Canopy Temperature and Heat Flux Prediction by Leaf Area Index of Bell Pepper in a Greenhouse Environment: Experimental Verification and Application
Abstract
:1. Introduction
1.1. Effects of Plant
1.2. Plant Models by Leaf Area Index (LAI)
2. Materials and Methods
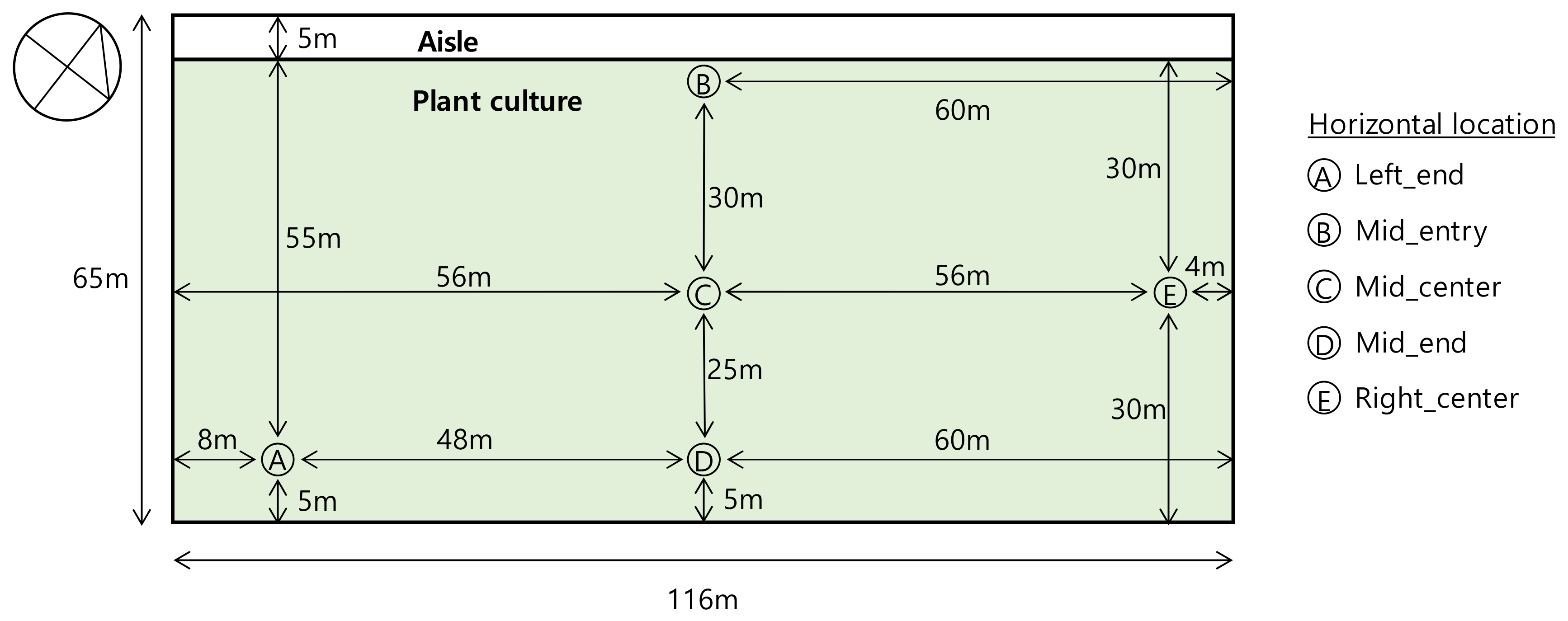
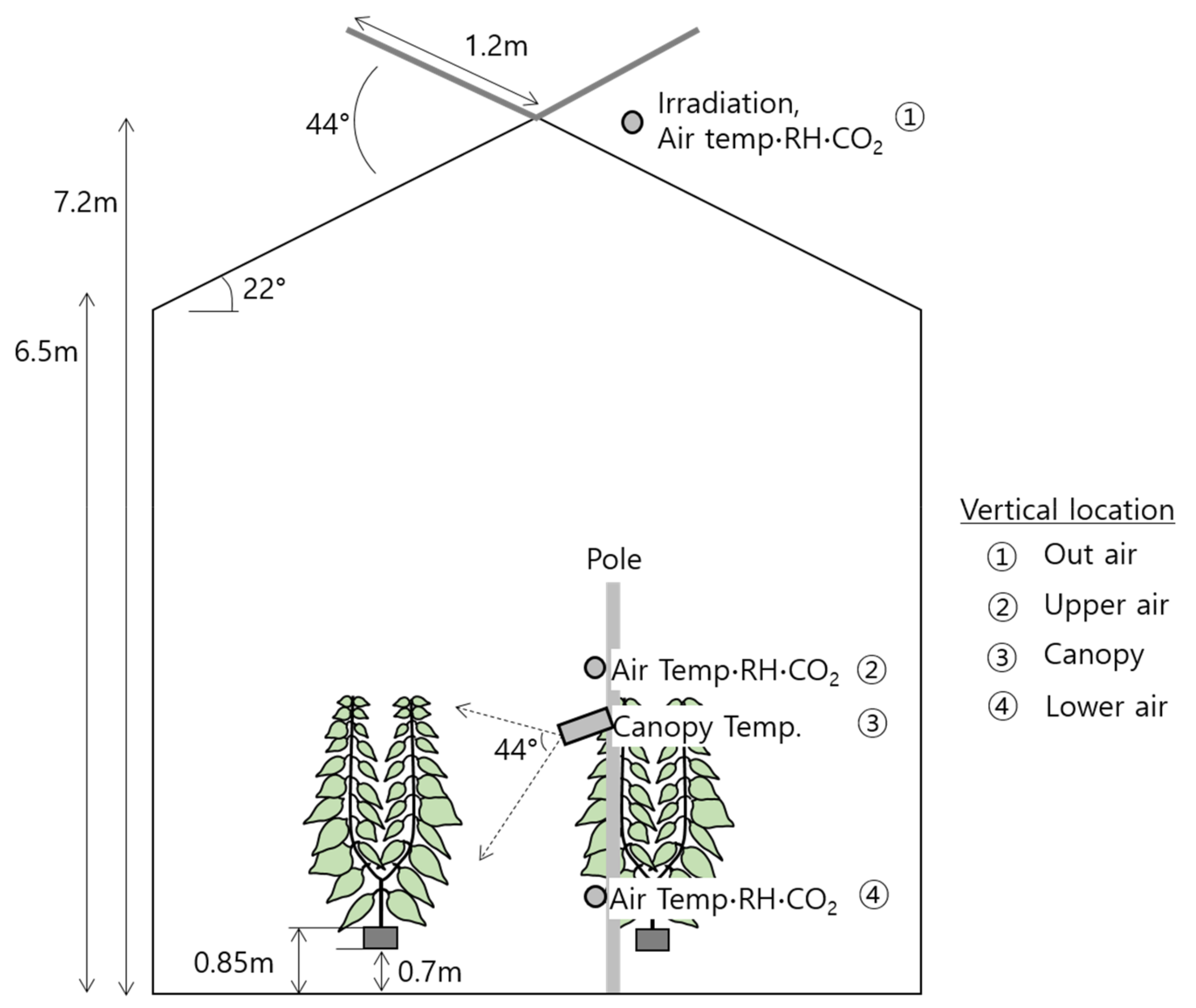
2.1. Target Greenhouse
2.2. Heterogeneously Scaled Models of Plant
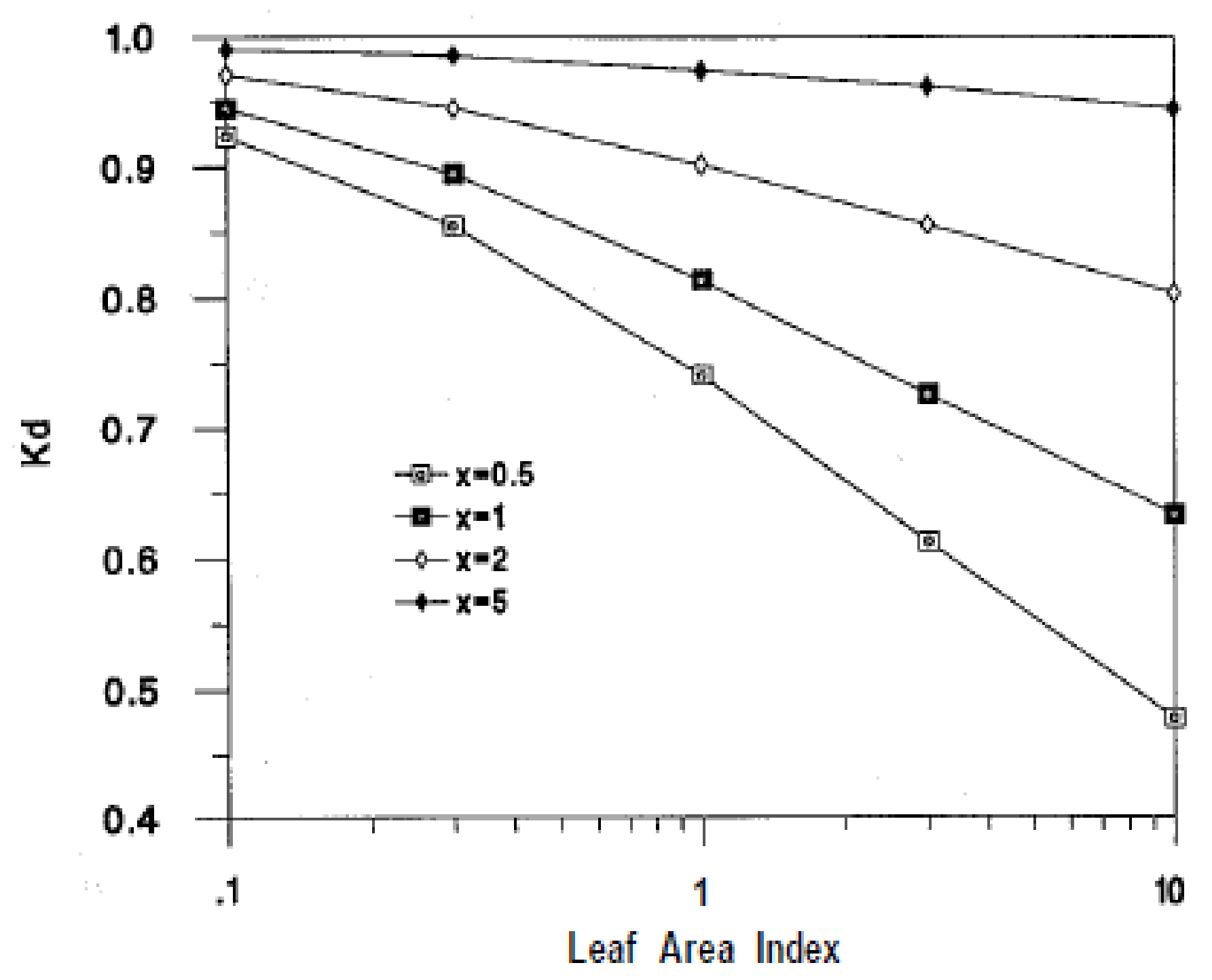
2.2.1. Extinction Coefficient
2.2.2. Boundary Layer Conductance
2.2.3. Wind Speed
2.2.4. Irradiation
2.3. Theoretical Mechanism
2.3.1. Transpiration and Canopy Temperature
2.3.2. Energy Flux between the Canopy and Air
2.3.3. Stomatal Conductance
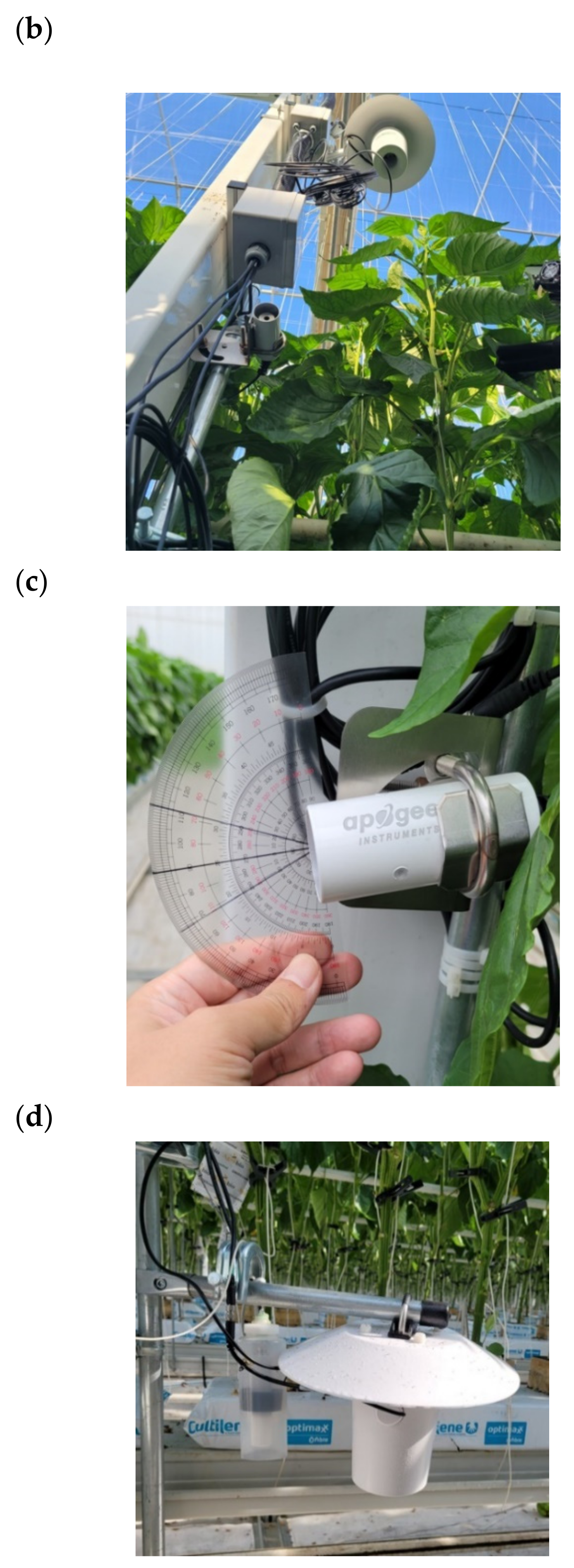
2.4. Measurements
2.5. Parametrization
3. Results
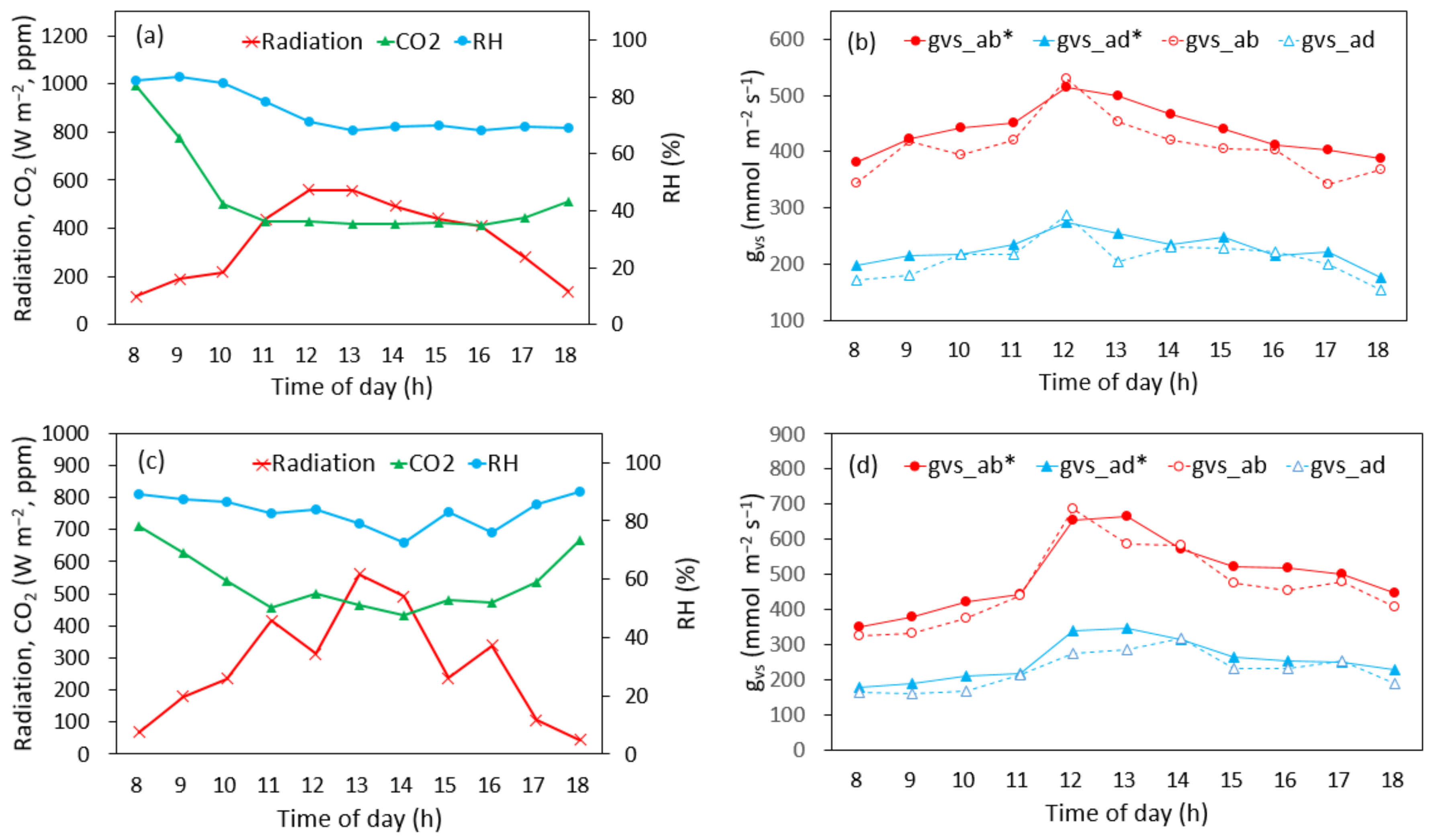
3.1. Actual Measurements
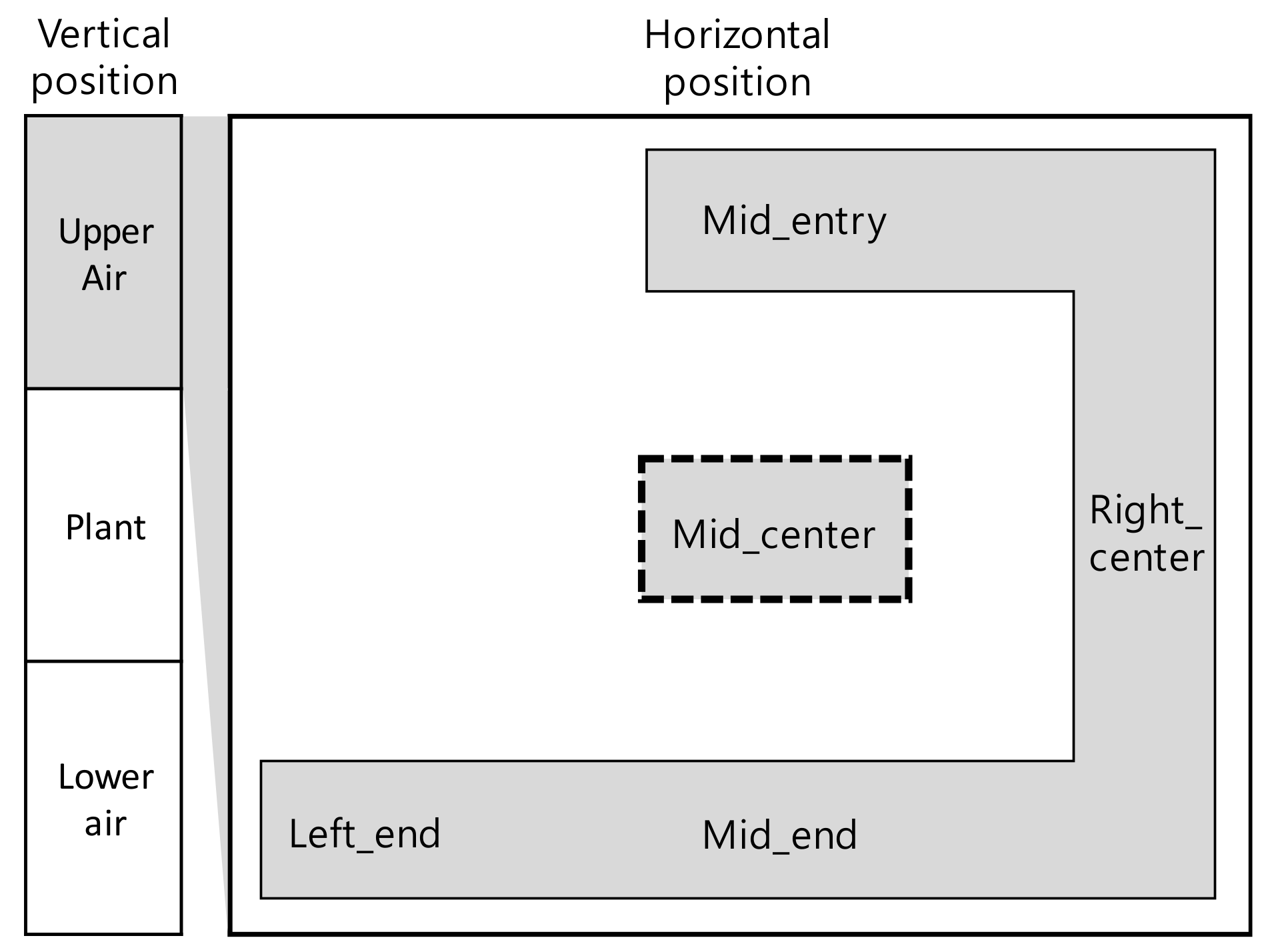
3.1.1. Measurements by Position
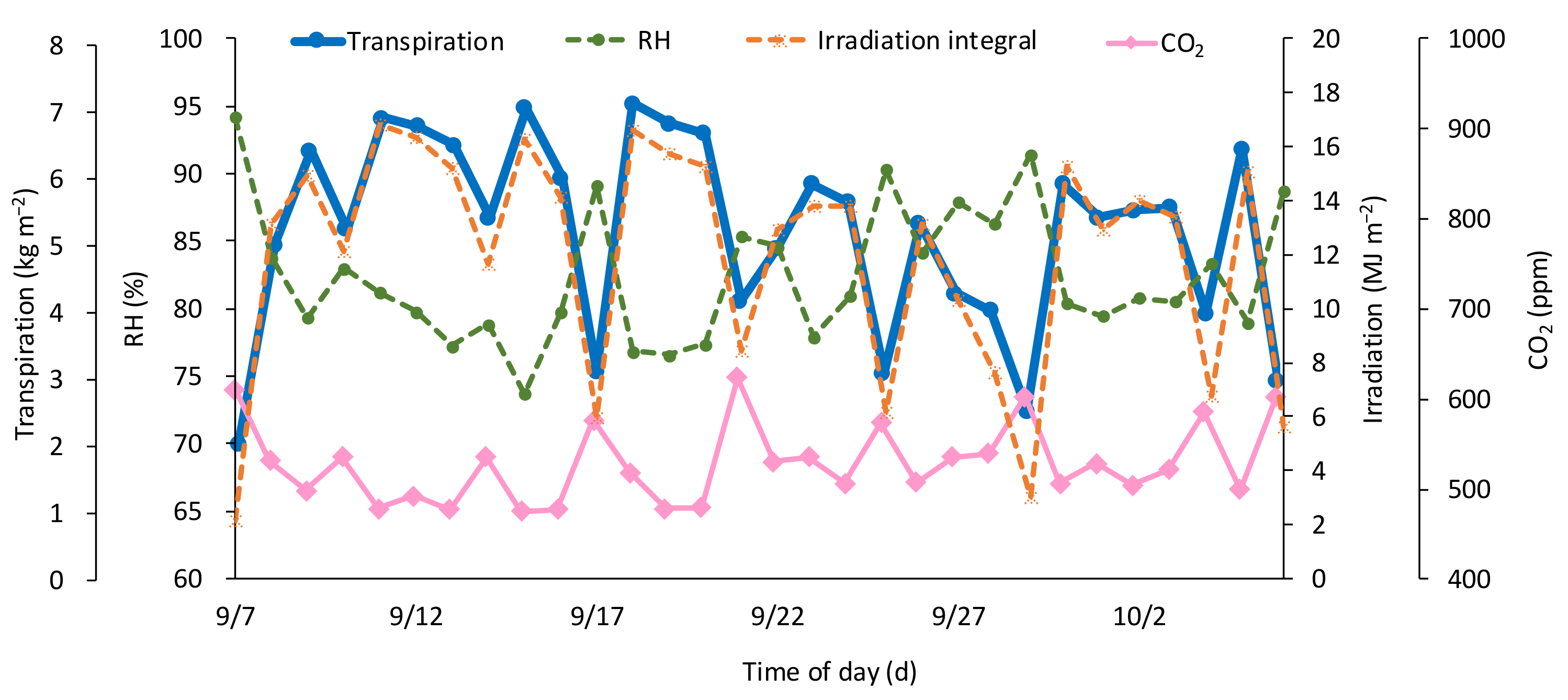
3.1.2. Other Measurements
3.2. Model Calculation by LAI
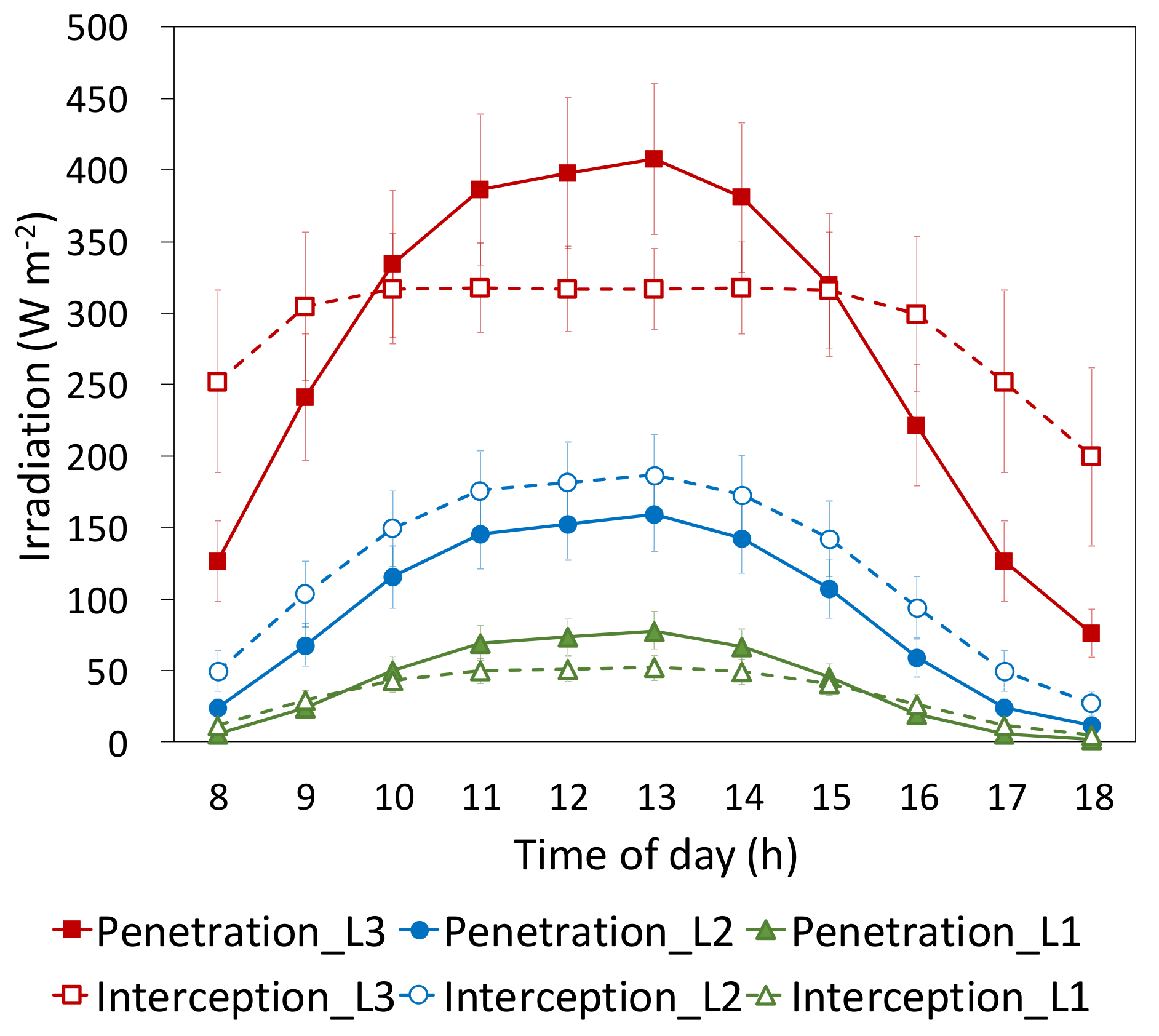
3.2.1. Irradiation
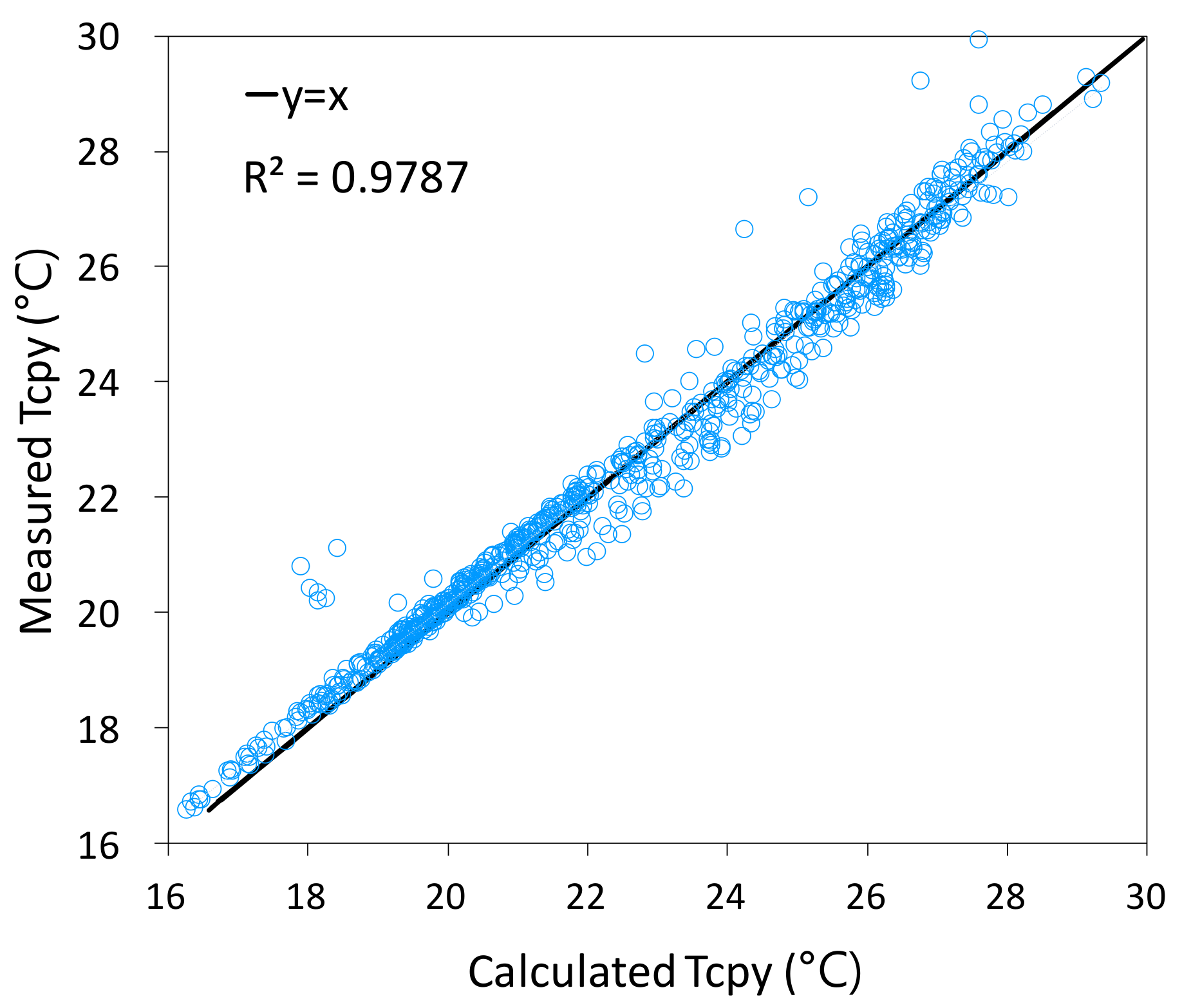
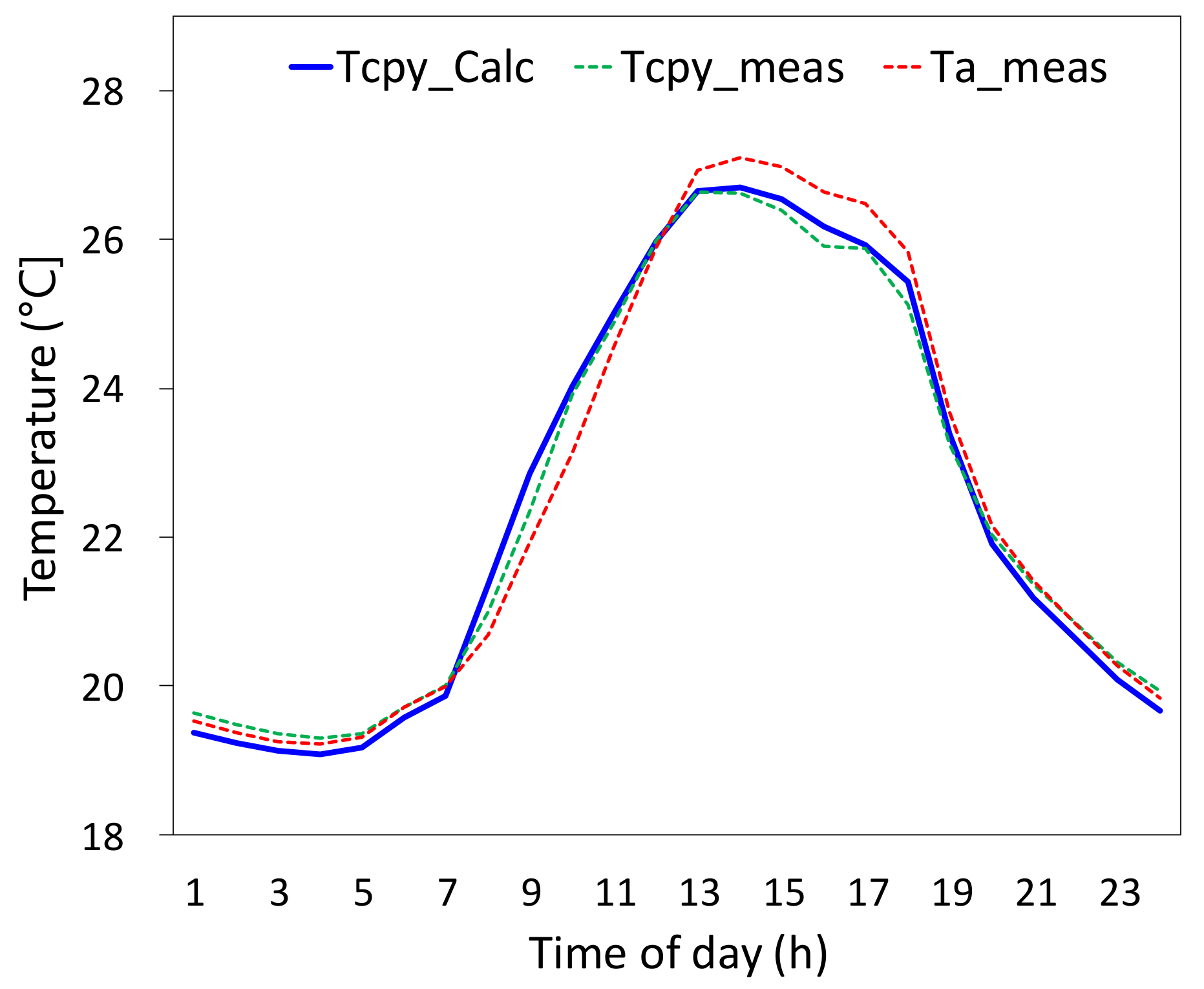
3.2.2. Canopy Temperature and Comparison to Measurements
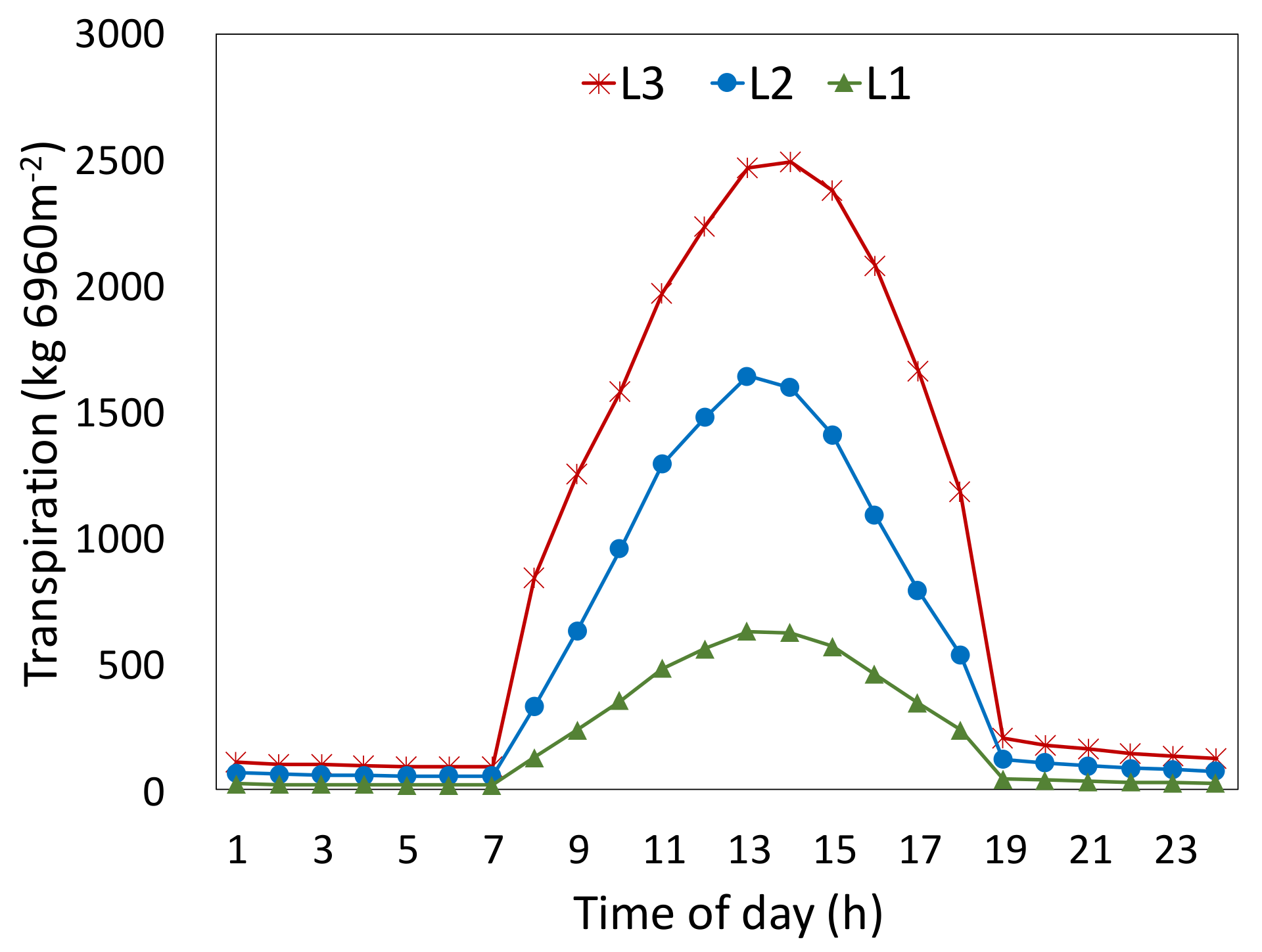
3.2.3. Transpiration
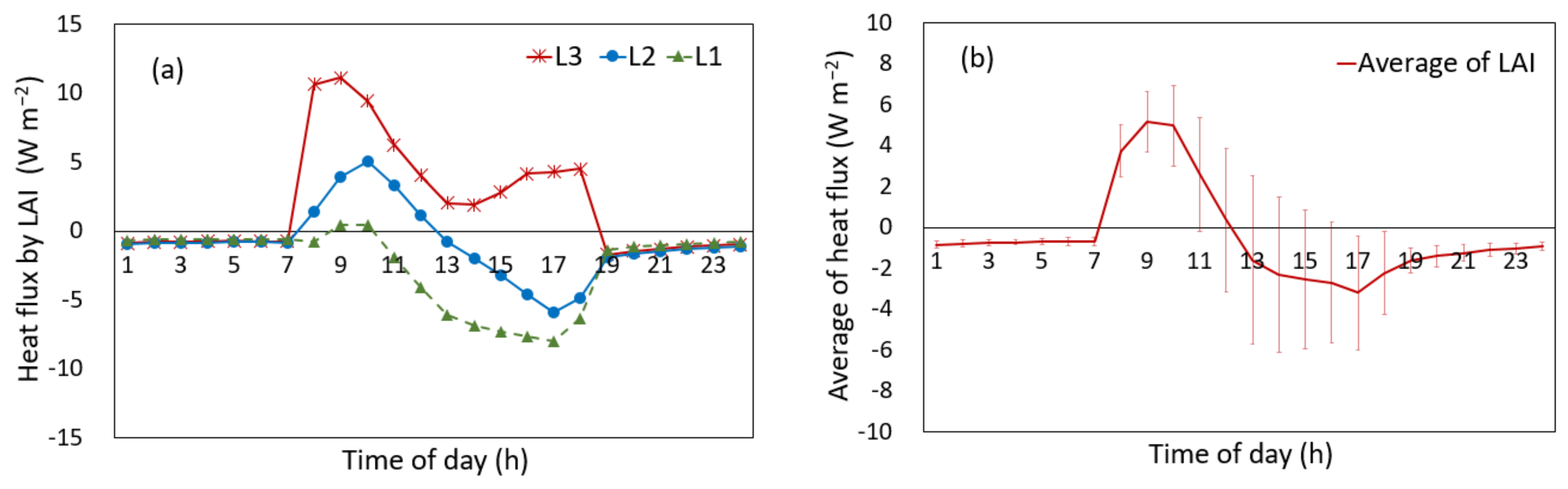
3.2.4. Heat Flux, Impact of Transpiration, and Canopy Temperature
4. Discussion
4.1. Approximation of Zero Plane Displacement and Roughness Length for Wind Profile
4.2. Heat Flux by the Temperature Difference between the Canopy and Air
4.3. Transpiration under the Crop Development Stage in Practice
4.4. Input Variables for Crop Model
4.5. Regression of Stomatal Vapor Conductance
4.6. Implication for Modeling the Interactions between Plants and a Field-Based Greenhouse
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, H.-J. Effects of Stem Density on Fruit Load; Yield Fluctuation and Production of Sweet Pepper; Graduate School, Chonnam National University: Gwangju, Korea, 2016. [Google Scholar]
- Joung, K.H.; Jin, H.J.; An, J.U.; Yoon, H.S.; Oh, S.S.; Lim, C.S.; Um, Y.C.; Kim, H.D.; Hong, K.P.; Park, S.M. Analysis of Growth Characteristics and Yield Pattern of ‘Cupra’ and ‘Fiesta’ Paprika for Yield Prediction. Prot. Hortic. Plant Fact. 2018, 27, 349–355. [Google Scholar] [CrossRef]
- Gupta, S.K.; Ram, J.; Singh, H. Comparative Study of Transpiration in Cooling Effect of Tree Species in the Atmosphere. J. Geosci. Environ. Prot. 2018, 6, 151–166. [Google Scholar] [CrossRef]
- Lin, H.; Chen, Y.; Zhang, H.; Fu, P.; Fan, Z. Stronger cooling effects of transpiration and leaf physical traits of plants from a hot dry habitat than from a hot wet habitat. Funct. Ecol. 2017, 31, 2202–2211. [Google Scholar] [CrossRef]
- Van Westreenen, A.; Van Westreenen, A.; Zhang, N.; Zhang, N.; Douma, J.C.; Douma, J.C.; Evers, J.B.; Anten, N.P.R.; Marcelis, L.F.M. Substantial differences occur between canopy and ambient climate: Quantification of interactions in a greenhouse-canopy system. PLoS ONE 2020, 15, e0233210. [Google Scholar] [CrossRef] [PubMed]
- Albright, L.D.; Seginer, I.; Marsh, L.S.; Oko, A. In situ thermal calibration of unventilated greenhouses. J. Agric. Eng. Res. 1985, 31, 265–281. [Google Scholar] [CrossRef]
- Tiwari, G.N.; Sharma, P.K.; Goyal, R.K.; Sutar, R.F. Estimation of an efficiency factor for a greenhouse: A numerical and experimental study. Energy Build. 1998, 28, 241–250. [Google Scholar] [CrossRef]
- Boulard, T.; Baille, A. A simple greenhouse climate control model incorporating effects of ventilation and evaporative cooling. Agric. For. Meteorol. 1993, 65, 145–157. [Google Scholar] [CrossRef]
- Allen, R.; Smith, M.; Perrier, A.; Pereira, L. An Update for the Definition of Reference Evapotranspiration AND An Update for the Calculation of Reference Evapotranspiration. ICID Bull. Int. Comm. Irrig. Drain. 1994, 43, 1–34. [Google Scholar]
- Pieters, J.G.; Deltour, J.M. Modelling solar energy input in greenhouses. Sol. Energy 1999, 67, 119–130. [Google Scholar] [CrossRef]
- Bailey, B.J. Microcliamte, physical processes and greenhouse technology. Acta Hortic. 1985, 174, 35–42. [Google Scholar]
- Elsner, B.V. Elsner The influence of heating systems on micro climate in crops and possibilities of energy saving. Acta Hortic. 1977, 70, 104–110. [Google Scholar] [CrossRef]
- Yang, X.; Short, T.H.; Bauerle, W.L. The Microclimate and Transpiration of a Greenhouse Cucumber Crop. Am. Soc. Agric. Eng. 1989, 32, 2143–2150. [Google Scholar] [CrossRef]
- Raupach, M.R.; Finnigan, J.J. Single-Layer models of evaporation from plant canopies are incorrect but useful, whereas multilayer models are correct but useless: Discuss. Aust. J. Plant Physiol. 1988, 15, 705–716. [Google Scholar] [CrossRef]
- Demrati, H.; Boulard, T.; Fatnassi, H.; Bekkaoui, A.; Majdoubi, H.; Elattir, H.; Bouirden, L. Microclimate and transpiration of a greenhouse banana crop. Biosyst. Eng. 2007, 98, 66–78. [Google Scholar] [CrossRef]
- Papadakis, G.; Frangoudakis, A.; Kyritsis, S. Experimental Investigation and Modelling of Heat and Mass Transfer between a Tomato Crop and the Greenhouse Environment. J. Agric. Eng. Res. 1994, 57, 217–227. [Google Scholar] [CrossRef]
- Yang, X. Greenhouse micrometeorology and estimation of heat and water vapour fluxes. J. Agric. Eng. Res. 1995, 61, 227–238. [Google Scholar] [CrossRef]
- Morille, B.; Migeon, C.; Bournet, P.E. Is the Penman-Monteith model adapted to predict crop transpiration under greenhouse conditions? Application to a New Guinea Impatiens crop. Sci. Hortic. 2013, 152, 80–91. [Google Scholar] [CrossRef]
- Katsoulas, N.; Stanghellini, C. Modelling Crop Transpiration in Greenhouses: Different Models for Different Applications. Agronomy 2019, 9, 392. [Google Scholar] [CrossRef]
- Campbell, G.S.; Norman, J.M. An Introduction to Environmental Biophysics; Springer: Berlin/Heidelberg, Germany, 1998; ISBN 0387949372. [Google Scholar]
- de Pury, G.G.; Farquhar, G.D. Simple scaling of photosynthesis from leaves to canopies without the errors of big-leaf models. Plant. Cell Environ. 1997, 20, 537–557. [Google Scholar] [CrossRef]
- Monteith, J.; Unsworth, M. Principles of Environmental Physics: Plants, Animals, and the Atmosphere, 5th ed.; Academic Press: Cambridge, MA, USA, 2013; ISBN 9780123869104. [Google Scholar]
- Jones, H.G. Plants and Microclimate A Quantitative Approach to Environmental Plant Physiology; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Yang, R.; Friedl, M.A. Determination of roughness lengths for heat and momentum over boreal forests. Bound.-Layer Meteorol. 2003, 107, 581–603. [Google Scholar] [CrossRef]
- Nobel, P.S. Physicochemical and Environmental Plant Physiology, 5th ed.; Academic Press: San Diego, CA, USA, 2020; pp. 1–654. ISBN 978-0-12-819146-0. [Google Scholar]
- Cionco, R.M. A Mathematical Model for Air Flow in a Vegetative Canopy. J. Appl. Meteorol. Climatol. 1965, 4, 517–522. [Google Scholar] [CrossRef]
- Oliver, H.R. Wind profiles in and above a forest canopy. Q. J. R. Meteorol. Soc. 1971, 97, 548–553. [Google Scholar] [CrossRef]
- Goudriaan, J. Crop Micrometeorology a Simulation Study; Wageningen University and Research: Wageningen, The Netherlands, 1977; ISBN 902200614X. [Google Scholar]
- Bodin, P.; Franklin, O. Efficient modeling of sun/shade canopy radiation dynamics explicitly accounting for scattering. Geosci. Model Dev. 2012, 5, 535–541. [Google Scholar] [CrossRef]
- Monteith, J. Evaporation and Environment. Symposia of the Society for Experimental Biology. Symp. Soc. Exp. Biol. 1965, 19, 205–234. [Google Scholar]
- Ou, L.J.; Zhang, Z.Q.; Dai, X.Z.; Zou, X. Photooxidation Tolerance Characters of a New Purple Pepper. PLoS ONE 2013, 8, e63593. [Google Scholar] [CrossRef] [PubMed]
- Fitter, A.H.; Hay, R.K.M. Environmental Physiology of Plants, 3rd ed.; Academic Press: London, UK, 2002; pp. v–vi. ISBN 978-0-12-257766-6. [Google Scholar]
- KOZLOWSKI, T.T. Water Deficits and Plant Growth; Academic Press: Cambridge, MA, USA, 1983. [Google Scholar]
- Ball, J.T.; Wood, I.E.; Berry, J.A. A Model Predicting Stomatal Conductance and its Contribution to the Control of Photosynthesis Under Different Environmental Conditions. Prog. Photosynth. Res. 1987, IV, 221–224. [Google Scholar]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef]
- Buckley, T.N.; Mott, K.A. Modelling stomatal conductance in response to environmental factors. Plant Cell Environ. 2013, 36, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Caird, M.A.; Richards, J.H.; Donovan, L.A. Nighttime stomatal conductance and transpiration in C3 and C4 plants. Plant Physiol. 2007, 143, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Shafizadeh, F.; Fu, Y. Pyrolysis Of cellulose. Carbohydr. Res. 1973, 29, 113–122. [Google Scholar] [CrossRef]
- Fu, Q.S.; Zhao, B.; Wang, Y.J.; Ren, S.; Guo, Y.D. Stomatal development and associated photosynthetic performance of capsicum in response to differential light availabilities. Photosynthetica 2010, 48, 189–198. [Google Scholar] [CrossRef]
- Knapp, A.K.; Smith, W.K. Stomatal and photosynthetic responses to variable sunlight. Physiol. Plant. 1990, 78, 160–165. [Google Scholar] [CrossRef]
- Dai, Y.; Dickinson, R.E.; Wang, Y.P. A two-big-leaf model for canopy temperature, photosynthesis, and stomatal conductance. J. Clim. 2004, 17, 2281–2299. [Google Scholar] [CrossRef]
- Jifon, J.L.; Syvertsen, J.P. Erratum: Moderate shade can increase net gas exchange and reduce photoinhibition in citrus leaves (Tree Physiology 22 (1079–1092)). Tree Physiol. 2003, 23, 719. [Google Scholar] [CrossRef]
- Heuvelink, T.K.E. Plant Physiology in Greenhouses; Horti-Text: Woerden, The Netherland, 2015; ISBN 9789082332506. [Google Scholar]
- Ilahi, W.F.F. Evapotranspiration Models in Greenhouse. Master Thesis, Agricultural Bioresearch Engineering, Wageningen University, Wageningen, The Netherlands, 2009; p. 52. [Google Scholar]
- Kozlov, K.K.; Shaliapin, V.G.; Mamontov, V.V.; Filippov, A.A.; Ostroukhov, N.F.; Korzhuk, M.S.; Pilipenko, A.P. Effect of aerodynamic resistance on energy balance and Penman-Monteith estimates of evapotranspiration in greenhouse conditions. Agric. For. Meteorol. 1992, 58, 209–228. [Google Scholar]
- Seginer, I. The Penman-Monteith evapotranspiration equation as an element in greenhouse ventilation design. Biosyst. Eng. 2002, 82, 423–439. [Google Scholar] [CrossRef]
- Willits, D.H. The Penman-Monteith Equation As a Predictor of Transpiration in a Greenhouse Tomato Crop. Soc. Eng. Agric. food, Biol. Syst. 2013, 0300, 034095. [Google Scholar] [CrossRef]
- Valdés, H.; Ortega-Farias, S.; Argote, M.; Leyton, B.; Olioso, A.; Paillán, H. Estimation of evapotranspiration over a greenhouse tomato crop using the penman-monteith equation. Acta Hortic. 2004, 664, 477–482. [Google Scholar] [CrossRef]
- Yan, H.; Acquah, S.J.; Zhang, C.; Wang, G.; Huang, S.; Zhang, H.; Zhao, B.; Wu, H. Energy partitioning of greenhouse cucumber based on the application of Penman-Monteith and Bulk Transfer models. Agric. Water Manag. 2019, 217, 201–211. [Google Scholar] [CrossRef]
- Kustas, W.P.; Choudhury, B.J.; Kunkel, K.E.; Gay, L.W. Estimate of the aerodynamic roughness parameters over an incomplete canopy cover of cotton. Agric. For. Meteorol. 1989, 46, 91–105. [Google Scholar] [CrossRef]
- Verhoef, A.; McNaughton, K.G.; Jacobs, A.F.G. A parameterization of momentum roughness length and displacement height for a wide range of canopy densities. Hydrol. Earth Syst. Sci. 1997, 1, 81–91. [Google Scholar] [CrossRef]
- Jacobs, A.F.G.; Van Boxel, J.H. Changes of the displacement height and roughness length of maize during a growing season. Agric. For. Meteorol. 1988, 42, 53–62. [Google Scholar] [CrossRef]
- Hatfield, J.L. Aerodynamic properties of partial canopies. Agric. For. Meteorol. 1989, 46, 15–22. [Google Scholar] [CrossRef]
- Stanghellini, C. Transpiration of Greeenhouse Crops an Aid to Climate Management; Institute of Agricultural Engineering (IMAG): Wageningen, The Netherlands, 1987. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration (Guidelines for Computing Crop Water Requirements); FAO Irrigation and Drainage Paper No. 56; Food and Agriculture Organisation of the United Nations: Rome, Italy, 1998; Volume 300. [Google Scholar]
- Perera, R.S.; Cullen, B.R.; Eckard, R.J. Using leaf temperature to improve simulation of heat and drought stresses in a biophysical model. Plants 2020, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Penman, H.L. Natural evaporation from open water, bare soil and grass. Proc. R. Soc. Lond. Ser. A. Math. Phys. Sci. 1948, 193, 120–145. [Google Scholar]
- An, S.; Park, S.W.; Kwack, Y. Growth of cucumber scions, rootstocks, and grafted seedlings as affected by different irrigation regimes during cultivation of ‘joenbaekdadagi’ and ‘heukjong’ seedlings in a plant factory with artificial lighting. Agronomy 2020, 10, 1943. [Google Scholar] [CrossRef]
- Lee, S.-B.; Lee, I.-B.; Homg, S.-W.; Seo, I.-H.; Bitog, P.J.; Kwon, K.-S.; Ha, T.-H.; Han, C.-P. Prediction of Greenhouse Energy Loads using Building Energy Simulation (BES). J. Korean Soc. Agric. Eng. 2012, 54, 113–124. [Google Scholar] [CrossRef]
- Seong No, L. Design of a Greenhouse Energy Model Including Energy Exchange of Internal Plants and Its Application for Energy Loads Estimation; Seoul National University Graduate School: Seoul, Korea, 2017. [Google Scholar]
- Chou, S.K.; Chua, K.J.; Ho, J.C.; Ooi, C.L. On the study of an energy-efficient greenhouse for heating, cooling and dehumidification applications. Appl. Energy 2004, 77, 355–373. [Google Scholar] [CrossRef]
- Van Beveren, P.J.M.; Bontsema, J.; Van Straten, G.; Van Henten, E.J. Minimal heating and cooling in a modern rose greenhouse. Appl. Energy 2015, 137, 97–109. [Google Scholar] [CrossRef]
- Iddio, E.; Wang, L.; Thomas, Y.; McMorrow, G.; Denzer, A. Energy efficient operation and modeling for greenhouses: A literature review. Renew. Sustain. Energy Rev. 2020, 117, 109480. [Google Scholar] [CrossRef]
- Kephe, P.N.; Ayisi, K.K.; Petja, B.M. Challenges and opportunities in crop simulation modelling under seasonal and projected climate change scenarios for crop production in South Africa. Agric. Food Secur. 2021, 10, 10. [Google Scholar] [CrossRef]
- Savary, S.; Teng, P.S.; Willocquet, L.; Nutter, F.W. Quantification and modeling of crop losses: A review of purposes. Annu. Rev. Phytopathol. 2006, 44, 89–112. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.J.; Squire, G.R. Comparative measurements with two water vapour diffusion porometers (dynamic and steady-state). J. Exp. Bot. 1981, 32, 1143–1156. [Google Scholar] [CrossRef]
- Toro, G.; Flexas, J.; Escalona, J.M. Contrasting leaf porometer and infra-red gas analyser methodologies: An old paradigm about the stomatal conductance measurement. Theor. Exp. Plant Physiol. 2019, 31, 483–492. [Google Scholar] [CrossRef]
- Idso, S.B.; Allen, S.G.; Choudhury, B.J. Problems with porometry: Measuring stomatal conductances of potentially transpiring plants. Agric. For. Meteorol. 1988, 43, 49–58. [Google Scholar] [CrossRef]
- McDermitt, D.K. Sources of Error in the Estimation of Stomatal Conductance and Transpiration from Porometer Data. HortScience 1990, 25, 1538–1548. [Google Scholar] [CrossRef]
- Lombardini, L.; Restrepo-Diaz, H.; Volder, A. Photosynthetic light response and epidermal characteristics of sun and shade pecan leaves. J. Am. Soc. Hortic. Sci. 2009, 134, 372–378. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Babani, F.; Navrátil, M.; Buschmann, C. Chlorophyll fluorescence kinetics, photosynthetic activity, and pigment composition of blue-shade and half-shade leaves as compared to sun and shade leaves of different trees. Photosynth. Res. 2013, 117, 355–366. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.W.; Ahn, T.I.; Shin, J.H.; Park, K.S.; Son, J.E. Sweet pepper (Capsicum annuum L.) canopy photosynthesis modeling using 3D plant architecture and light ray-tracing. Front. Plant Sci. 2016, 7, 1321. [Google Scholar] [CrossRef]
- Durand, M.; Murchie, E.H.; Lindfors, A.V.; Urban, O.; Aphalo, P.J.; Robson, T.M. Diffuse solar radiation and canopy photosynthesis in a changing environment. Agric. For. Meteorol. 2021, 311, 108684. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Van Haaften, M.; Li, L.; Lu, S.; Wen, W.; Zheng, X.; Pan, J.; Qian, T. Interactions between Diffuse Light and Cucumber (Cucumis sativus L.) Canopy Structure, Simulations of Light Interception in Virtual Canopies. Agronomy 2022, 12, 602. [Google Scholar] [CrossRef]






| Intercept | Penetrate | ||||
|---|---|---|---|---|---|
| Sunlit | Beam | (21) | (26) | ||
| Scattered diffuse | (22) | (27) | |||
| Scattered beam | (23) | (28) | |||
| Shade | Scattered diffuse | (24) | (29) | ||
| Scattered beam | (25) | (30) | |||
| Sensor | Picture | Measurement | Accuracy | Operating Temperature | Model Name | Manufacturer |
|---|---|---|---|---|---|---|
| Pyranometer |  | Spectral range: 285~3000 nm | ±5% | -40~80 °C | SR05 | Hukseflux |
| -10~40 °C | LI-19 | |||||
 | Spectral range: 385~2105 nm | ±5% | -50~80 °C | SP-510-SS | Apogee | |
| Thermometer, hygrometer |  | Air temperature | ±0.2% | -40~80 °C | ATMOS 14 | Meter |
| Air humidity | ±2% | 0~80 °C, 100% RH | ||||
| Aspirated radiation shield | - | -40~70 °C | TS-100 | Apogee | ||
| Infrared sensor |  | Canopy temperature, spectral range: 8~14 μm | 0.2 °C at -30~65 °C | -50~80 °C, 100% RH | SI-111-SS | Apogee |
| 0.5 °C at -40~80 °C | ||||||
| Porometer |  | Leaf conductance range: 0~1000 mmol m−2 s−1 | ±10% at 0~500 mmol | 5~40 °C, 100% RH | SC-1 | Meter |
| Ceptometer |  | Leaf area index, Spectral range: 400~700 nm | ±5% | 0~50 °C, 100% RH | LP-80 | Meter |
| Anemometer |  | Wind velocity | ±3% | 0~50 °C | TES1340 | TES |
| CO2 |  | Carbon dioxide | ±40 ppm | -40~60 °C | GMP-252 | Vaisala |
| Data logger |  | Data logging | Processor: 32 bit, 100 MHz | -40~80 °C | CR1000X | Campbell scientific |
| Item | Symbol | Value | Unit | Remark |
|---|---|---|---|---|
| LAI | L | 2.50 | - | Measure |
| Wind speed a | u(z) | 0.80 | m s−1 | Measure |
| Covering material Transmissivity b | τcv.b | 0.90 | - | Specification of manufacturer |
| τcv.d | 0.90 | - | ||
| τcv.bs | 0.90 | - | ||
| Canopy view factor | Fb | cosΨ | - | Campbell and Norman, 1998 |
| Fd | 1.00 | - | ||
| Fbs | 1.00 | - | ||
| Fa | 0.50 | |||
| Fe | 0.50 | - | ||
| Radiation absorptivity | αlf | 0.85 | - | Pury and Farqhar, 1997 |
| αsb c | - | - | ||
| αl | 0.95 | - | Campbell and Norman, 1998 | |
| αd | 0.95 | - | ||
| Leaf angle distribution | χ | 0.96 | - | |
| Canopy emissivity | εcpy | 0.95 | m | |
| Plant height d | h | 2.50 | m | Measure |
| Leaf width | w | 0.15 | m | Measure |
| Air temperature | Ta | - | °C | Measure |
| Relative humidity | RH | - | % | Measure |
| Stomatal conductance | - | mol m−2 s−1 | Measure | |
| - | Measure |
| Horizontal | Vertical | ||||||
|---|---|---|---|---|---|---|---|
| 5 Full a | Mid b | F c | Upper d | Lower e | F c | ||
| Ta | Upper air | 22.8 | 22.6 | 1.0 | 22.6 | 22.7 | 1.1 |
| Lower air | 22.9 | 22.8 | 1.0 | ||||
| RH | Upper air | 86.4 | 88.3 | 1.2 *** | 88.3 | 87.2 | 1.9 *** |
| Lower air | 86.3 | 87.2 | 1.1 ** | ||||
| CO2 | Upper air | 572.0 | 589.1 | 1.0 ** | 589.1 | 610.9 | 1.0 *** |
| Lower air | 583.6 | 610.9 | 1.0 ** | ||||
| Tcpy | Plant | 22.8 | 22.5 | 1.0 ** | - | - | - |
| R-Squared Score | Correlation Coefficient | RMSE | Residual Sum |
|---|---|---|---|
| 0.979 | 0.989 | 0.457 | −6.889 × 10−12 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, Y.; Cho, L.; Park, S.; Kim, S.; Lee, C.; Kim, D. Canopy Temperature and Heat Flux Prediction by Leaf Area Index of Bell Pepper in a Greenhouse Environment: Experimental Verification and Application. Agronomy 2022, 12, 1807. https://doi.org/10.3390/agronomy12081807
Jeon Y, Cho L, Park S, Kim S, Lee C, Kim D. Canopy Temperature and Heat Flux Prediction by Leaf Area Index of Bell Pepper in a Greenhouse Environment: Experimental Verification and Application. Agronomy. 2022; 12(8):1807. https://doi.org/10.3390/agronomy12081807
Chicago/Turabian StyleJeon, Youngkwang, Lahoon Cho, Sunyong Park, Seokjun Kim, Chunggeon Lee, and Daehyun Kim. 2022. "Canopy Temperature and Heat Flux Prediction by Leaf Area Index of Bell Pepper in a Greenhouse Environment: Experimental Verification and Application" Agronomy 12, no. 8: 1807. https://doi.org/10.3390/agronomy12081807
APA StyleJeon, Y., Cho, L., Park, S., Kim, S., Lee, C., & Kim, D. (2022). Canopy Temperature and Heat Flux Prediction by Leaf Area Index of Bell Pepper in a Greenhouse Environment: Experimental Verification and Application. Agronomy, 12(8), 1807. https://doi.org/10.3390/agronomy12081807





