dev. 2014, 12, 635–640. [Google Scholar]
- Poorter, L.; Markesteijn, L. Seedling traits determine drought tolerance of tropical tree species. Biotropica 2008, 40, 321–331. [Google Scholar] [CrossRef]
- Kadioglu, A.; Terzi, R.A. Dehydration Avoidance Mechanism: Leaf Rolling. Bot. Rev. 2007, 73, 290–302. [Google Scholar] [CrossRef]
- Chozin, M.A.; Lubis, I.; Junaedi, A.; Ehara, H. Some physiological character responses of rice under drought conditions in a paddy system. J. Int. Soc. Southeast Asian Agric. Sci. 2014, 20, 104–114. [Google Scholar]
- Sikuku, P.; Onyango, J.; Netondo, G.W. Physiological and biochemical responses of five nerica rice varieties (Oryza sativa L.) to water deficit at vegetative and reproductive stage. Agric. Biol. J. N. Am. 2012, 3, 93–104. [Google Scholar] [CrossRef]
- Asma, A.; Hussain, L.; Ashraf, M.Y.; Rasheed, R.; Iqbal, M.; Anwar, S.; Shereen, A.; Khan, M.K. Assessment of rice (Oryza sativa L.) genotypes for drought stress tolerance using morpho-physiological indices as a screening technique. Pak. J. Bot. 2021, 53, 45–58. [Google Scholar] [CrossRef]
- Abdel-Hafez, A.G.; Abdallah, A.A.; Ghazy, M.I.; El-Degwy, I.S. Genetic analysis of water deficit and heat tolerance in rice under Egyptian conditions. Plant Breed. 2017, 21, 202–218. [Google Scholar]
- Abdallah, A.A. Development of high yielding rice lines tolerant to drought and heat stress conditions in Egypt. World Rural Obs. 2015, 7, 58–64. [Google Scholar]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, F.; Zohaib, S.; Sadia, W.; Nasim, S.; Adkins, S.; Saud, M.Z.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Eltaher, S.; Baenziger, P.S.; Belamkar, V.; Emara, H.A.; Nower, A.A.; Salem, K.F.B.; Alqudah, A.M.; Sallam, A. GWAS revealed effect of genotype×environment interactions for grain yield of Nebraska winter wheat. BMC Genom. 2021, 22, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gaballah, M.M.; Abu El-Ezz, E. Genetic Behavior of Some Rice Genotypes under Normal and High Temperature Stress. Alex. Sci. Exch. J. 2019, 40, 370–384. [Google Scholar] [CrossRef][Green Version]
- Jeevanapriya, P.; Saraswathi, R.; Thiruvengadam, V.; Surendar, K.K. Assessment of Genetic Diversity in New Restorer Lines of Hybrid Rice. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 530–536. [Google Scholar] [CrossRef]
- Kang, D.; Futakuchi, K. Effect of moderate drought-stress on flowering time of interspecific hybrid progenies (Oryza sativa L. × Oryza glaberrima Steud). J. Crop Sci. Biotech. 2019, 22, 75–81. [Google Scholar] [CrossRef]
- Lakshmi, I.; Sreedhar, V.; Vanisri, M.; Anantha, S.; Subba, M.; Rao, L.; Gireesh, C. Multivariate analysis and selection criteria for identification of African rice (Oryza glaberrima) for genetic improvement of indica rice cultivars. Plant Genet. Resour. Charact. Util. 2019, 7, 499–505. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).