Optimized Phosphorus Application Alleviated Adverse Effects of Short-Term Low-Temperature Stress in Winter Wheat by Enhancing Photosynthesis and Improved Accumulation and Partitioning of Dry Matter
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sampling and Measurement
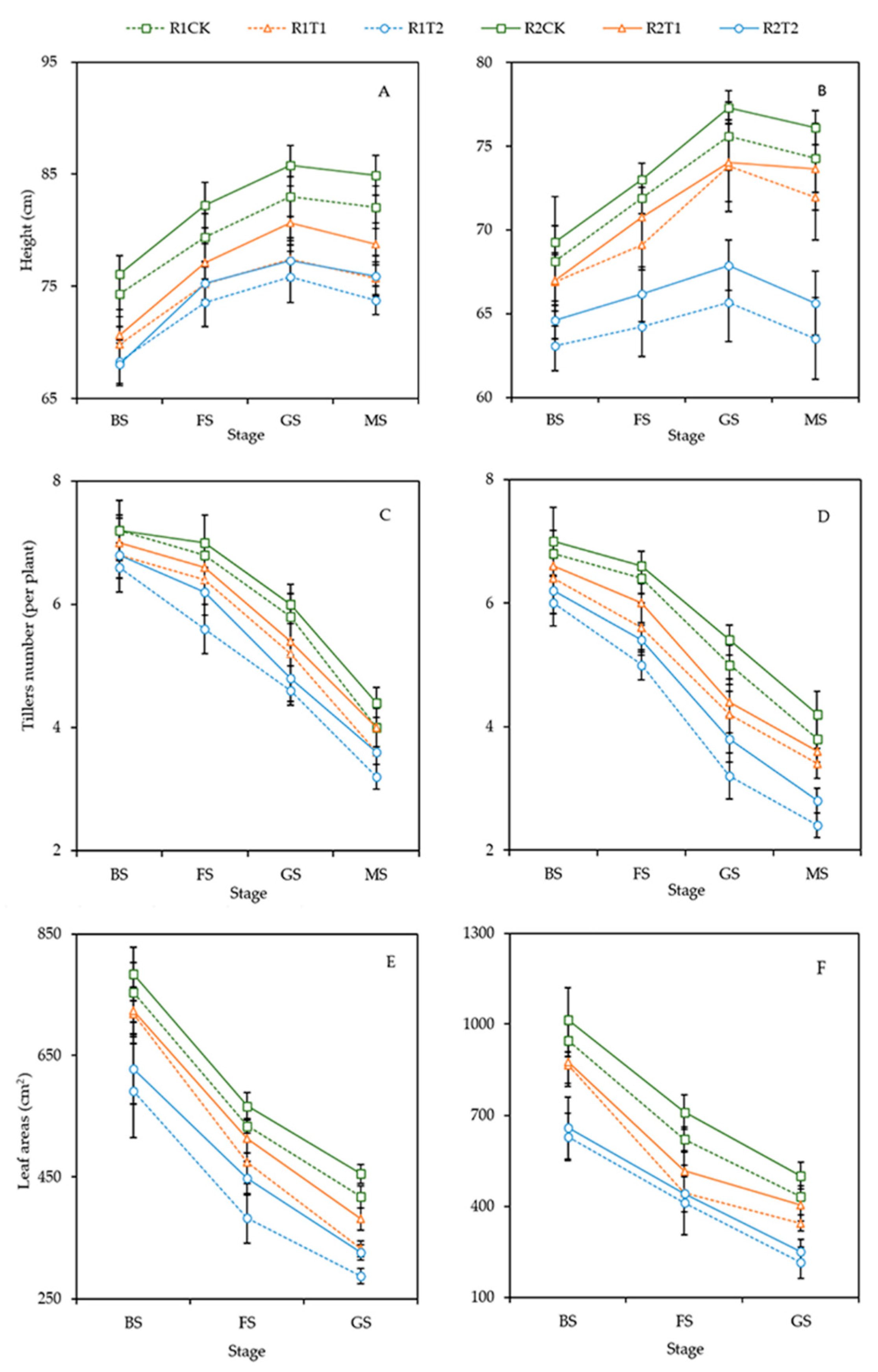
2.2.1. Plant Morphology
2.2.2. Determination of Photosynthetic Parameters
2.2.3. Dry Matter Accumulation, Translocation, and Distribution
- (1)
- Dry matter transportation of vegetative organs before flowering stage (DMT) = Dry matter of vegetative organs at the flowering stage—dry matter of vegetative organs at maturity stage (g).
- (2)
- Proportion of dry matter transportation of vegetative organs before flowering stage (%) (PDMT) = (DMT/dry matter of vegetative organs at the flowering stage) × 100%.
- (3)
- Contribution of dry matter transportation of vegetative organs before the flowering stage to dry matter of grains (%) (CDMT) = (DMT/dry matter of grains at maturity stage) × 100%.
- (4)
- Dry matter accumulation of vegetative organs after flowering stage (DMAA) = Dry matter of grains at maturity stage—DMT (g).
- (5)
- Contribution of dry matter after flowering stage to dry matter of grains (%) (CDMAA) = (DMAA/dry matter of grains at maturity stage) × 100%.
2.2.4. Grain Yield Components
2.3. Statistical Analysis
3. Results
3.1. Plant Morphology
3.2. Photosynthetic Parameters
3.3. Dry Matter
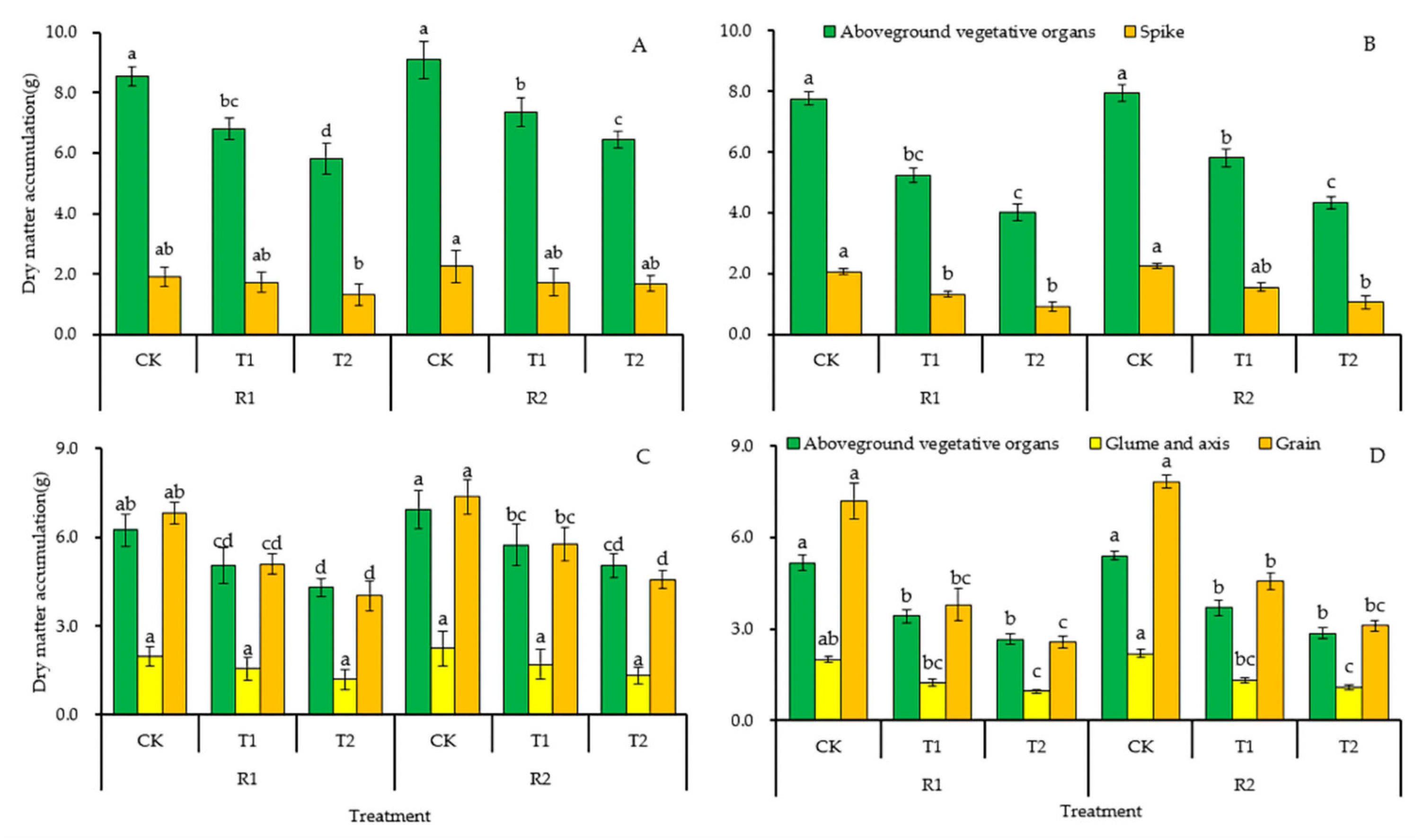
3.3.1. Dry Matter Accumulation
3.3.2. Dry Matter Accumulation in Different Wheat Organs
3.3.3. Dry Matter Redistribution in Wheat
3.4. Yield and Yield Components
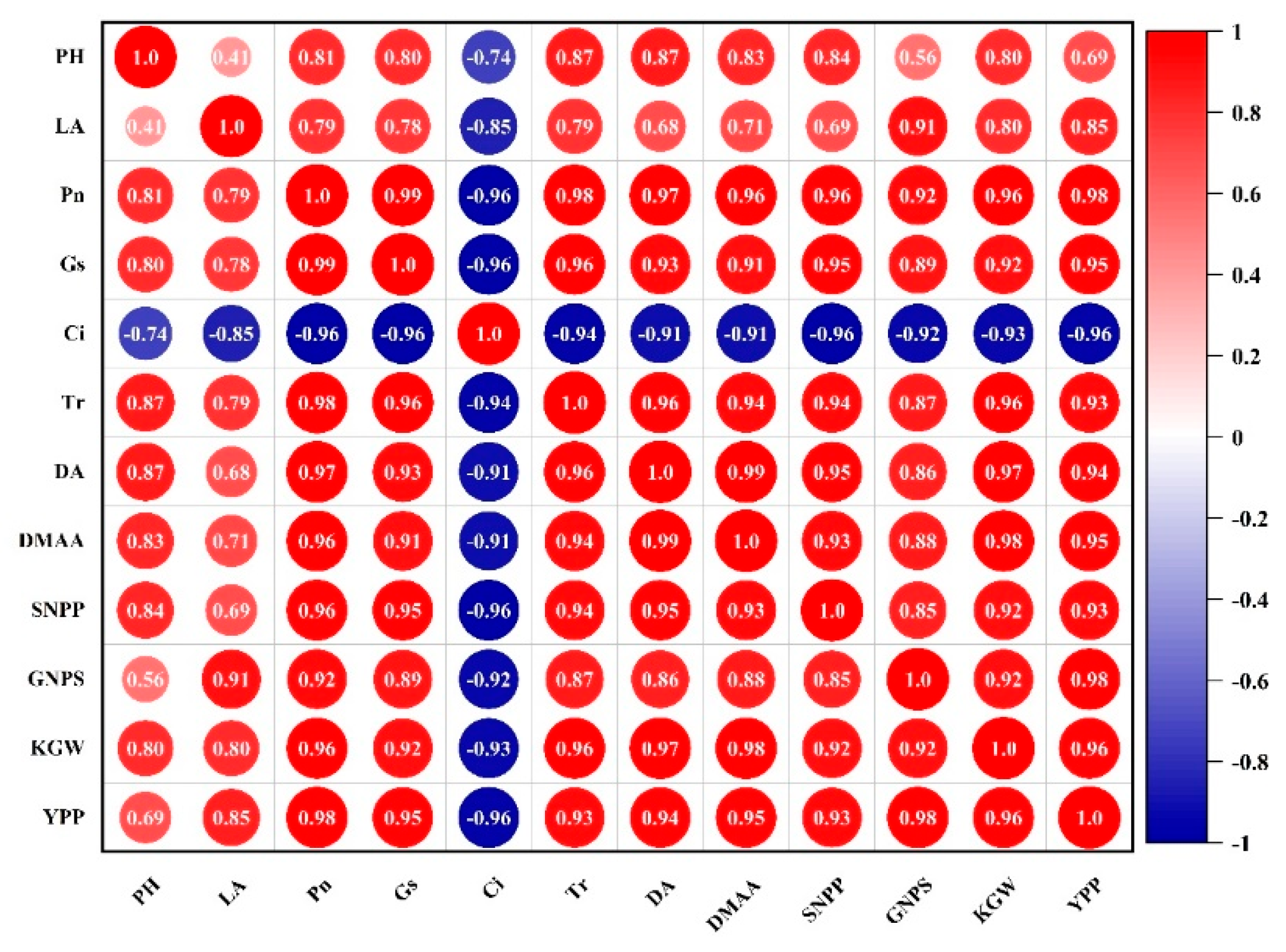
3.5. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agnolucci, P.; Rapti, C.; Alexander, P.; De Lipsis, V.; Holland, R.A.; Eigenbrod, F.; Ekins, P. Impacts of rising temperatures and farm management practices on global yields of 18 crops. Nat. Food 2020, 1, 562–571. [Google Scholar] [CrossRef]
- Kulundzic, A.M.; Viljevac Vuletic, M.; Kocar, M.M.; Mijic, A.; Varga, I.; Sudaric, A.; Cesar, V.; Lepedus, H. The combination of increased temperatures and high irradiation causes changes in photosynthetic efficiency. Plants 2021, 10, 2076. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, S.; Sidhu, G.P.S. Climate change regulated abiotic stress mechanisms in plants: A comprehensive review. Plant Cell Rep. 2022, 41, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.L.; Song, H.; Shi, K.J.; Liu, B.; Zhang, Y.; Tang, L.; Cao, W.X.; Zhu, Y. Response of wheat grain quality to low temperature during jointing and booting stages-On the importance of considering canopy temperature. Agric. For. Meteorol. 2019, 278, 107658. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Chen, X.; Li, J. Effects of low-temperature stress during the anther differentiation period on winter wheat photosynthetic performance and spike-setting characteristics. Plants 2022, 11, 389. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Hassan, M.A.; Sun, D.; Wu, Z.; Jiang, G.; Liu, B.B.; Ni, Q.; Yang, W.; Fang, H.; Jincai, L. Effects of low temperature stress on source-sink organs in wheat and phosphorus mitigation strategies. Front. Plant Sci. 2022, 13, 807844. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Chapman, S.C.; Christopher, J.T.; Frederiks, T.M.; Chenu, K. Frost trends and their estimated impact on yield in the Australian wheatbelt. J. Exp. Bot. 2015, 66, 3611–3623. [Google Scholar] [CrossRef]
- Osman, R.; Zhu, Y.; Ma, W.; Zhang, D.; Ding, Z.; Liu, L.; Tang, L.; Liu, B.; Cao, W. Comparison of wheat simulation models for impacts of extreme temperature stress on grain quality. Agric. For. Meteorol. 2020, 288, 107995. [Google Scholar] [CrossRef]
- Liu, L.; Xia, Y.; Liu, B.; Chang, C.; Xiao, L.; Shen, J.; Tang, L.; Cao, W.; Zhu, Y. Individual and combined effects of jointing and booting low-temperature stress on wheat yield. Eur. J. Agron. 2020, 113, 125989. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, J.; Huang, Z.; Mi, L.; Xu, K.; Wu, J.; Fan, Y.; Ma, S.; Jiang, D. Effects of low temperature at booting stage on sucrose metabolism and endogenous hormone contents in winter wheat spikelet. Front. Plant Sci. 2019, 10, 498. [Google Scholar] [CrossRef]
- Hassan, M.A.; Xiang, C.; Farooq, M.; Muhammad, N.; Yan, Z.; Hui, X.; Ke, Y.Y.; Bruno, A.K.; Zhang, L.L.; Li, J.C. Cold stress in wheat: Plant acclimation responses and management strategies. Front. Plant Sci. 2021, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.J.; Ort, D.R. Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends Plant Sci. 2001, 6, 36–42. [Google Scholar] [CrossRef]
- Bhattacharya, A. Effect of low temperature stress on photosynthesis and allied traits: A review. In Physiological Processes in Plants Under Low Temperature Stress; Bhattacharya, A., Ed.; Springer: Singapore, 2022; pp. 199–297. [Google Scholar] [CrossRef]
- Gan, P.; Liu, F.; Li, R.; Wang, S.; Luo, J. Chloroplasts—beyond energy capture and carbon fixation: Tuning of photosynthesis in response to chilling stress. Int. J. Mol. Sci. 2019, 20, 5046. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; He, Y.; Irfan, A.R.; Liu, X.; Yu, Q.; Zhang, Q.; Yang, D. Exogenous brassinolide enhances the growth and cold resistance of maize (Zea mays L.) seedlings under chilling stress. Agronomy 2020, 10, 488. [Google Scholar] [CrossRef]
- Fuller, M.P.; Fuller, A.M.; Kaniouras, S.; Christophers, J.; Fredericks, T. The freezing characteristics of wheat at ear emergence. Eur. J. Agron. 2007, 26, 435–441. [Google Scholar] [CrossRef]
- Ji, H.T.; Xiao, L.J.; Xia, Y.M.; Song, H.; Liu, B.; Tang, L.; Cao, W.X.; Zhu, Y.; Liu, L.L. Effects of jointing and booting low temperature stresses on grain yield and yield components in wheat. Agric. For. Meteorol. 2017, 243, 33–42. [Google Scholar] [CrossRef]
- Zhong, X.; Mei, X.; Li, Y.; Yoshida, H.; Zhao, P.; Wang, X.; Han, L.; Hu, X.; Huang, S.; Huang, J.; et al. Changes in frost resistance of wheat young ears with development during jointing stage. J. Agron. Crop. Sci. 2008, 194, 343–349. [Google Scholar] [CrossRef]
- Thakur, P.; Kumar, S.; Malik, J.A.; Berger, J.D.; Nayyar, H. Cold stress effects on reproductive development in grain crops: An overview. Environ. Exp. Bot. 2010, 67, 429–443. [Google Scholar] [CrossRef]
- Yaakob, M.A.; Mohamed, R.M.S.R.; Al-Gheethi, A.; Ravishankar, G.A.; Ambati, R.R. Influence of nitrogen and phosphorus on microalgal growth, biomass, lipid, and fatty acid production: An overview. Cells 2021, 10, 393. [Google Scholar] [CrossRef]
- Poirier, Y.; Jaskolowski, A.; Clua, J. Phosphate acquisition and metabolism in plants. Curr. Biol. 2022, 32, 623–629. [Google Scholar] [CrossRef]
- Noor, I.; Sohail, H.; Hasanuzzaman, M.; Hussain, S.; Li, G.; Liu, J. Phosphorus confers tolerance against manganese toxicity in Prunus persica by reducing oxidative stress and improving chloroplast ultrastructure. Chemosphere 2022, 291, 132999. [Google Scholar] [CrossRef] [PubMed]
- Bouain, N.; Krouk, G.; Lacombe, B.; Rouached, H. Getting to the root of plant mineral nutrition: Combinatorial nutrient stresses reveal emergent properties. Trends Plant Sci. 2019, 24, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.X.; Wang, C.J.; Qi, L.L.; Zhang, X.L.; Tang, G.M.; Li, L.; Guo, J.H.; Jia, Y.J.; Dou, X.L.; Lu, M. Phosphorus is more effective than nitrogen in restoring plant communities of heavy metals polluted soils. Environ. Pollut. 2020, 266, 9. [Google Scholar] [CrossRef] [PubMed]
- Attarzadeh, M.; Balouchi, H.; Rajaie, M.; Dehnavi, M.M.; Salehi, A. Improving growth and phenolic compounds of Echinacea purpurea root by integrating biological and chemical resources of phosphorus under water deficit stress. Ind. Crop. Prod. 2020, 154, 7. [Google Scholar] [CrossRef]
- Li, H.Y.; Qiu, Y.Z.; Yao, T.; Ma, Y.C.; Zhang, H.R.; Yang, X.L. Effects of PGPR microbial inoculants on the growth and soil properties of Avena sativa, Medicago sativa, and Cucumis sativus seedlings. Soil Tillage Res. 2020, 199, 10. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.F.; Wu, W.H. Potassium and phosphorus transport and signaling in plants. J. Integr. Plant Biol. 2021, 63, 34–52. [Google Scholar] [CrossRef]
- Li, S.X.; Cheng, Z.H.; Dong, S.M.; Li, Z.B.; Zou, L.P.; Zhao, P.J.; Guo, X.; Bao, Y.; Wang, W.Q.; Peng, M. Global identification of full-length cassava lncRNAs unveils the role of cold-responsive intergenic lncRNA 1 in cold stress response. Plant Cell Environ. 2022, 45, 412–426. [Google Scholar] [CrossRef]
- Guo, X.Y.; Liu, D.F.; Chong, K. Cold signaling in plants: Insights into mechanisms and regulation. J. Integr. Plant Biol. 2018, 60, 745–756. [Google Scholar] [CrossRef]
- Xie, Y.K.; Wang, C.; Yang, W.D.; Feng, M.C.; Qiao, X.X.; Song, J.Y. Canopy hyperspectral characteristics and yield estimation of winter wheat (Triticum aestivum) under low temperature injury. Sci. Rep. 2020, 10, 244. [Google Scholar] [CrossRef]
- Han, Q.X.; Kang, G.Z.; Guo, T.C. Proteomic analysis of spring freeze-stress responsive proteins in leaves of bread wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2013, 63, 236–244. [Google Scholar] [CrossRef]
- Li, X.N.; Pu, H.C.; Liu, F.L.; Zhou, Q.; Cai, J.; Dai, T.B.; Cao, W.X.; Jiang, D. Winter wheat photosynthesis and grain yield responses to spring freeze. Agron. J. 2015, 107, 1002–1010. [Google Scholar] [CrossRef]
- Seleiman, M.F. Use of plant nutrients in improving abiotic stress tolerance in wheat. In Wheat Production in Changing Environments; Springer: Berlin/Heidelberg, Germany, 2019; pp. 481–495. [Google Scholar] [CrossRef]
- Školníková, M.; Škarpa, P.; Ryant, P.; Kozáková, Z.; Antošovský, J. Response of winter wheat (Triticum aestivum L.) to fertilizers with nitrogen-transformation inhibitors and timing of their application under field conditions. Agronomy 2022, 12, 223. [Google Scholar] [CrossRef]
- Chen, X.X.; Zhang, W.; Liang, X.Y.; Liu, Y.M.; Xu, S.J.; Zhao, Q.Y.; Du, Y.F.; Zhang, L.; Chen, X.P.; Zou, C.Q. Physiological and developmental traits associated with the grain yield of winter wheat as affected by phosphorus fertilizer management. Sci. Rep. 2019, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Izhar Shafi, M.; Adnan, M.; Fahad, S.; Wahid, F.; Khan, A.; Yue, Z.; Danish, S.; Zafar-ul-Hye, M.; Brtnicky, M.; Datta, R. Application of single superphosphate with humic acid improves the growth, yield and phosphorus uptake of wheat (Triticum aestivum L.) in calcareous soil. Agronomy 2020, 10, 1224. [Google Scholar] [CrossRef]
- Jahan, M.; Amiri, M.B. Optimizing application rate of nitrogen, phosphorus and cattle manure in wheat production: An approach to determine optimum scenario using response-surface methodology. J. Soil Sci. Plant Nutr. 2018, 18, 13–26. [Google Scholar] [CrossRef][Green Version]
- Gururani, M.A.; Venkatesh, J.; Tran, L.S.P. Regulation of photosynthesis during abiotic stress-induced photoinhibition. Mol. Plant 2015, 8, 1304–1320. [Google Scholar] [CrossRef]
- Hussain, S.; Ulhassan, Z.; Brestic, M.; Zivcak, M.; Zhou, W.J.; Allakhverdiev, S.I.; Yang, X.H.; Safdar, M.E.; Yang, W.Y.; Liu, W.G. Photosynthesis research under climate change. Photosynth. Res. 2021, 150, 5–19. [Google Scholar] [CrossRef]
- Khan, T.A.; Fariduddin, Q.; Yusuf, M. Low-temperature stress: Is phytohormones application a remedy? Environ. Sci. Pollut. Res. 2017, 24, 21574–21590. [Google Scholar] [CrossRef]
- Venzhik, Y.V.; Titov, A.F.; Talanova, V.V.; Frolova, S.A.; Talanov, A.V.; Nazarkina, Y.A. Influence of lowered temperature on the resistance and functional activity of the photosynthetic apparatus of wheat plants. Biol. Bull. 2011, 38, 132–137. [Google Scholar] [CrossRef]
- Nevyl, S.F.; Battaglia, M.E. Developmental plasticity in Arabidopsis thaliana under combined cold and water deficit stresses during flowering stage. Planta 2021, 253, 13. [Google Scholar] [CrossRef]
- Yamori, W.; Noguchi, K.; Hikosaka, K.; Terashima, I. Cold-tolerant crop species have greater temperature homeostasis of leaf respiration and photosynthesis than cold-sensitive species. Plant Cell Physiol. 2009, 50, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Waraich, E.A.; Ahmad, R.; Ashraf, M. Role of mineral nutrition in alleviation of drought stress in plants. Aust. J. Crop. Sci. 2011, 5, 764–777. [Google Scholar] [CrossRef]
- Rafiullah; Khan, M.J.; Muhammad, D.; Fahad, S.; Adnan, M.; Wahid, F.; Alamri, S.; Khan, F.; Dawar, K.M.; Irshad, I.; et al. Phosphorus nutrient management through synchronization of application methods and rates in wheat and maize crops. Plants 2020, 9, 1389. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Li, S.; Hu, C.; Sun, X.; Tan, Q.; Liu, H. Effects of molybdenum and phosphorus fertilizers on cold resistance in winter wheat. J. Plant Nutr. 2015, 38, 808–820. [Google Scholar] [CrossRef]
- Mahmood, I.A.; Arshad, A. Response of direct seeded rice and wheat crops to phosphorus application with crop residue incorporation in saline-sodic soil. Int. J. Agric. Biol. 2015, 17, 1219–1224. [Google Scholar] [CrossRef]
- Wang, W.L.; Wang, X.; Huang, M.; Cal, J.; Zhou, Q.; Dai, T.B.; Jiang, D. Alleviation of field low-temperature stress in winter wheat by exogenous application of salicylic acid. J. Plant Growth Regul. 2021, 40, 811–823. [Google Scholar] [CrossRef]
- Liu, L.; Ji, H.; An, J.; Shi, K.; Ma, J.; Liu, B.; Tang, L.; Cao, W.; Zhu, Y. Response of biomass accumulation in wheat to low-temperature stress at jointing and booting stages. Environ. Exp. Bot. 2019, 157, 46–57. [Google Scholar] [CrossRef]
- Craufurd, P.Q.; Vadez, V.; Jagadish, S.V.K.; Prasad, P.V.V.; Zaman-Allah, M. Crop science experiments designed to inform crop modeling. Agric. For. Meteorol. 2013, 170, 8–18. [Google Scholar] [CrossRef]
- Ma, S.; Gai, P.; Wang, Y.; Ullah, N.; Zhang, W.; Fan, Y.; Shan, Y.; Huang, Z.; Hu, X. Carbohydrate assimilation and translocation regulate grain yield formation in wheat crops (Triticum aestivum L.) under post-flowering waterlogging. Agronomy 2021, 11, 2209. [Google Scholar] [CrossRef]
- Cong, W.F.; Suriyagoda, L.D.B.; Lambers, H. Tightening the phosphorus cycle through phosphorus-efficient crop genotypes. Trends Plant Sci. 2020, 25, 967–975. [Google Scholar] [CrossRef]
- El Mazlouzi, M.; Morel, C.; Robert, T.; Yan, B.; Mollier, A. Phosphorus uptake and partitioning in two durum wheat cultivars with contrasting biomass allocation as affected by different P supply during grain filling. Plant Soil 2020, 449, 179–192. [Google Scholar] [CrossRef]
- Zhang, C.A.H.; Gu, K.J.; Gu, D.X.; Zhang, S.M.; Wu, J.J. Quantifying the effect of low-temperature events on the grain quality formation of wheat. J. Cereal Sci. 2021, 100, 9. [Google Scholar] [CrossRef]
- Frederiks, T.M.; Christopher, J.T.; Harvey, G.L.; Sutherland, M.W.; Borrell, A.K. Current and emerging screening methods to identify post-head-emergence frost adaptation in wheat and barley. J. Exp. Bot. 2012, 63, 5405–5416. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Hassan, M.A.; Muhammad, N.; Arshad, M.; Chen, X.; Xu, Y.H.; Xu, H.; Ni, Q.Q.; Liu, B.B.; Yang, W.K.; et al. Comparative physiology and transcriptome analysis of young spikes in response to late spring coldness in wheat (Triticum aestivum L.). Front. Plant Sci. 2022, 13, 811884. [Google Scholar] [CrossRef]
- Zheng, D.X.; Yang, X.G.; Minguez, M.I.; Mu, C.Y.; He, Q.; Wu, X. Effect of freezing temperature and duration on winter survival and grain yield of winter wheat. Agric. For. Meteorol. 2018, 260, 1–8. [Google Scholar] [CrossRef]
- Qiu, Y.H.; Fall, T.; Su, Z.H.; Bortolozo, F.; Mussoline, W.; England, G.; Dinkins, D.; Morgan, K.; Clark, M.; Liu, G.D. Effect of phosphorus fertilization on yield of chipping potato grown on high legacy phosphorus soil. Agronomy 2022, 12, 812. [Google Scholar] [CrossRef]

| Variety | MPA | Temperature Regimes | Number of Experimental Pots | ||
|---|---|---|---|---|---|
| CK (11 °C) | T1 (4 °C) | T2 (−4 °C) | |||
| YN19 | R1 | YNR1CK | YNR1T1 | YNR1T2 | 36 pots |
| R2 | YNR2CK | YNR2T1 | YNR2T2 | 36 pots | |
| XM26 | R1 | XMR1CK | XMR1T1 | XMR1T2 | 36 pots |
| R2 | XMR2CK | XMR2T1 | XMR2T2 | 36 pots | |
| Stage | MPA | TEMP | YN19 | XM26 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pn | Gs | Ci | Tr | Pn | Gs | Ci | Tr | |||
| μmol·m−2·s−1 | mol·m−2·s−1 | μmol·mol−1 | mmol·m−2·s−1 | μmol·m−2·s−1 | mol·m−2·s−1 | μmol·mol−1 | mmol·m−2·s−1 | |||
| FS | R1 | CK | 25.1 ± 0.7 a | 0.231 ± 0.005 ab | 141.7 ± 4.1 cd | 4.89 ± 0.34 a | 24.7 ± 0.4 a | 0.219 ± 0.010 a | 148.3 ± 5.5 de | 4.74 ± 0.14 a |
| T1 | 20.3 ± 0.3 bc | 0.179 ± 0.003 c | 154.0 ± 2.3 bc | 3.78 ± 0.13 abc | 18.3 ± 0.5 bc | 0.153 ± 0.007 bc | 170.7 ± 7.4 bc | 3.46 ± 0.10 b | ||
| T2 | 16.8 ± 0.7 c | 0.124 ± 0.013 d | 175.7 ± 3.7 a | 2.55 ± 0.10 c | 14.8 ± 0.5 d | 0.110 ± 0.005 d | 195.0 ± 5.7 a | 2.24 ± 0.10 d | ||
| R2 | CK | 26.4 ± 0.8 a | 0.255 ± 0.014 a | 132.0 ± 2.5 d | 5.11 ± 0.23 a | 25.6 ± 0.8 a | 0.230 ± 0.009 a | 135.7 ± 2.8 e | 4.96 ± 0.08 a | |
| T1 | 23.6 ± 1.0 ab | 0.200 ± 0.007 bc | 146.3 ± 6.9 cd | 3.93 ± 0.44 ab | 19.9 ± 0.4 b | 0.168 ± 0.002 b | 162.0 ± 2.5 cd | 3.58 ± 0.08 b | ||
| T2 | 19.4 ± 0.2 c | 0.158 ± 0.006 cd | 169.0 ± 2.5 ab | 3.27 ± 0.25 bc | 17.5 ± 0.6 c | 0.133 ± 0.008 cd | 184.7 ± 8.4 ab | 2.89 ± 0.07 c | ||
| GS | R1 | CK | 12.3 ± 0.3 a | 0.134 ± 0.004 a | 174.0 ± 5.5 c | 3.35 ± 0.12 a | 11.7 ± 0.2 a | 0.119 ± 0.005 a | 175.3 ± 3.84 de | 3.12 ± 0.09 ab |
| T1 | 9.0 ± 0.3 bc | 0.091 ± 0.003 bc | 198.7 ± 6.8 bc | 2.71 ± 0.07 bc | 7.7 ± 0.4 bc | 0.082 ± 0.003 bc | 210.7 ± 3.53 bc | 2.53 ± 0.16 c | ||
| T2 | 7.1 ± 0.3 c | 0.065 ± 0.004 c | 242.7 ± 6.0 a | 2.42 ± 0.09 c | 5.3 ± 0.4 d | 0.054 ± 0.008 c | 247.7 ± 6.98 a | 1.74 ± 0.07 d | ||
| R2 | CK | 13.1 ± 0.3 a | 0.145 ± 0.004 a | 166.7 ± 5.8 c | 3.52 ± 0.13 a | 12.6 ± 0.3 a | 0.126 ± 0.006 a | 162.7 ± 3.84 e | 3.36 ± 0.08 a | |
| T1 | 9.8 ± 0.2 b | 0.101 ± 0.007 b | 187.0 ± 7.5 c | 2.88 ± 0.07 b | 8.9 ± 0.4 b | 0.098 ± 0.007 ab | 197.7 ± 4.98 cd | 2.81 ± 0.07 bc | ||
| T2 | 8.0 ± 0.3 cd | 0.075 ± 0.002 cd | 224.0 ± 6.2 ab | 2.55 ± 0.07 bc | 6.5 ± 0.5 cd | 0.066 ± 0.003 c | 234.3 ± 6.36 ab | 1.95 ± 0.12 d | ||
| Variety | MPA | TEMP | BS | FS | GS | MS |
|---|---|---|---|---|---|---|
| YN19 | R1 | CK | 7.41 ± 0.57 a | 10.45 ± 0.65 ab | 12.19 ± 0.52 ab | 15.01 ± 1.22 ab |
| T1 | 6.95 ± 0.54 a | 8.54 ± 0.71 cd | 9.99 ± 0.27 c | 11.70 ± 1.31 cd | ||
| T2 | 6.37 ± 0.07 a | 7.13 ± 0.88 d | 9.26 ± 0.28 c | 9.52 ± 1.17 d | ||
| R2 | CK | 7.55 ± 0.44 a | 11.35 ± 1.16 a | 12.66 ± 0.38 a | 16.53 ± 1.83 a | |
| T1 | 7.14 ± 0.40 a | 9.09 ± 0.94 bc | 10.62 ± 0.17 bc | 13.20 ± 1.76 bc | ||
| T2 | 6.72 ± 0.16 a | 8.15 ± 0.52 cd | 9.75 ± 0.42 c | 10.93 ± 0.98 cd | ||
| XM26 | R1 | CK | 7.14 ± 0.45 ab | 9.83 ± 0.15 a | 11.31 ± 0.62 a | 14.37 ± 0.22 a |
| T1 | 5.63 ± 0.24 cd | 6.55 ± 0.22 bc | 7.41 ± 0.57 bc | 8.47 ± 0.15 bc | ||
| T2 | 4.62 ± 0.18 d | 4.91 ± 0.12 d | 5.48 ± 0.30 c | 6.20 ± 0.48 d | ||
| R2 | CK | 7.37 ± 0.34 a | 10.18 ± 0.35 a | 12.57 ± 0.51 a | 15.44 ± 0.68 a | |
| T1 | 6.27 ± 0.23 bc | 7.35 ± 0.35 b | 8.43 ± 0.45 b | 9.60 ± 0.18 b | ||
| T2 | 5.18 ± 0.12 d | 5.39 ± 0.41 cd | 6.25 ± 0.25 bc | 7.06 ± 0.22 cd |
| Variety | MPA | TEMP | Dry Matter BF | Dry Matter AF | ||
|---|---|---|---|---|---|---|
| PDMT | CDMT | DMAA | CDMAA | |||
| YN19 | R1 | CK | 26.96 ± 0.57 a | 33.81 ± 0.92 a | 4.51 ± 0.07 ab | 66.19 ± 0.92 a |
| T1 | 26.06 ± 4.82 a | 34.79 ± 6.25 a | 3.32 ± 0.32 cd | 65.21 ± 6.25 a | ||
| T2 | 26.06 ± 1.74 a | 37.78 ± 2.52 a | 2.50 ± 0.18 d | 62.22 ± 2.51 a | ||
| R2 | CK | 23.65 ± 0.22 a | 29.22 ± 0.61 a | 5.20 ± 0.05 a | 70.78 ± 0.62 a | |
| T1 | 22.34 ± 3.43 a | 28.67 ± 4.73 a | 4.13 ± 0.38 bc | 71.33 ± 4.73 a | ||
| T2 | 22.01 ± 1.67 a | 31.26 ± 2.89 a | 3.15 ± 0.22 d | 68.74 ± 2.89 a | ||
| XM26 | R1 | CK | 33.65 ± 5.29 a | 36.11 ± 4.27 a | 4.59 ± 0.48 a | 63.89 ± 4.26 a |
| T1 | 34.50 ± 2.51 a | 48.21 ± 2.97 a | 2.00 ± 0.38 b | 51.79 ± 2.97 a | ||
| T2 | 33.22 ± 1.79 a | 51.73 ± 2.98 a | 1.24 ± 0.11 b | 48.27 ± 2.98 a | ||
| R2 | CK | 31.78 ± 2.90 a | 32.49 ± 4.27 a | 5.30 ± 0.42 a | 67.51 ± 4.27 a | |
| T1 | 35.60 ± 7.21 a | 45.30 ± 9.18 a | 2.46 ± 0.33 b | 54.70 ± 9.18 a | ||
| T2 | 33.80 ± 5.01 a | 48.62 ± 11.22 a | 1.63 ± 0.42 b | 51.38 ± 11.22 a | ||
| Variety | MPA | TEMP | Spike Number | Grains per Spike | 1000-Grain Weight (g) | Yield per Plant (g) | Yield Reduction Rate (%) |
|---|---|---|---|---|---|---|---|
| YN19 | R1 | CK | 3.5 ± 0.2 ab | 46.4 ± 2.0 a | 40.03 ± 1.27 ab | 6.52 ± 0.50 ab | — |
| T1 | 3.3 ± 0.2 ab | 40.3 ± 3.2 a | 36.59 ± 1.73 ab | 4.95 ± 0.58 bc | 24.1 | ||
| T2 | 2.7 ± 0.2 b | 35.8 ± 2.1 a | 35.44 ± 1.05 b | 3.38 ± 0.17 c | 48.2 | ||
| R2 | CK | 3.7 ± 0.2 a | 46.9 ± 2.2 a | 41.22 ± 0.63 a | 7.08 ± 0.42 a | −8.6 | |
| T1 | 3.3 ± 0.3 ab | 41.4 ± 2.8 a | 39.59 ± 0.68 ab | 5.46 ± 0.36 abc | 16.3 | ||
| T2 | 2.8 ± 0.2 ab | 36.3 ± 2.9 a | 36.09 ± 0.94 ab | 3.73 ± 0.38 c | 42.8 | ||
| XM26 | R1 | CK | 3.3 ± 0.2 a | 52.4 ± 4.3 a | 41.13 ± 0.59 a | 7.19 ± 0.63 a | — |
| T1 | 2.8 ± 0.2 ab | 38.7 ± 1.3 b | 34.77 ± 1.74 bc | 3.80 ± 0.08 bc | 47.1 | ||
| T2 | 2.5 ± 0.2 b | 33.1 ± 0.3 b | 31.18 ± 1.42 d | 2.58 ± 0.11 c | 64.1 | ||
| R2 | CK | 3.5 ± 0.2 a | 52.8 ± 2.6 a | 42.44 ± 0.28 a | 7.83 ± 0.35 a | −8.9 | |
| T1 | 3.0 ± 0.0 ab | 41.5 ± 4.8 b | 36.66 ± 1.24 b | 4.56 ± 0.57 b | 36.6 | ||
| T2 | 2.7 ± 0.2 b | 36.0 ± 2.4 b | 32.38 ± 0.55 cd | 3.11 ± 0.25 bc | 56.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Wu, Z.; Xu, B.; Sun, D.; Hassan, M.A.; Cai, H.; Wu, Y.; Yu, M.; Chen, A.; Li, J.; et al. Optimized Phosphorus Application Alleviated Adverse Effects of Short-Term Low-Temperature Stress in Winter Wheat by Enhancing Photosynthesis and Improved Accumulation and Partitioning of Dry Matter. Agronomy 2022, 12, 1700. https://doi.org/10.3390/agronomy12071700
Xu H, Wu Z, Xu B, Sun D, Hassan MA, Cai H, Wu Y, Yu M, Chen A, Li J, et al. Optimized Phosphorus Application Alleviated Adverse Effects of Short-Term Low-Temperature Stress in Winter Wheat by Enhancing Photosynthesis and Improved Accumulation and Partitioning of Dry Matter. Agronomy. 2022; 12(7):1700. https://doi.org/10.3390/agronomy12071700
Chicago/Turabian StyleXu, Hui, Zhaochen Wu, Bo Xu, Dongyue Sun, Muhammad Ahmad Hassan, Hongmei Cai, Yu Wu, Min Yu, Anheng Chen, Jincai Li, and et al. 2022. "Optimized Phosphorus Application Alleviated Adverse Effects of Short-Term Low-Temperature Stress in Winter Wheat by Enhancing Photosynthesis and Improved Accumulation and Partitioning of Dry Matter" Agronomy 12, no. 7: 1700. https://doi.org/10.3390/agronomy12071700
APA StyleXu, H., Wu, Z., Xu, B., Sun, D., Hassan, M. A., Cai, H., Wu, Y., Yu, M., Chen, A., Li, J., & Chen, X. (2022). Optimized Phosphorus Application Alleviated Adverse Effects of Short-Term Low-Temperature Stress in Winter Wheat by Enhancing Photosynthesis and Improved Accumulation and Partitioning of Dry Matter. Agronomy, 12(7), 1700. https://doi.org/10.3390/agronomy12071700






