Abstract
Free and glycosidically-bound aromatic characterization of 21 minority red grapevine varieties was carried out, along three consecutive vintages, using solid phase microextraction followed by gas chromatography-mass spectrometry methodology (SPME-GC-MS). The two main study aims were to evaluate the possibility of aromatically differentiated varieties based on their origin and to test the aromatic profile for being used as a chemotaxonomic tool. Based on the results obtained in this research, it would be also interesting to verify in future studies if this varietal diversity could translate into a diversification of quality products in the current globalized wine market. A volatile profile was established grouping aroma compounds into thirteen families: acids, alcohols, esters, C6 compounds, thiols, ketones, aldehydes, phenols, terpenes, C13-norisoprenoids, lactones, polycyclic aromatic hydrocarbons (PAHs), and sesquiterpenes. Significant differences were found among varieties for esters, phenols, terpenes, and total compounds in the free fraction and for alcohols, acids, C6 compounds, C13-norisoprenoids, terpenes, sesquiterpenes, and total compounds in the glycosidically-bound fraction. Subtle differentiation between different groups of varieties with common genetic origin was achieved by free aromatic profile (PCA) component analysis. Nevertheless, more in-depth studies are considered necessary to confirm the usefulness of the aromatic profile as a chemotaxonomic tool.
1. Introduction
Despite the large number of Vitis vinifera L. varieties grown along the world, estimated at least 6.000 [1], for production or research objectives, only 33 of these varieties occupy 50 % of the total worldwide vineyard surface [2]. Spain is the biggest viticulture surface area country in the world, occupying approximately 13% of the surface, and is the third-biggest country in wine production [3]. However, only 10 varieties occupy 75% of the total surface of this country, with two of them, ‘Airén’ and ‘Tempranillo’, occupying around 43% of it. The remaining 25% of the vineyard surface is occupied by those named minority varieties [2]. Many of them are being characterized nowadays with the main aim of discovering their oenological potential and recovering diversity by means of including them in the corresponding vineyard register.
The diverse Spanish viticultural panorama includes 96 Protected Designation of Origin (PDOs) and 42 Protected Geographical Indication (PGIs). Galicia, located in the NW of the Iberian Peninsula, is highlighted by its special climatic conditions, given that Atlantic and Mediterranean currents influence the territory depending on the mountain slopes and/or the distance from the sea. It is also a wine-growing area with small plots, and with centuries-old vines, many of which can only be worked by hand. This translates into a high cost in production and in the resulting wine. Sharing borders with Portugal, Galicia also shares certain climatic conditions with it, as well as some wine grapevine varieties and viticultural areas and traditional practices with the neighbouring Portugal [4]. Vineyard in Galicia is characterized by a varietal richness, a mixture of allochthonous and autochthonous grapevine varieties, mainly established in the area with the monastic order’s arrival in the Middle Ages [5]. However, because of the phytosanitary vineyard damages occasioned in the nineteen century by the phylloxera (Daktulosphaira vitifoliae (Fitch)), powdery mildew (Uncinula necator (Burr.)) and downy mildew (Plasmopara vitícola (Berk. & Curtis) Berl. & de Toni), the broad varietal diversity has remained relegated for decades by few of the most productive allochthonous varieties, mainly ‘Palomino’ and ‘Garnacha Tintorera’, and even by direct producer hybrids, which had led to the production of common quality wines for several years [6]. This situation was not an extraordinary fact that only affected Galicia; contrary to this, the phylloxera almost eradicated the European viticulture and triggered the most drastic change in viticultural practices in the last two centuries [7].
In contrast to this, a change in trend has been observed from a few decades ago up to these days, looking for the study of local ancient cultivars trying to promote the recovery of geographical origin varieties as a differentiation mechanism [8,9].
Climate change, wine market globalization, and overwhelming phytosanitary threats are some of the challenges that viticulture could face these days, which apart from the direct incomes that are produced from wine, it has also other important indirect benefits in terms of landscape conformation and ecosystem services, acting as a key factor in the cultural, environmental, and socioeconomic sectors in traditional viticulturist countries such as Spain [10]. With respect to climate change, Droulia et al. [11] predicted impacts on the phenology of up to 40 days in regions such as northern Iberia, with its corresponding negative impact over the ripening period, which was also described by Duchêne [12], who refers to genetic variability, among other options, to face an earlier ripening or to maintain a better sugar/acidity ratio, as temperature increases triggered an important rise in sugar and pH content while there is an acidity decrease. Because of that, varieties with a higher tartaric/malic ratio would be better adapted to warmer climatic conditions. At the same time, this last study also highlighted the genetic and phenotypic diversity embraced by germplasm banks and existing clone collections, as a useful strategy to ameliorate these threats, using new or different existing genotypes for both rootstock and scion. This varietal richness could be also useful to promote the regional wine typicity leading to product diversification in markets [13], which should also be complemented with new vinification guides that lead to the elaboration of less alcoholic degree wines without compromising other wine characteristics such as texture or sensory properties, to satisfy new consumer and market demands [14]. This increase in value in that minority varieties, would be also the best way to protect them from extinction [15]. In this same research line, Bigard et al. [16] showed that sugarless phenotype reaches higher malic/tartaric acid, resulting a more balanced composition in the berry and in the corresponding wine, being a future solution to climate change.
In Galicia, from the 80’s decade onwards, a varietal recovery program has been promoted by the autonomous community in terms of recovering the Galician wines’ quality and typicity. As a result of this, the ‘Estación de Viticultura y Enología de Galicia’ (EVEGA) vineyard germplasm bank was established in 1985 with the main objectives of maintaining, protecting, and recovering the diverse grapevine resources [17]. From that moment up to these days, the characterization of the grapevine varietal diversity in the Galician vineyard became a priority in terms of evaluating their potential to produce quality wines [18].
The genetic diversity of the EVEGA vineyard germplasm bank has been largely studied through SSRs analysis; as well, its phenotypic diversity has also been explored through the ampelographic and ampelometry description [18,19,20,21]. Nowadays varietal description is being completed with chemical characterization based on the phenolic description, which has been conducted, up to this moment, in the red varieties [22]. Given that the active aromatic compounds composition is one of the main differential strategies to distinguish wines based on their variety, vinification procedures or origin [23], the aromatic characterization both in white and red grapevine varieties is also being developed [24]. Their basic ripening physicochemical parameters are also being described, as one of the main objectives to avoid the negative impact of climate change would be to maintain good acidity values at harvest with a good equilibrium among both main organic acids of the berry [25].
In this study, a deep aromatic composition characterization of 21 red grapevine varieties of traditional cultivation in Galicia, has been developed.
The main aims of this research were: (1) to evaluate if it is possible to aromatically differentiate them based on their origin, and (2) to test if their aromatic profile could be a useful chemotaxonomic tool for the differentiation and classification of grapevine varieties comparing their aromatic and genetic population classification.
Finally, a global discussion of the results obtained in this, and other similar research works will determine if this varietal biodiversity could provide tools for the viticulturist and oenologist to elaborate alternative high-quality wines by applying the best viticulture and elaboration procedures for each variety depending on their aromatic profiles.
2. Materials and Methods
2.1. Vegetal Material
Grapes from 21 red genotypes of Vitis vinifera L. from EVEGA germplasm bank: ‘Albarín Tinto’—VIVC code 277 [26], ‘Corbillón’—VIVC code 3612, ‘Espadeiro’—VIVC code 2107, ‘Evega 3′, ‘Evega 4′, ‘Evega 6′, ‘Ferrón’—VIVC code 7340, ‘Gran Negro’—VIVC code 5012, ‘Garnacha’—VIVC code 4980, ‘Híbrido’, ‘Mandón’—VIVC code 7326, ‘Mencía’—VIVC code 7623,‘Merenzao’—VIVC code 12668, ‘Moscatel de Hamburgo’—VIVC code 8226, ‘Mouratón’—VIVC code 8082, ‘Picapoll Negro’—VIVC code 9298, ‘Pan y Carne’—VIVC code 26281, ‘Pedral’—VIVC code 9078, ‘Tempranillo’—VIVC code 12350 and ‘Zamarrica’, were analyzed in three different vintages: 2015, 2016 and 2017. All varieties except for ‘Moscatel de Hamburgo’, which is a table grape, are wine grapes. ‘Híbrido’ is a direct producer hybrid that was used in the past for wine elaboration and varieties named ‘Evega 3, 4 and 6′ are not identified yet with any other variety already known [21]. Exceptionally ‘Xafardán’ was studied in 2019 and 2020 vintages as it entered later in the germplasm bank for being a new characterized variety.
Of the total varieties analyzed, ‘Espadeiro’, ‘Ferrón’, ‘Gran Negro’, ‘Mencía’, ‘Merenzao’, ‘Mouratón’, and ‘Pedral’ are included within the different PDO’ s of the community.
The experimental vineyard is in Ourense (42°21′34.5′′ N, 8°07′08.2′′ W, elevation 87 MAMSL), Galicia (Spain).
Vines are around 30 years old, grafted on 196-17C rootstock and trained into a vertical trellis. With a planting frame of 1.8 m between rows and 1.2 m within the row and faces East-West. Varieties are in duplicate plots, from 6 to 11 plants.
Grapes were weekly monitored from veraison to harvest. At ripening stage, around 20–23 °Brix and according to their health status, they were manually harvested. Approximately 500 berries were collected from the top, bottom, and central parts of the bunch. From those samples, 100 berries in duplicate were frozen at −20 °C until the aroma volatile compounds extraction. Two other aliquots of 100 berries were saved to obtain the corresponding must for the physicochemical parameter’s analysis.
2.2. Climatic Conditions
Meteorological conditions (mean, maximum and minimum temperature as well as rainfall) were registered by an automatic meteorological station (iMETOS, Pessl Instruments GmbH, Weiz, Austria) located in the same vineyard. Two climate indices related to thermal conditions, Heliothermal Index (HI) [27] and Cool Night index (CI) [28] were calculated.
2.3. Must Basic Chemical Composition
Analysis of the musts for the assessment of the oenological parameters was carried out as previously described by Díaz-Fernández et al. [24]. Two aliquots of 100 berries from the different varieties were crushed and obtained juices’ characteristics: TSS (°Brix), titratable acidity (g tartaric acidxL−1) and pH were determined by Fourier transform infrared spectrometry (FTIR, OENOFOSS ™, FOSS, Hilleroed, Denmark). Tartaric and malic acid (g·L−1) were determined by an autoanalyzer (LISA 2000, HYCEL DIAGNOSTICS, Massy, France). Two maturation indexes were estimated: Baragiola & Scuppli (MI-BS) and Cillis and Odifredi (MI-CO) [29] as well as the tartaric: malic acid relation.
2.4. Volatile Composition
2.4.1. Chemicals
Internal standards and referenced compounds were from Sigma-Aldrich (Steinheim, Germany). Pure water was obtained from a Mili-Q purification system (Milipore, Bedford, MA, USA). Dichloromethane, absolute ethanol, and methanol were purchased from Merck (Darmstadt, Germany).
Fibers used for volatile fractions analysis were Divinylbenzene/Carboxen/Polydimethylsiloxane (DVB/CAR/PDMS) of 2 cm 50/30 μm were purchased from Supelco, Bellefonte, PA, USA; C-18 cartridges (Hypersep Spe 1000 mg C-18) were from Thermo Scientific, Waltham, MA, USA and enzyme AR2000 from Rapidase, DSM food specialties, Seclin, France.
Compounds separation, identification and semi-quantification were carried out on a GC 7820 A gas chromatograph (Agilent Technologies, Santa Clara, CA, USA), with a ZB-Wax column (Phenomenex; 60 m 0.25 mm 0.25 m film thickness), coupled with a 5975 Series MSD, Agilent mass spectrometer detector.
2.4.2. Determination of Varietal Volatile Compounds (SPME-GC-MS)
Free and bound volatile compounds were determined according to the methodology described by Díaz-Fernández et al. [24].
Free volatile compounds were directly extracted from a 10 mL vial containing 5 mL of clear juice, 20 µL of each internal standard, 3-octanol (1 g·L−1 in ethanol) and 4-methyl-2-pentanol (10 mg·L−1 in ethanol), 1.5 g of NaCl and a magnetic stirrer. Vials were equilibrated at 60 °C for 2 min and 500 rpm in a water bath.
For the glycosidically bound fraction, volatile compounds were previously separated in a 1 g C-18 cartridge using 25 mL of clear juice diluted 1:1 with distilled water. Free volatiles were released with 10 mL of dichloromethane and water-soluble compounds were washed with 25 mL of distilled water. Finally, glycosidically bound compounds were released with 10 mL of methanol which evaporated afterwards, and the sample was reconstituted with 5 mL of citrate-phosphate buffered solution. A total of 200 µL of the enzyme AR 2000 was added and enzymatic hydrolysis was carried out for 16 h at 40 °C. Afterwards, the obtained extracts containing the hydrolyzed bound compounds were prepared in the same way as the sample of free compounds.
The extraction and desorption of free and released glycosidically bound compounds were carried out by Solid Phase Microextraction (SPME), using the previously conditioned fiber for 25 min at 60 °C and 500 rpm. Samples desorption was carried out in the injection port of the gas chromatograph at 250 °C for 5 min in splitless mode.
Volatile compounds separation, identification, and semi-quantification were carried out on a GC 7820 A gas chromatograph (Agilent Technologies, Santa Clara, CA, USA) coupled with a 5975 Series MSD, Agilent mass spectrometer detector in chromatographic conditions reported in a previous paper [24].
2.4.3. Statistical Analysis
Data were subjected to one-way analysis of variance (ANOVA) using XLstat-Basic+ (Addinsoft, Paris, France) with the objective of identifying if there were significant differences among the values obtained for the three vintages mean value of each parameter analysed. Afterwards, Fisher’s least significant difference method was applied for significance of comparisons. Pearson’s correlations between climatic parameters and oenological and volatile compounds were also calculated. Principal component analysis (PCA) was applied to attempt the separation of the 21 grape varieties according to their content in free and glycosidically bound compounds.
3. Results & Discussion
3.1. Climatic Conditions
Figure 1 reflects that temperatures were quite similar among the three different vintages, while 2016 was a much rainier year than 2015 and 2017 with 1211 mm against 659 and 869 mm, respectively, and also had almost double the number of days with mean temperatures rising higher than 35 °C than the other two vintages [24].
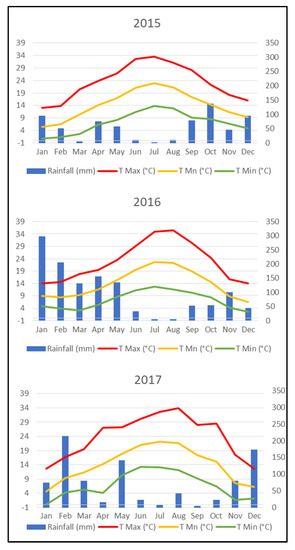
Figure 1.
Climatic conditions in 2015, 2016, and 2017 vintages. Maximum T, Mean T and Minimum T are average values of the maximum, mean and minimum temperatures respectively.
Heliothermal Index (HI) values showed that the three vintages were characterized by warm climate (HI + 2) and Cool Night Index (CI) showed that 2015 had cool nights (CI + 1) while 2016 and 2017 showed very cool nights (CI + 2) [24].
3.2. Must Basic Chemical Composition
Table 1 shows all varieties analyzed with their corresponding analytical maturity parameters. Every variety was harvested on different dates for their optimal maturation and sanitary state.

Table 1.
Oenological parameters of the grapes in 2015, 2016 and 2017 vintages.
Intended °Brix were among 20–23 °Brix, with every variety being around those values except for ‘Merenzao’, ‘Pan y Carne’, and ‘Xafardán’, with 24.2, 25.2, and 26.1 °Brix, respectively, as the highest values, because of their quite earlier maturation rates, and in the opposite side ‘Gran Negro’ with 19.2 °Brix.
ANOVA showed significant differences for every parameter studied among varieties except for the Baragiola & Scuppli (MI-BS) maturation index, which shows the tartaric acid percentage with respect to the total acidity. Nevertheless, tartaric acid and the ratio among tartaric and malic acid showed lower significant differences than for the sugar and °Brix degree. Tartaric acid was among 4.6 g·L−1 in ‘Mouratón’, with the lowest value, and 7.8 g·L−1 in ‘Xafardán’, which despite achieving quite a high °Brix, is capable of maintaining high acid values. Malic acid had a higher variability range than the tartaric one, being 1.3 g·L−1 in ‘Garnacha’ and 7.6 g·L−1 in ‘Ferrón’ with the highest value. According to the De Cillis and Odifredi maturation index, which assesses the relationship between sugar and total acidity, almost every variety was among the correct theoretical range of industrial maturity, which is established between three and five points depending on the variety considered.
3.3. Volatile Composition
370 volatile compounds were identified among the 21 varieties studied, 191 identified as free forms and 294 as glycosidically bound compounds. Compounds were grouped in thirteen volatile families: alcohols, acids, esters, phenols, thiols, C6 compounds, aldehydes, ketones, terpenes, sesquiterpenes, C13-norisoprenoids, polycyclic aromatic hydrocarbons (PAH’s) and lactones.
Table 2 shows the results obtained for the free volatile compounds identified in the 21 grape varieties. C6 compounds in the free fraction were the highest contribution family in all varieties, except for ‘Moscatel de Hamburgo’, in which the family of terpenes showed the greatest contribution to the volatile profile of this aromatic variety.

Table 2.
Free volatile compounds (values are expressed as µg·L−1).
Table 3 shows the results obtained for the bound compounds in the same 21 grape varieties. In this fraction, alcohols and phenols were the highest contribution families depending on the variety in their aromatic precursor fraction except for ‘Moscatel de Hamburgo’ in which terpene, in the same way as in the free fraction, was the family with the greatest contribution to the aroma profile.

Table 3.
Glycosidically bound fraction (Values are expressed as µg·L−1).
Table 2 shows, for each grape variety and vintage, the mean concentration of the volatile compounds detected in free form in the corresponding volatile family. To complete the above-mentioned results about the aroma profile of each variety and to establish similitudes or differences among them, Table 4 shows the major volatile compounds identified in each family, both in free and glycosidically bound form.

Table 4.
Major volatile compounds in free and bound form.
3.3.1. Free volatile composition
Results in Table 2 show significant differences among varieties for esters, phenols, and terpenes. Mean total free volatile content was also significantly different, being ‘Mandón’ the variety with the highest concentration (10,269 µg·L−1) while ‘Mouratón’ showed the lowest values (2699 µg·L−1).
The contribution of individual free-form volatile families to the global aromatic potential of each variety was estimated with their percentage out of the total of free volatile compounds (Table S1).
Terpenes are secondary plant metabolites that have a wide range of tasks among them; they are the communication between plants and other organisms, different defensive role strategies, or an important contribution to grapes and wines aroma [30]. They are known for being the axis of the sensory expression of wines, typical of its variety, being the reason why they could be used for varietal characterization. There are five major monoterpene alcohols, above all in Muscat varieties, geraniol, linalool, citronellol, nerol and α-terpineol [31].
In this study, terpenes were detected in every variety in the free form, from which ‘Moscatel de Hamburgo’ stood out with a very high total concentration of 5823 µg·L−1, distantly followed by ‘Zamarrica’ with 202 µg·L−1. Facing this, ‘Evega 6′ was the variety that showed the lowest mean terpenes content, less than 1 µg·L−1.
Table 4 shows that linalool was the major terpene in ‘Moscatel de Hamburgo’ and ‘Zamarrica’, described with floral and citrus odor [30], while cis geraniol, which contributes to floral and fruity notes [32], was the major one in ‘Albarín Tinto’, ‘Espadeiro’, ‘Garnacha’ and ‘Picapoll Negro’. α terpinyl acetate, with sweet, lavender, and herbaceous floral odor [33] and different cymene isomers, such as p-cymene, which is considered to be the most important monoterpene in aromatic plants and possesses antifungal, antiviral, and antibacterial activities [34] and minty notes [35], were the major terpenes in the other five varieties studied.
Sesquiterpenes are a terpene subclass with C15, generally present in plant essential oils. Aroma-active ones have been identified in numerous plant species. A total of 97 sesquiterpenes, identified in grapes, wine, and pomace have been recompiled by Li et al. [36], highlighting their importance to wine aroma profile and their potential health benefits.
In this research, sesquiterpenes were not detected in all varieties. The major ones identified were: 4βH-eudesmane, with eudesmane skeleton [36] in ‘Corbillón’, ‘Evega 6′ and ‘Merenzao’, humulene epoxide II in ‘Evega 4′, ‘Híbrido’ and ‘Tempranillo’, caryophyllene oxide in ‘Picapoll Negro’, aromadendrene oxide in ‘Pan y Carne’ and ‘Zamarrica’, trans-Z-α Bisabolene epoxide in ‘Pedral’ and (Z)-β-Farnesene in ‘Xafardán’. α-caryophyllene was also identified by Perestrelo et al. [37] in ‘Bastardo’ grapes,
C13-norisoprenoids, varietal aroma compounds resulted from the breakage of carotenoids that are of high interest as their presence is thought to improve wine quality [38]. Table 4 shows that they were identified in almost every variety except for: ‘Evega 4′, ‘Gran Negro’, ‘Mencía’, and ‘Moscatel de Hamburgo’. α and β-damascenone, were the major norisoprenoids detected in the free fraction in the studied varieties (Table 4). α-damascenone is characterized by its sweet, fruity, and floral description [39], whereas β-damascenone, which generally has a direct significant impact on red wine aromas [40], possesses high floral intensity and red berry notes [38].
Organic acids are with terpenoids, tannins, and some precursors of aldehydes, thiols, and esters, one of the most important families of flavor and aroma compounds [41]. They improve the freshness of wines and help to equilibrate their fruity notes [42]. ‘Ferrón’ and ‘Mandón’ are the varieties with higher acid values, despite also having high standard deviations due to the very high content of this group of compounds, in both varieties, in 2015 vintage. Table 4 shows that hexanoic acid is the major one in most of the varieties studied.
C6 compounds are described with greasy and potentially herbaceous notes [41] and include alcohols and aldehydes derived from membrane lipids [43]. They were the major group of free volatile compounds in all varieties except for ‘Moscatel de Hamburgo’ in which terpenes are in higher concentration. Values ranged from 5033 µg·L−1 in ‘Evega 3′ with the highest value to 1391 µg·L−1 in ‘Moscatel de Hamburgo’ with the lowest one. Table 4 shows that, depending on the variety, major C6 compounds were 1-hexanol, hexanal and 2-hexenal, (E)-. These compounds are generally responsible for green or herbaceous aromas in wines.
In general, the group of alcohols contributed with a high content to the volatile profile in free form for most of the grape varieties studied. They are generally composed of n-alcohols of C6 chain length as well as aromatic compounds, mainly benzyl alcohol and 2-phenylethanol [44]. ‘Moscatel de Hamburgo’ stood out with the higher content (1148 µg·L−1) and ‘Xafardán’ with the lowest one (404 µg·L−1). The clear major compound almost in every variety was the 1-hexanol, 2-ethyl-, with citrus, fresh, floral, oily, and sweet aroma [39], except for ‘Corbillón’ and ‘Mandón’ with 1-heptanol, with musty, leafy, herbal, or green descriptors [39].
Aldehydes are organic compounds that are very common in different foods and as flavoring agents [45]. Saturated aldehydes such as hexanal, heptanal, or nonanal, have green, floral, and grassy aromas [46]. They were identified in low quantities almost in every variety, being ‘Ferrón’ and ‘Mandón’, the varieties with higher values, with 118 and 171 µg·L−1, respectively. ‘Mencía’ also showed higher aldehydes values, but as in the case of ‘Ferrón’, showed a high standard deviation between vintages.
Esters are related to the fruity character of wines [47]. They were not detected in ‘Evega 4′ neither in ‘Xafardán’. As could be expected, hexanoic acid, ethyl ester was the major compound in almost every variety studied, corresponding with hexanoic as the major acid in almost every variety.
Referring to phenols, many of them are particularly odor-active compounds and the more complex ones have the most desirable aroma qualities [48]. They were identified in all varieties apart from ‘Xafardán’, being estragole, a natural phenol present in different spices such as anise, fennel, basil, or tarragon [49] and 4,6-di-tert-Butyl-m-cresol major compounds as it could be seen in Table 4. Phenols generally have low content, being ‘Mandón’ the one with the highest content.
Ketones tend to appear in all varieties in a similar content, except for ‘Ferrón’ in which they were not detected. The most complex ketones tend to have a key role in aroma and, depending on their structure, they could have a broad range of aromas, such as blue cheese through 2-heptanone or mushroom notes in the case of 3-octanone [48]. They also generally present lower deviation values among years. The major ketone for every variety studied was methyl isobutyl ketone, which has been also identified in grapes and in other foods such as orange or lemon juice, vinegar, papaya, or ginger, among others [50]. Isobutyl ketone shows solvent-like, green, fruity, herbal, and dairy nuances [39].
Among PAH’s, ‘Mencía’ and ‘Moscatel de Hamburgo’ statistically stood out for being those varieties with higher content, being mesitylene and hemimellitene, both isomers of trimethylbenzene, the major compounds for the varieties studied.
Finally, lactones and thiols are those families in minor content. Lactones are cyclic esters that could provide aromas such as peachy, coconut, or creamy ones [48]. On the other hand, thiols, have been identified as key molecules of young wines, with a positive varietal aroma contribution [51]. Both tend to have also high deviations which could be related to big differences in the different vintages. Thiols were identified in ‘Corbillón’, ‘Evega 6′, ‘Mandón’, ‘Mencía’, ‘Merenzao’, ‘Moscatel de Hamburgo’, ‘Mouratón’, ‘Tempranillo’ and ‘Zamarrica’ while lactones appeared in all varieties except for ‘Garnacha’, ‘Híbrido’ and ‘Mouratón’, being furaneol the major one in almost all varieties (Table 4). Furaneol is a very interesting volatile compound regarding its olfactory properties, providing a strawberry or caramel-like odor depending on its concentration [52].
3.3.2. Glycosidically-Bound Volatile Composition
Table 3 shows, for each grape variety, the results obtained for the content of glycosidically bound volatile compounds grouped in the corresponding family. In the same way as for volatile compounds in free form, major compounds identified in each family for each variety, in their glycosidically bound form are listed in Table 4.
Significant differences were observed among varieties for alcohols, acids, C6 compounds, terpenes, sesquiterpenes, C13-norisoprenoids being terpenes the family with the biggest significant differences among varieties.
Table 3, shows that there is a significant broad range of contents among varieties for the total bound volatile compound’s concentration, being ‘Moscatel de Hamburgo’ the one with the highest content of 109,912 µg·L−1 against ‘Albarín Tinto’ with the lowest one, 15,684 µg·L−1.
The contribution of each volatile family with the components in bound form to the global aromatic potential of each variety was estimated with their percentage out of the total of free volatile compounds (Table S2) being alcohols and phenols the families that showed higher percentages, while thiols, lactones, PAH’s, and ketones were those showing lower percentages among the bound forms.
In most grapes, the total content of volatile compounds in bound form was more abundant than the corresponding concentration in the free one. Moreover, some volatile compounds were only detected in bound form, increasing the aroma profile of the corresponding variety after their enzymatic release, such as thiols and sesquiterpenes. Thiols were not detected in their free form for ‘Albarín tinto’, ‘Espadeiro’, ‘Evega 3′, ‘Evega 4′, ‘Ferrón’, ‘Gran Negro’, ‘Pedral’, ‘Pan y Carne’, ‘Picapoll Negro’ and ‘Xafardán’, whereas sesquiterpenes were not detected for ‘Albarín tinto’, ‘Espadeiro’, ‘Evega 3′, ‘Evega 6′, ‘Garnacha’, ‘Gran Negro’, ‘Mandón’, ‘Mencía’ and ‘Moscatel de Hamburgo’, however, both compound families were present in their bound form for those same varieties.
As well as with what happened with the free fraction, terpenes were detected in every variety, standing out once again with ‘Moscatel Hamburgo’, with its high content with 71,566 µg·L−1, having almost the same % of terpenes in the free fraction profile (62.9%) that in the bound fraction profile (68.6%) (Tables S1 and S2). ‘Zamarrica’ is the second variety with higher terpene content with 7264 µg·L−1. In the other side, ‘Evega 6′, ‘Mencía’ and ‘Corbillón’ were those with lower values, with 304, 416, and 449 µg·L−1, respectively. As it happened in the free fraction, both varieties with higher content of terpenes showed once again linalool as the major content terpene, being dihydro-citronellol the major terpene in most of the varieties studied (Table 4).
Sesquiterpenes in the bound form were identified in more varieties than the free ones, lacking once again in ‘Ferrón’. They were detected in neither ‘Evega 6′ or ‘Merenzao’.
Trans-Z-α-bisabolene epoxide and cis-bisabolene were the major sesquiterpenes in most varieties (Table 4), having both a bisabolene skeleton [36]. Trans-Z-α-bisabolene epoxide has been already identified in Merlot wines by Welke et al. [53], while α-bisabolene was a predominant sesquiterpene in ‘Bual’ grapes [37].
C13-norisoprenoids were not detected in their bound form in several varieties such as: ‘Evega 3′, ‘Evega 4′, ‘Evega 6′, ‘Ferrón’, ‘Gran Negro’, ‘Híbrido’, ‘Mencía’, ‘Merenzao’, ‘Moscatel de Hamburgo’, ‘Mouratón’ and ‘Picapoll Negro’. Summed to the other two major norisoprenoids detected in the free form, α and β-damascenone, dihydro-β-ionol, with a woody-flowery and camphoraceous odor [54] was identified in this fraction as an important compound in some varieties such as ‘Albarín tinto’, ‘Garnacha’, ‘Mandón’, ‘Pan y Carne’, ‘Tempranillo’ and ‘Xafardán’. C13-norisoprenoids are considered as those compounds with highest contribution in the aroma of wines that are made with non-aromatic grapes [55].
Organic acids content was not quite different among varieties except for ‘Moscatel de Hamburgo’, which showed the highest content with 6576 µg·L−1. Nonanoic and octanoic acids, with buttery, cheesy, and sweaty-like odors [42], were the major ones in this fraction.
C6 compounds showed lower contents than in the free fraction, in which they were the major family almost in every variety. In this case ‘Híbrido’ and ‘Mandón’ were the varieties with higher levels. 1-hexanol was the major compound in all varieties studied in their bound form (Table 4), with grass, cream and resinous odor descriptors associated [56].
Alcohols was the highest content family in: ‘Albarín tinto’, ‘Espadeiro’, ‘Ferrón’, ‘Garnacha’, ‘Mandón’, ‘Merenzao’, ‘Mouratón’, ‘Picapoll Negro’, ‘Pan y Carne’, ‘Tempranillo’ ‘Xafardán’ and ‘Zamarrica’, being ‘Merenzao’ the one with highest content. Phenylethyl alcohol, with rose and honey descriptors and benzyl alcohol, with roasted and toasted descriptors [57] were those major compounds in all varieties (Table 4).
Aldehydes where only not detected in ‘Garnacha’ and ‘Híbrido’, being present in the rest of varieties. ‘Zamarrica’ and ‘Evega 4’ stood out over the rest of varieties, with 2024 and 1152 µg·L−1. Benzaldehyde, with roasted and almond descriptors [58] or cherry and almond [48], was the major aldehyde in almost all varieties studied.
Esters showed much higher contents in the bound from than in the free one. ‘Evega 4’, ‘Híbrido’, ‘Moscatel de Hamburgo’ and ‘Zamarrica’ stand out for their high contents, statistically grouped. Salicylic acid methyl ester, a volatile compound with green and mint-like flavor nuances [58] and nonanoic acid methyl ester, with wine and coconut-like odor, and in low concentrations with a sweet and coconut-like flavor [54], were the major ones in all varieties studied (Table 4). As it happened in the free fraction, these results could be expected for being nonanoic acid the major organic acid in most of the varieties studied.
Together with alcohols, phenols family was the one with higher contents in the bound aromatic fraction for ‘Corbillón’, ‘Evega 3’, ‘Evega 4’, ‘Evega 6’, ‘Híbrido’ and ‘Mencía’. Despite this, they showed quite high deviation because of a high interannual difference. Phenol, 2,4-di-tert-butyl- and p-propyl guaiacol were the major phenols for almost all varieties studied.
Ketones were identified in all varieties studied and as it happened in the free aromatic form, methyl isobutyl ketone was the major ketone in almost every variety (Table 4).
PAH’s were detected in all varieties, in small contents being ‘Evega 4’ and ‘Moscatel de Hamburgo’ those varieties with lower contents while ‘Zamarrica’ showed the highest one.
In this fraction, lactones were only detected in three varieties: ‘Mencía’, ‘Mouratón’ and ‘Tempranillo’ with γ-undecalactone, γ-dodecalactone, with fruity and sweet floral descriptors [59] and 3,4,5-trimethyl dihydrofuran-2-one as major compounds respectively (Table 4).
Finally, thiols were not detected in ‘Garnacha’, ‘Híbrido’, ‘Merenzao’, ‘Moscatel de Hamburgo’ and ‘Zamarrica’ being for the rest of varieties 2-undecanethiol, 2-methyl- the major compound.
3.3.3. Pearson’s Correlation
Pearson’s correlations values (r) between climatic parameters and the % of volatile compounds in free and glycosidically bound forms in each family and with the oenological parameters are reported in Table 5.

Table 5.
Pearson’s correlation among climatic conditions, volatile content (%) and oenological parameters.
In a previous study, Antalick et al [60] showed the complex relation among the berry sugar acumulation (mg/berry) and the subsequent volatile profile of the wine. This resarch concluded that some metabolites are present in the grapes at diiferent stages harvest, irrespective of grape genotype and environment, whereas other volatile compounds depends on the climatic conditions and specially on the grape variety. According with this results the aroma composition of each grape can be predectible to obtain a determined sensory profile in the wine.
The results in Table 5 show that pH and total acidity were the only two oenological parameters correlated with the climatic conditions, mainly with the temperature.
With respect to the accumulation of volatile compounds in the grape, Table 5 shows that mean T was the climatic parameter with more significant correlation with the aroma composition. Mean T was positively correlated with % acids, % norisoprenoids and % sesquiterpenes in free form and with % aldehydes in bound form. However, this parameter was negatively correlated with % alcohols, % esters in free form and with % acids and PAH’s in bound form.
The results in Table 5, also showed that rainfall showed a negative influence in the contribution of norisoprenoids and sesquiterpenes in free form as well as days with mean temperature upper than 35 °C.
Maximum T was positively correlated with % thiols in grapes, both in free and bound form.
It was important to note that the contribution of terpenes in free and glycosidically bound form was not correlated with any climatic parameter, as were norisoprenoids and sesquiterpenes in bound form.
3.3.4. Aromatic Relationships between Varieties
To better interpret the results and looking to understand which kind of relationships are established among different varieties based on their aromatic profiles, two principal component analyses (PCA) were carried out. There were used the percentages of each aromatic compound family in their free or bound form, also known as free and bound aromatic profile, as was performed in a previous study [24]. Percentages instead of total concentration values were used, given that according to other kind of compounds’ studies, non-genetic factors had a higher influence on the quantitative amounts of compounds more than in their qualitative composition [61,62,63].
Annual means and not annual data would be used for the PCA’s construction given that the main aim of this study was to know which kind of relationships were established among varieties and not so much how yearly effect influenced the varieties distribution.
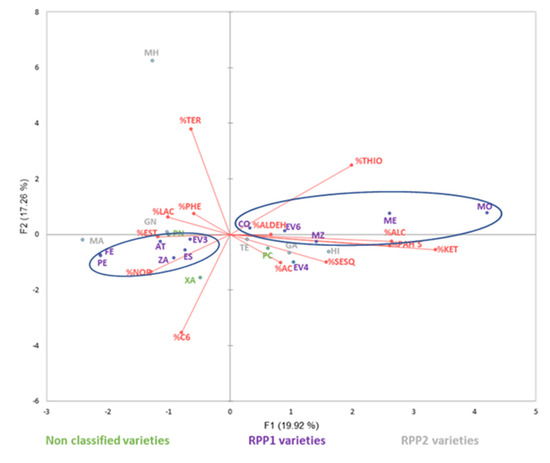
Figure 2.
Principal component analysis on free aromatic fraction profile (percentage) of grapes. NOR: norisoprenoids, AC: acids, EST: esters, KET: ketones, PAHs: polycyclic aromatic hydrocarbons, ALC: alcohols, ALDH: aldehydes, C6: C6 compounds, THIO: thiols, SESQ: sesquiterpenes, PHE: phenols, TER: terpenes, LAC: lactones. AT: ‘Albarín Tinto’, CO: ‘Corbillón’, ES: ‘Espadeiro’, EV3: ‘Evega 3’, EV4: ‘Evega 4’, EV6: ‘Evega 6’, FE: ‘Ferrón’, GN: ‘Gran Negro’, GA: ‘Garnacha’, HI: ‘Híbrido’, MA: ‘Mandón’, ME: ‘Mencía’, MZ: ‘Merenzao’, MH: ‘Moscatel de Hamburgo’, MO: ‘Mouratón’, PN: ‘Picapoll Negro’, PC: ‘Pan y Carne’, PE: ‘Pedral’, TE: ‘Tempranillo’, XA: ‘Xafardán’, ZA: ‘Zamarrica’. Varieties in purple correspond with Reconstructed Population (RPP) RPP1; varieties in grey correspond with RPP2 and varieties in green correspond with non-classified varieties in the genetical-geographical structure stablished by Díaz-Losada et al. [21].
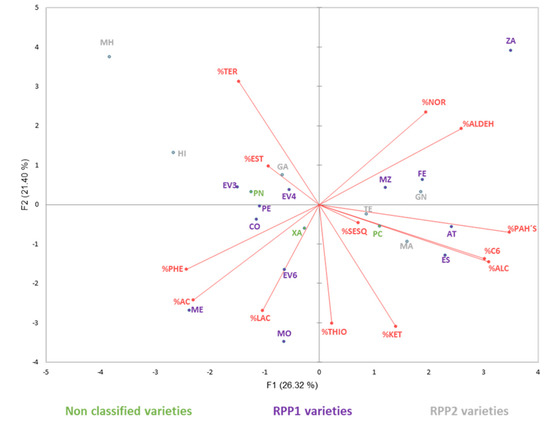
Figure 3.
Principal component analysis on glycosidically bound aromatic fraction profile (percentage) of grapes. NOR: norisoprenoids, AC: acids, EST: esters, KET: ketones, PAHs: polycyclic aromatic hydrocarbons, ALC: alcohols, ALDH: aldehydes, C6: C6 compounds, THIO: thiols, SESQ: sesquiterpenes, PHE: phenols, TER: terpenes, LAC: lactones. AT: ‘Albarín Tinto’, CO: ‘Corbillón’, ES: ‘Espadeiro’, EV3: ‘Evega 3’, EV4: ‘Evega 4’, EV6: ‘Evega 6’, FE: ‘Ferrón’, GN: ‘Gran Negro’, GA: ‘Garnacha’, HI: ‘Híbrido’, MA: ‘Mandón’, ME: ‘Mencía’, MZ: ‘Merenzao’, MH: ‘Moscatel de Hamburgo’, MO: ‘Mouratón’, PN: ‘Picapoll Negro’, PC: ‘Pan y Carne’, PE: ‘Pedral’, TE: ‘Tempranillo’, XA: ‘Xafardán’, ZA: ‘Zamarrica’. Varieties in purple correspond with Reconstructed Population (RPP) RPP1; varieties in grey correspond with RPP2 and varieties in green correspond with non-classified varieties in the genetical-geographical structure stablished by Díaz-Losada et al. [21].
In the first PCA (Figure 2), made with the percentages of the free aromatic fraction volatile families, the first two principal components explained 37.22% of the total variance.
The first PCA (Figure 2) achieves a good differentiation between varieties. From varieties included in the RPP1 [21], the aromatic profile of ‘Mouratón’, ‘Mencía’, ‘Evega 6′, ‘Corbillón’ and ‘Merenzao’ is mainly determined by thiol, ketones, PAHs and alcohols families while the one of ‘Evega 3′, ‘Espadeiro’, ‘Zamarrica’, ‘Albarín Tinto’, ‘Ferrón’ and ‘Pedral’ is mainly defined by ester, norisoprenoid, and C6 families.
With respect to the RPP2 varieties [21], the proximity among ‘Tempranillo’ and ‘Garnacha’ can be highlighted, because of the sesquiterpene family.
In the second PCA (Figure 3), made with the percentages of the bound aromatic fraction volatile families, the first two principal components explained 47.72% of the total variance.
Norisoprenoids, aldehydes, PAHs, C6 and alcohols mainly defined the aromatic profile of ‘Ferrón’, ‘Zamarrica’, ‘Albarín tinto’, ‘Espadeiro’, with these varieties being also grouped together in Figure 2. Phenols, acids, and lactons defined the aromatic profile of ‘Corbillón’, ‘Evega 6′, ‘Mouratón’ and ‘Mencía’, maintaining almost the same group as in Figure 2. From this last group, ‘Mencía’ and ‘Mouratón’ were also established in the same genetic lineage by Díaz-Losada et al. [19].
4. Conclusions
This aromatic study has achieved the characterization and differentiation of studied varieties. Varieties included in the RPP1 [21] maintained almost the same aggrupation in both PCAs (Figure 2 and Figure 3). Moreover, in this study, it was verified that there are minority grapevine varieties that are interesting for their higher aromatic potential, even more than some varieties already included in the community PDOs. They could contribute richness and diversity when making wines with differential qualities. This latter fact should still be corroborated through further studies of their corresponding monovarietal wines.
Supplementary Materials
The following supporting information can be downloaded at: www.mdpi.com/article/10.3390/agronomy12081799/s1, Table S1: Free aromatic fraction profile (Values are expressed as percentages) and Table S2: Glycosidically bound fraction profile (Values are expressed as percentages).
Author Contributions
Conceptualization, Á.D.-F., E.D.-L. and S.C.-D.; methodology, E.D.-L. and S.C.-D.; software, Á.D.-F. and S.C.-D.; validation, Á.D.-F., E.D.-L. and S.C.-D.; formal analysis, Á.D.-F. and S.C.-D.; investigation, Á.D.-F., E.D.-L. and S.C.-D.; resources, E.D.-L. and S.C.-D.; data curation, Á.D.-F. and S.C.-D.; writing—original draft preparation, Á.D.-F. and S.C.-D.; writing—review and editing, Á.D.-F., E.D.-L. and S.C.-D.; supervision, S.C.-D.; project administration, E.D.-L.; funding acquisition, E.D.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by financial assistance from the European Agricultural Fund for Rural Development (EAFRD), within the framework of sub-measure 10.2.2. “Conservation of genetic resources in agriculture” of the Galician Rural Development program, project number 12/32/408/280318/16. A.D.F. contract was funded by ‘Agencia Estatal de Investigación (AEI)–Ministerio de Ciencia e Innovación de España’, grant number BES-2017-082396.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or Supplementary Material.
Acknowledgments
A predoctoral contract in ‘Estación Enológica y de Viticultura de Galicia’ (EVEGA) supplied by ‘Ministerio de Ciencia e Innovación-Agencia Estatal de Investigación (AEI)’ is gratefully acknowledged by Angela Díaz Fernández. She also thanks to ‘BiotecnIA’ personal for welcoming and helping her during her several working periods in there.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lacombe, T. Contribution á l´étude de l´histoire Evolutive de la Vigne Cultivée (Vitis vinifera L.) par l´analyse de la Diversité Génétique Neuter et de Genes d´intérêt, Centre International d’Etudes Supérieures en Sciences Agronomiques, Montpellier SupAgro, 18 December 2012. Available online: https://www.supagro.fr/theses/extranet/12-0040_Lacombe.pdf (accessed on 30 March 2022).
- Organisation of Vine and Wine (OIV). Report on the World Vitivinicultural Situation. 2017. Available online: https://www.oiv.int/en/oiv-life/oiv-2017-report-on-the-world-vitivinicultural-situation (accessed on 10 January 2022).
- Organisation of Vine and Wine (OIV). State of the world vitivinicultural sector in 2020. 2021. Available online: https://www.oiv.int/es/normas-y-documentos-tecnicos/analisis-estadisticos/analisis-de-la-coyuntura (accessed on 10 January 2022).
- Blanco-Ward, D.; Queijeiro, J.M.G.; Jones, G.V. Spatial climate variability and viticulture in the Miño River Valley of Spain. Vitis 2007, 46, 63–70. [Google Scholar] [CrossRef]
- Huetz de Lemps, A. Vignobles et Vins du Nord-Ouest de l’Espagne; Impressions: Bordeaux, France, 1967. [Google Scholar]
- Vilanova, M.; Oliveira, J.M.; Rivas, R.; Alonso, J.C.; Martínez-Zapater, J.M.; Ibáñez, J.; Cacho, J. Primera parte: El sector vitivinícola gallego en el siglo XXI. El triunfo de las variedades de cultivo tradicional. In El Potencial Aromático de las Variedades de Vid Cultivadas en Galicia; Xunta de Galicia-Consellería de Medio Rural: Santiago de Compostela, Spain, 2017; Available online: https://libraria.xunta.gal/sites/default/files/downloads/publicacion/libro_vid_i_castellano.pdf (accessed on 23 May 2022).
- Tello, J.; Mammerler, R.; Čajić, M.; Forneck, A. Major outbreaks in the nineteenth century shaped grape phylloxera contemporary genetic structure in Europe. Sci. Rep. 2019, 9, 17540. [Google Scholar] [CrossRef]
- García-Muñoz, S.; Asproudi, A.; Cabello, F.; Borsa, D. Aromatic characterization and enological potential of 21 minor varieties (Vitis vinifera L.). Eur. Food Res. Technol. 2011, 233, 473. [Google Scholar] [CrossRef]
- Loureiro, M.D.; Moreno-Sanz, P.; Suárez, B. Agronomical characterization of minority grapevine cultivars from Asturias (Spain). Ciência Téc. Vitiv. 2017, 32, 102–114. [Google Scholar] [CrossRef]
- Santos, J.A.; Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Dinis, L.T.; Correia, C.; Moriondo, M.; Leolini, L.; Dibari, C.; Costafreda-Aumedes, S.; et al. A review of the potential climate change impacts and adaptation options for European viticulture. Appl. Sci. 2020, 10, 3092. [Google Scholar] [CrossRef]
- Droulia, F.; Charalampopoulos, I. Future climate change impacts on European viticulture: A review on recent scientific advances. Atmosphere 2021, 12, 495. [Google Scholar] [CrossRef]
- Duchêne, E. How can grapevine genetics contribute to the adaptation to climate change? Oeno One 2016, 50, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Antolín, M.C.; Toledo, M.; Pascual, I.; Irigoyen, J.J.; Goicoechea, N. The Exploitation of Local Vitis vinifera L. biodiversity as a valuable tool to cope with climate change maintaining berry quality. Plants 2021, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Pallioti, A.; Tombesi, S.; Silvestroni, O.; Lanari, V.; Gatti, M.; Poni, S. Changes in vineyard establishment and canopy management urged by earlier climate-related grape ripening: A review. Sci. Hortic. 2014, 178, 43–54. [Google Scholar] [CrossRef]
- Urrestarazu, J.; Miranda, C.; Santesteban, L.G.; Royo, J.B. Recovery and identification of grapevine varieties cultivated in old vineyards from Navarre (Northeastern Spain). Sci. Hortic. 2015, 191, 65–73. [Google Scholar] [CrossRef]
- Bigard, A.; Romieu, C.; Ojeda, H.; Torregrosa, L.J.-M. The sugarless grape trait characterised by single berry phenotyping. OENO One 2022, 56, 89–102. [Google Scholar] [CrossRef]
- Díaz-Losada, E.; Fernández, I.O.; Martínez, F.R.; Salgado, A.T.; Cabrer, A.R.; Lorenzo, S.P. A Colección de Vides da Estación de Viticultura e Enoloxía de Galicia; Xunta de Galicia-Consellería do Medio Rural: Santiago de Compostela, Spain, 2011; Available online: https://libraria.xunta.gal/sites/default/files/documents/11-0247_0.pdf (accessed on 15 February 2022).
- Díaz-Losada, E.; Cortés-Diéguez, S.; Rodríguez-Torres, I.; Mirás-Avalos, J.M.; Orriols-Fernández, I.; Pereira-Lorenzo, S. Characterization of the nearly extinct ‘Albilla’ cultivar from Galicia and its relationships with other Spanish ‘Albillos’. J. Int. Sci. Vigne Vin 2013, 47, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Losada, E.; Salgado, A.T.; Ramos-Cabrer, A.M.; Río Segade, S.; Diéguez, S.C.; Pereira-Lorenzo, S. Twenty microsatellites (SSRs) reveal two main origins of variability in grapevine cultivars from Northwestern Spain. Vitis. 2010, 49, 55–62. [Google Scholar] [CrossRef]
- Díaz-Losada, E.; Salgado, A.T.; Ramos-Cabrer, A.M.; Pereira-Lorenzo, S. Determination of genetic relationships of Albariño and Loureira cultivars with the Caíño group by microsatellites. Am. J. Enol. Viticult. 2011, 62, 3. [Google Scholar] [CrossRef]
- Díaz-Losada, E.; Salgado, A.T.; Ramos-Cabrer, A.M.; Díaz-Hernández, B.; Pereira-Lorenzo, S. Genetic and geographical structure in grapevines from northwestern Spain. Ann. Appl. Biol. 2012, 161, 24–35. [Google Scholar] [CrossRef]
- Díaz-Fernández, A.; Díaz-Losada, E.; Moreno, D.; Valdés, M.E. Anthocyanin profile of Galician endangered varieties. A tool for varietal selection. Food Res. Int. 2022, 154, 110983. [Google Scholar] [CrossRef]
- Furdíková, K.; Bajnoclová, L.; Malík, F.; Špánik, I. Investigation of volatile profile of varietal Gewürztraminer wines using two-dimensional gas chromatography. J. Food Nutr. Res. 2017, 56, 73–85. Available online: https://www.vup.sk/en/download.php?bulID=1927 (accessed on 4 December 2021).
- Díaz-Fernández, A.; Díaz-Losada, E.; Cortés-Diéguez, S. Approach to the chemotaxonomic characterization of traditional cultivation grape varieties through their varietal aroma profiles. Foods 2022, 11, 1427. [Google Scholar] [CrossRef]
- Frioni, T.; Bertoloni, G.; Squeri, C.; Garavani, A.; Ronney, L.; Poni, S.; Gatti, M. Biodiversity of local Vitis vinifera L. germplasm: A powerful tool toward adaptation to global warming and desired grape composition. Front. Plant Sci. 2020, 11, 608. [Google Scholar] [CrossRef]
- Vitis International Variety Catalogue VIVC. Available online: https://www.vivc.de/ (accessed on 14 April 2022).
- Huglin, P. Nouveau mode d´évaluation des possibilités héliothermiques d´en milieu viticole. Comptes Rendus Acad. Agric. Fr. 1978, 64, 1117–1126. [Google Scholar]
- Tonietto, J.; Carbonneau, A. A multicriteria climatic classification system for grape growing regions worldwide. Agric. Meteolo. 2004, 124, 81–97. [Google Scholar] [CrossRef] [Green Version]
- Togores, J.H. Chapter 3: Vendimia. Recepción de uva en la bodega. In Tomo I: Tratado de Enología, 2nd ed; Mundi-Prensa Libros: Madrid, Spain, 2010. [Google Scholar]
- Black, C.A.; Paarker, M.; Siebert, T.E.; Capone, D.L.; Francis, I.L. Terpenoids and their role in wine flavour: Recent advances. Aust. J. Grape Wine Res. 2015, 21, 582–600. [Google Scholar] [CrossRef]
- Mateo, J.J.; Jiménez, M. Review: Monoterpenes in grape juice and wines. J. Chromatogr. A 2000, 881, 557–567. [Google Scholar] [CrossRef]
- Porat, R.; Deterre, S.; Giampaoli, P.; Plotto, A. Chapter 1: The flavour of citrus fruits. In Biotechnology in Flavour Production, 2nd ed.; Havkin-Frenkel, D., Dudai, N., Eds.; Wiley: Hoboken, NJ, USA, 2016; Available online: https://media.wiley.com/product_data/excerpt/60/11183540/1118354060-77.pdf (accessed on 14 April 2022).
- Vaičiulytė, V.; Ložienė, K.; Švedienė, J.; Raudonienė, V.; Paškevičius, A. α-Terpinyl acetate: Occurrence in essential oils bearing Thymus pulegioides, Phytotoxicity and antimicrobial effects. Molecules 2021, 26, 1065. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Arciola, C.R.; Barbieri, R.; Silva, A.S.; Nabavi, S.F.; Tsetegho Sokeng, A.J.; Izadi, M.; Jafari, N.J.; Suntar, I.; Daglia, M.; et al. Update on monoterpenes as antimicrobial agents: A particular focus on p-Cymene. Materials 2017, 10, 947. [Google Scholar] [CrossRef]
- Ruiz Pérez-Cacho, P.; Rouseff, R. Processing and storage effects on orange juice aroma: A review. J. Agric. Food Chem. 2008, 56, 9785–9796. [Google Scholar] [CrossRef]
- Li, Z.; Howell, K.; Fang, Z.; Zhang, P. Sesquiterpenes in grapes and wines: Occurrence, biosynthesis, functionality, and influence of winemaking process. Compr. Rev. Food Sci. Food Saf. 2019, 19, 247–281. [Google Scholar] [CrossRef]
- Perestrelo, R.; Barros, A.S.; Câmara, J.S.; Rocha, S.M. In depth search focused on furans, lactones, volatile phenols and acetal as potential age markers of Madeira wines by comprenhensive two-dimensional gas chromatography with time-of-flight mass spectrometry combined with solid pase microextraction. J. Agric. Food Chem. 2011, 59, 3186–3204. [Google Scholar] [CrossRef] [Green Version]
- Tomasino, E.; Bolman, S. The potential effect of β-Ionone and β-damascenone on sensory perception of Pinot Noir wine aroma. Molecules 2021, 26, 1288. [Google Scholar] [CrossRef]
- The Good Scent Company. Available online: https://www.thegoodscentcompany.com (accessed on 20 May 2022).
- Pineau, B.; Barbe, J.C.; Van Leeuwen, C.; Dubordieu, D. Which impact for β-damascenone on red wines aroma? J. Agric. Food Chem. 2007, 55, 4103–4108. [Google Scholar] [CrossRef]
- González-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. Wine aroma compounds in grapes: A critical review. Crit. Rev. Food Sci. Nutr. 2015, 55, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Etievant, P. Wine. In Volatile Compounds in Foods and Beverages; Maarse, H., Ed.; Marcel Dekker: New York, NY, USA, 1991; pp. 483–546. [Google Scholar]
- Oliveira, J.M.; Faria, M.; Sá, F.; Barros, F.; Araújo, I.M. C6-alcohols as varietal markers for assessment of wine origin. Anal. Chim. Acta 2006, 563, 300–309. [Google Scholar] [CrossRef] [Green Version]
- Perestrelo, R.; Fernandes, A.; Alburquerque, F.F.; Marques, J.C.; Câmara, J.S. Analytical characterization of the aroma of ‘Tinta Negra Mole’ red wine: Identification of the main odorants compounds. Anal. Chim. Acta 2006, 563, 154–164. [Google Scholar] [CrossRef] [Green Version]
- Lomelí-Martín, A.; Martínez, L.M.; Welti-Chanes, J.; Escobedo-Avellaneda, Z. Induced Changes in Aroma Compounds of Foods Treated with High Hydrostatic Pressure: A Review. Foods 2021, 10, 878. [Google Scholar] [CrossRef]
- Mahajan, S.S.; Goddik, L.; Qian, M.C. Aroma Compounds in Sweet Whey Powder. J. Dairy Sci. 2004, 87, 4057–4063. [Google Scholar] [CrossRef]
- Slaghenaufi, D.; Peruch, E.; De Cosmi, M.; Nouvelet, L.; Ugliano, M. Volatile and phenolic composition of monovarietal red wines of Valpolicella appellations. OENO One 2021, 55, 279–294. [Google Scholar] [CrossRef]
- Parker, J.K. Chapter 1. Introduction to aroma compounds in foods. In Flavour Development, Analysis and Perception in Food and Beverages; Woodhead Publishing: Sawston, UK, 2015; pp. 3–30. [Google Scholar] [CrossRef]
- Zeller, A.; Rychlik, M. Impact of estragole and other odorants on the flavour of anise and tarragon. Flavour Fragr. J. 2007, 22, 105–113. [Google Scholar] [CrossRef]
- IARC Working Group of the Evaluation of Carcinogenic Risks to Humans. Some Chemicals Present in Industrial and Consumer Products, Food and Dringking Water; International Agency for Research on Cancer: Lyon, France, 2013. Available online: https://www.ncbi.nlm.nih.gov/books/NBK373195/ (accessed on 1 June 2022).
- Roland, A.; Schenider, R.; Razungles, A.; Cavelier, F. Varietal thiols in wine: Discovery, analysis and applications. Chem. Rev. 2011, 111, 7355–7376. [Google Scholar] [CrossRef]
- Genovese, A.; Piombo, P.; Lisanti, M.T.; Moio, L. Occurrence of furaneol (4-hydroxy-2,5-dimethyl-3(2H)-furanone) in some wines from Italian native grapes. Ann. Chim. 2005, 95, 415–419. [Google Scholar] [CrossRef] [Green Version]
- Welke, J.E.; Manfroi, V.; Zanus, M.; Lazarotto, M.; Zini, C.A. Characterization of the volatile profile of Brazilian Merlot wines through comprehensive two-dimensional gas chromatography time-of-flight mass spectrometric detection. J. Chromatogr. A 2012, 1226, 124–139. [Google Scholar] [CrossRef] [Green Version]
- Burdock, G.A. Fenaroli’s Handbook of Flavour Ingredients, 6th ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2010. [Google Scholar]
- Cabrita, M.J.; Freitas, A.M.C.; Laureano, O.; Di Stefano, R. Glycosidic aroma compounds of some Portuguese grape cul-tivars. J. Sci. Food Agric. 2006, 86, 922–931. [Google Scholar] [CrossRef]
- Noguerol-Pato, R.; González-Barreiro, C.; Cancho-Grande, B.; Santiago, J.L.; Martínez, M.C.; Simal-Gándara, J. Aroma potential of Brancellao grapes from different cluster positions. Food Chem. 2012, 132, 112–124. [Google Scholar] [CrossRef] [Green Version]
- Franco, M.; Peinado, R.A.; Medina, M.; Moreno, J. Off-vine grape drying effect on volatile compounds and aromatic se-ries in must from Pedro Ximénez grape variety. J. Agric. Food Chem. 2004, 52, 3905–3910. [Google Scholar] [CrossRef] [PubMed]
- Poitou, X.; Redon, P.; Pons, A.; Bruez, E.; Delière, L.; Marchal, A.; Cholet, C.; Geny-Denis, L.; Darriet, P. Methyl salicylate, a grape and wine chemical marker and sensory contributor in wines elaborated from grapes affected or not by cryptogrammic diseases. Food Chem. 2021, 360, 130120. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Olivero, S.J.; Pérez-Pont, M.L.; Conde, J.E.; Pérez-Trujillo, J.P. Determination of lactones in wines by headspace solid-phase microextraction and gas chromatography coupled with mass spectrometry. J. Anal. Methods Chem. 2014, 2014, 863019. [Google Scholar] [CrossRef] [Green Version]
- Antalick, G.; Šuklje, K.; Blackman, J.W.; Schmidtke, L.M.; Deloire, A. Performing sequential harvests based on berry sugar accumulation (mg/berry) to obtain specific wine sensory profiles. OENO One 2021, 55, 131–146. [Google Scholar] [CrossRef]
- Dimitrovska, M.; Bocevska, M.; Dimitrovski, D.; Murkovic, M. Anthocyanin composition of Vranec, Cabernet Sauvignon, Merlot and Pinot Noir grapes as indicator of their varietal differentiation. Eur. Food Res. Technol. 2011, 232, 591–600. [Google Scholar] [CrossRef]
- Mattivi, F.; Guzzon, F.; Vrhowsek, U.; Stefanini, M.; Velasco, R. Metabolite profiling of grape: Flavonols and anthocyanins. J. Agric. Food Chem. 2006, 54, 7692–7702. [Google Scholar] [CrossRef]
- Pomar, F.; Novo, M.; Masa, A. Varietal differences among the anthocyanin profiles of 50 red table grapes cultivars studied by high performance liquid chromatography. J. Chromatogr. A 2005, 1094, 34–41. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).