A Metal Chaperone Gene Regulates Rice Growth and Seed Development by Manganese Acquisition and Homeostasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth in Hydroponic and Field Experiments
2.2. mRNA Isolation and Expression Analysis
2.3. Generation of OsHIPP56 Overexpression and Knockout Rice
2.4. Yeast Complementation Assay
2.5. Determination of Chlorophyll Content and Electrolyte Leakage
2.6. Metal Quantification in Yeast and Plant Tissues
2.7. Statistical Analysis
3. Results
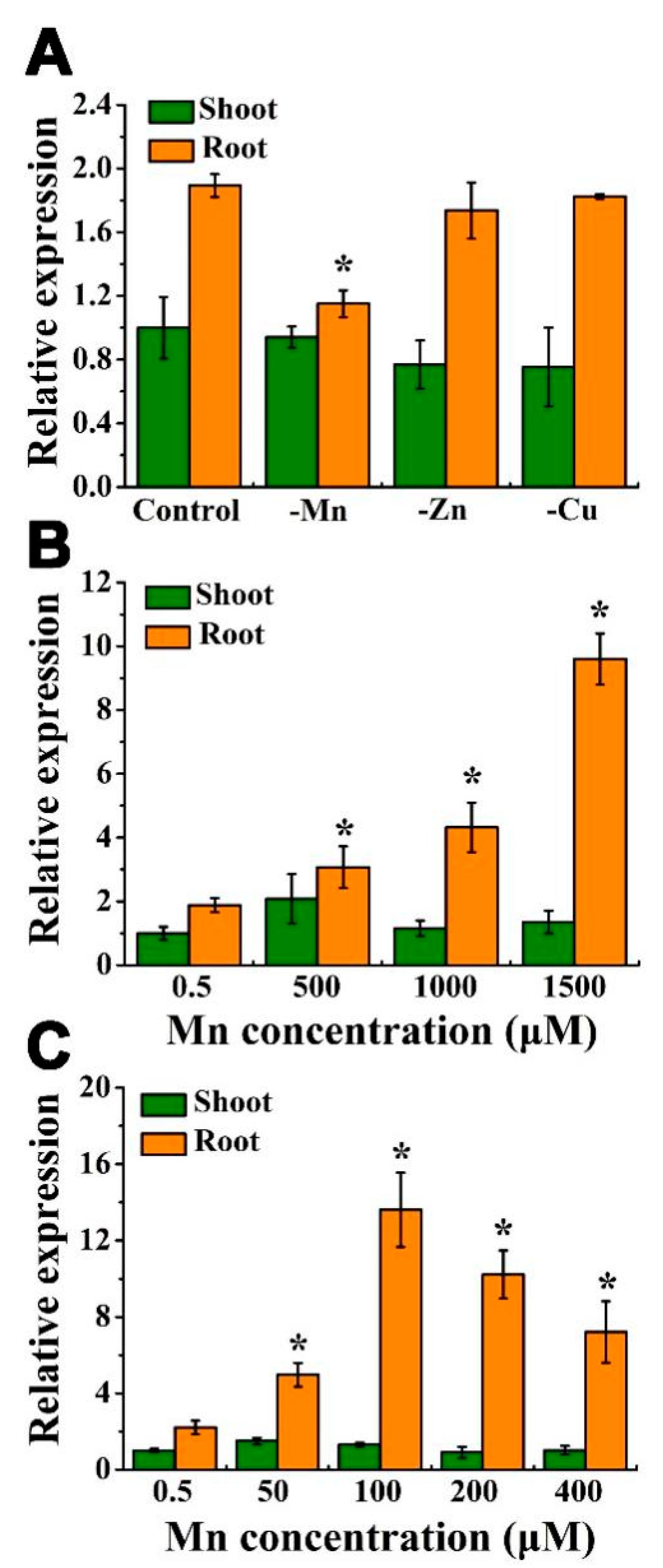
3.1. Expression of OsHIPP56 Was Induced by Excessive Mn Stress
3.2. Expressing OsHIPP56 in Yeast Conferred Cellular Mn Tolerance and Accumulation
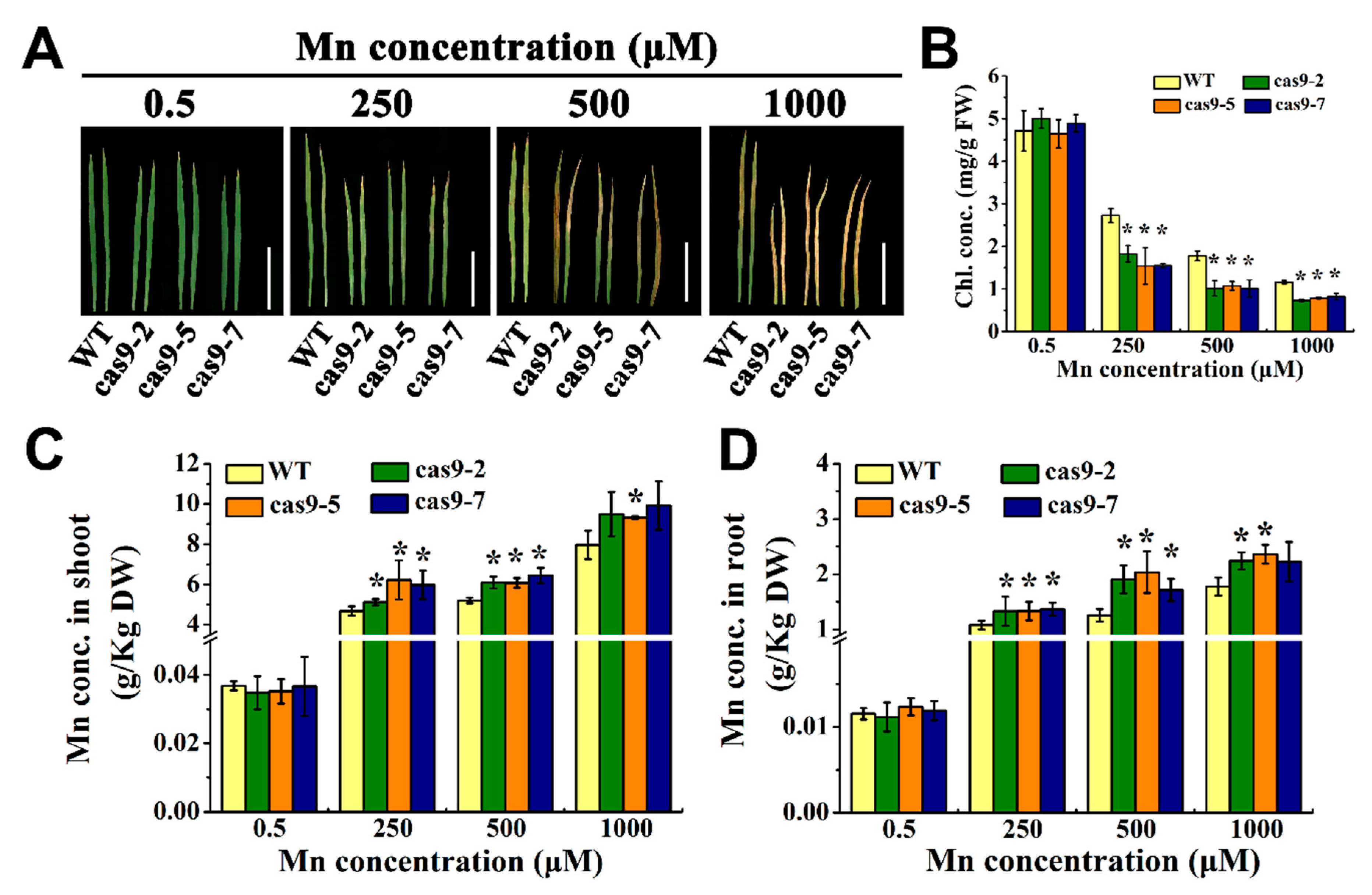
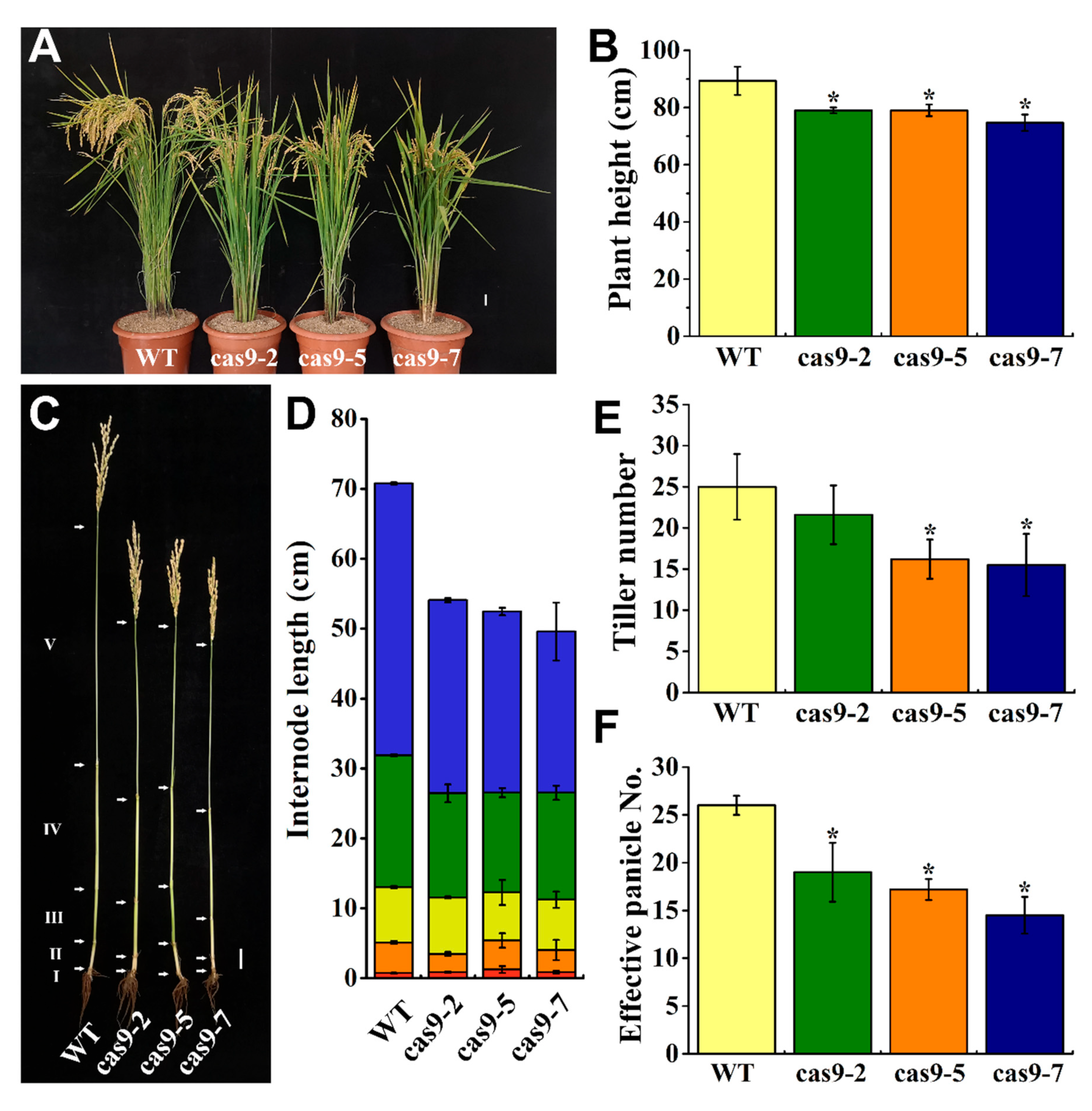
3.3. Knocking Out OsHIPP56 Compromised Rice Growth at Early Vegetative Stage
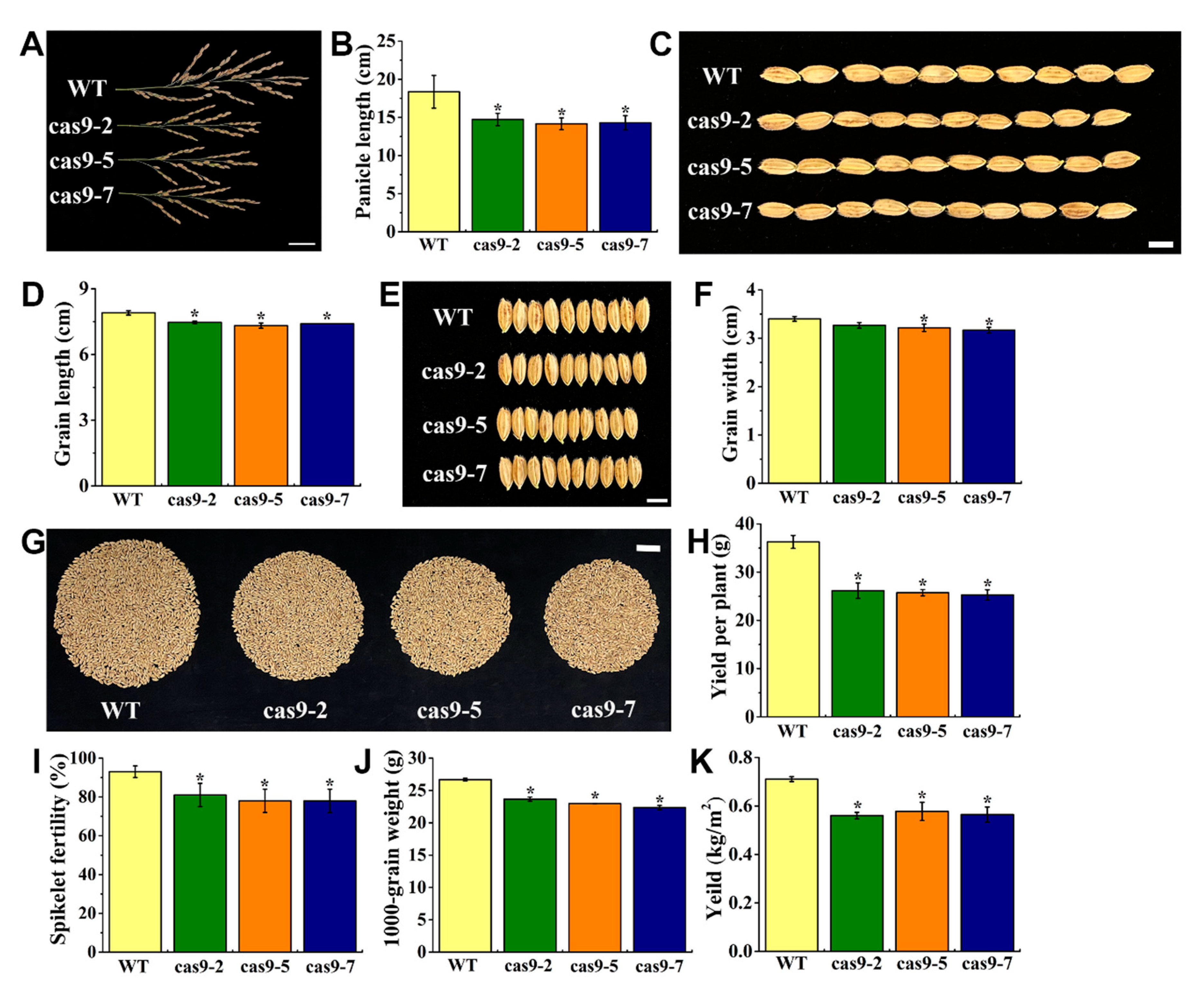
3.4. Knocking Out OsHIPP56 Impaired Rice Seed Development and Reduced Mn Concentrations in Maturity Rice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Andresen, E.; Peiter, E.; Küpper, H. Trace metal metabolism in plants. J. Exp. Bot. 2018, 69, 909–954. [Google Scholar] [CrossRef] [PubMed]
- Socha, A.L.; Guerinot, M.L. Mn-euvering manganese: The role of transporter gene family members in manganese uptake and mobilization in plants. Front. Plant Sci. 2014, 5, 106. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.F.; Yamaji, N.; Shen, R.F.; Ma, J.F. The key to Mn homeostasis in plants: Regulation of Mn transporters. Trends Plant Sci. 2017, 22, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H. Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 1986. [Google Scholar]
- Sasaki, A.; Yamaji, N.; Yokosho, K.; Ma, J.F. Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Plant Cell 2012, 24, 2155–2167. [Google Scholar] [CrossRef]
- Ueno, D.; Sasaki, A.; Yamaji, N.; Miyaji, T.; Fujii, Y.; Takemoto, Y.; Moriyama, S.; Che, J.; Moriyama, Y.; Iwasaki, K.; et al. A polarly localized transporter for efficient manganese uptake in rice. Nat. Plants 2015, 1, 15170. [Google Scholar] [CrossRef]
- Ishimaru, Y.; Masuda, H.; Bashir, K.; Inoue, H.; Tsukamoto, T.; Takahashi, M.; Nakanishi, H.; Aoki, N.; Hirose, T.; Ohsugi, R.; et al. Rice metal–nicotianamine transporter, OsYSL2, is required for the long-distance transport of iron and manganese. Plant J. 2010, 62, 379–390. [Google Scholar] [CrossRef]
- Sasaki, A.; Yamaji, N.; Xia, J.; Ma, J.F. OsYSL6 is involved in the detoxification of excess manganese in rice. Plant Physiol. 2011, 157, 1832–1840. [Google Scholar] [CrossRef]
- Chen, Z.; Fujii, Y.; Yamaji, N.; Masuda, S.; Takemoto, Y.; Kamiya, T.; Yusuyin, Y.; Iwasaki, K.; Kato, S.I.; Maeshima, M.; et al. Mn tolerance in rice is mediated by MTP8. 1, a member of the cation diffusion facilitator family. J. Exp. Bot. 2013, 64, 4375–4387. [Google Scholar] [CrossRef]
- Takemoto, Y.; Tsunemitsu, Y.; Fuji-Kashino, M.; Mitani-Ueno, N.; Yamaji, N.; Ma, J.F.; Kato, S.; Iwasaki, K.; Ueno, D. The tonoplastlocalized transporter MTP8.2 contributes to manganese detoxifcation in the shoots and roots of Oryza sativa L. Plant Cell Physiol. 2017, 58, 1573–1582. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, F.; Liu, J.L.; Wu, H.T.; Yang, H.; Shi, Y.; Liu, J.; Zhang, Y.F.; Luo, Y.R.; Chen, K.M. Heavy metal transporters: Functional mechanisms, regulation, and application in phytoremediation. Sci. Total Environ. 2022, 809, 151099. [Google Scholar] [CrossRef]
- Rono, J.K.; Sun, D.; Yang, Z.M. Metallochaperones: A critical regulator of metal homeostasis and beyond. Gene 2022, 822, 146352. [Google Scholar] [CrossRef]
- Robinson, N.J.; Winge, D.R. Copper Metallochaperones. Annu. Rev. Biochem. 2010, 79, 537–562. [Google Scholar] [CrossRef]
- Tehseen, M.; Cairns, N.; Sherson, S.; Cobbett, C.S. Metallochaperone-like genes in Arabidopsis thaliana. Metallomics 2010, 2, 556–564. [Google Scholar] [CrossRef]
- De Abreu-Neto, J.B.; Turchetto-Zolet, A.C.; de Oliveira, L.F.; Zanettini, M.H.; Margis-Pinheiro, M. Heavy metal-associated isoprenylated plant protein (HIPP): Characterization of a family of proteins exclusive to plants. FEBS J. 2013, 280, 1604–1616. [Google Scholar] [CrossRef]
- Barth, O.; Vogt, S.; Uhlemann, R.; Zschiesche, W.; Humbeck, K. Stress induced and nuclear localized HIPP26 from Arabidopsis thaliana interacts via its heavy metal associated domain with the drought stress related zinc finger transcription factor ATHB. Plant Mol. Biol. 2009, 69, 213–226. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, H.; Feng, C.; Xu, H.; Huang, X.; Wang, Q.; Duan, X.; Wang, X.; Wei, G.; Huang, L.; et al. Isolation and characterisation of cDNA encoding a wheat heavy metal-associated isoprenylated protein involved in stress responses. Plant Biol. 2015, 17, 1176–1186. [Google Scholar] [CrossRef]
- Abdel-Ghany, S.E.; Burkhead, J.L.; Gogolin, K.A.; Andrés-Colás, N.; Bodecker, J.R.; Puig, S.; Peñarrubia, L.; Pilon, M. AtCCS is a functional homolog of the yeast copper chaperone Ccs1/Lys. FEBS Lett. 2005, 579, 2307–2312. [Google Scholar] [CrossRef]
- Gao, W.; Xiao, S.; Li, H.Y.; Tsao, S.W.; Chye, M.L. Arabidopsis thaliana acyl-CoA-binding protein ACBP2 interacts with heavy-metal-binding farnesylated protein AtFP. New Phytol. 2009, 181, 89–102. [Google Scholar] [CrossRef]
- Shin, L.J.; Yeh, K.C. Overexpression of Arabidopsis ATX1 retards plant growth under severe copper deficiency. Plant Signal. Behav. 2012, 7, 1082–1083. [Google Scholar] [CrossRef][Green Version]
- Khan, I.U.; Rono, J.K.; Zhang, B.Q.; Liu, X.S.; Wang, M.Q.; Wang, L.L.; Wu, X.C.; Chen, X.; Cao, H.W.; Yang, Z.M. Identification of novel rice (Oryza sativa) HPP and HIPP genes tolerant to heavy metal toxicity. Ecotoxicol. Environ. Saf. 2019, 175, 8–18. [Google Scholar] [CrossRef]
- Zhang, B.Q.; Liu, X.S.; Feng, S.J.; Zhao, Y.N.; Wang, L.L.; Rono, J.K.; Li, H.; Yang, Z.M. Developing a cadmium resistant rice genotype with OsHIPP29 locus for limiting cadmium accumulation in the paddy crop. Chemosphere 2020, 247, 125958. [Google Scholar] [CrossRef]
- Chen, G.; Xiong, S. OsHIPP24 is a copper metallochaperone which affects rice growth. J. Plant Biol. 2021, 64, 145–153. [Google Scholar] [CrossRef]
- Zhao, Y.N.; Wang, M.Q.; Li, C.; Cao, H.W.; Rono, J.K.; Yang, Z.M. The metallochaperone OsHIPP56 gene is required for cadmium detoxification in rice crops. Environ. Exp. Bot. 2022, 193, 104680. [Google Scholar] [CrossRef]
- Khan, I.U.; Rono, J.K.; Liu, X.S.; Feng, S.J.; Li, H.; Chen, X.; Yang, Z.M. Functional characterization of a new metallochaperone for reducing cadmium concentration in rice crop. J. Clean. Prod. 2020, 272, 123152. [Google Scholar] [CrossRef]
- Feng, S.J.; Liu, X.S.; Cao, H.W.; Yang, Z.M. Identification of a rice metallochaperone for cadmium tolerance by an epigenetic mechanism and potential use for clean up in wetland. Environ. Pollut. 2021, 288, 117837. [Google Scholar] [CrossRef]
- Song, J.; Feng, S.J.; Chen, J.; Zhao, W.T.; Yang, Z.M. A cadmium stress-responsive gene AtFC1 confers plant tolerance to cadmium toxicity. BMC Plant Biol. 2017, 17, 187. [Google Scholar] [CrossRef]
- Uraguchi, S.; Fujiwara, T. Rice breaks ground for cadmium-free cereals. Curr. Opin. Plant Biol. 2013, 16, 328–334. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, K.; Zhao, F.J.; Sun, C.; Jin, C.; Shi, Y.; Sun, Y.; Li, Y.; Yang, M.; Jing, X.; et al. OsATX1 interacts with heavy metal P1B-type ATPases and affects copper transport and distribution. Plant Physiol. 2018, 178, 329–344. [Google Scholar] [CrossRef]
- Cao, H.W.; Li, C.; Zhang, B.Q.; Rono, J.K.; Yang, Z.M. A Metallochaperone HIPP33 Is Required for Rice Zinc and Iron Homeostasis and Productivity. Agronomy 2022, 12, 488. [Google Scholar] [CrossRef]
- Andrés-Colás, N.; Sancenón, V.; Rodríguez-Navarro, S.; Mayo, S.; Thiele, D.J.; Ecker, J.R.; Puig, S.; Peñarrubia, L. The Arabidopsis heavy metal P-type ATPase HMA5 interacts with metallochaperones and functions in copper detoxification of roots. Plant J. 2006, 45, 225–236. [Google Scholar] [CrossRef]
- Wu, Z.; Liang, F.; Hong, B.; Young, J.C.; Sussman, M.R.; Harper, J.F.; Sze, H. An endoplasmic reticulum-bound Ca2+/Mn2+ pump, ECA1, supports plant growth and confers tolerance to Mn2+ stress. Plant Physiol. 2002, 130, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Farthing, E.C.; Menguer, P.K.; Fett, J.P.; Williams, L.E. OsMTP11 is localised at the Golgi and contributes to Mn tolerance. Sci. Rep. 2017, 7, 15258. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Wang, Y.; Xue, D.; Wang, J.; Yan, M.; Liu, G.; Dong, G.; Zeng, D.; Lu, Z.; Zhu, X.; et al. Regulation of OsSPL14 by OsmiR156 defines ideal plant architecture in rice. Nat. Genet. 2010, 42, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.N.; Li, C.; Li, H.; Liu, X.S.; Yang, Z.M. OsZIP11 is a trans-Golgi-residing transporter required for rice iron accumulation and development. Gene 2022, 836, 146678. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J. Molecular basis of plant architecture. Annu. Rev. Plant Biol. 2008, 59, 253–279. [Google Scholar] [CrossRef]
- Jin, J.; Huang, W.; Gao, J.P.; Yang, J.; Shi, M.; Zhu, M.Z.; Luo, D.; Lin, H.X. Genetic control of rice plant architecture under domestication. Nat. Genet. 2008, 40, 1365–1369. [Google Scholar] [CrossRef]
- Wu, Q.; Li, D.; Li, D.; Liu, X.; Zhao, X.; Li, X.; Li, S.; Zhu, L. Overexpression of OsDof12 affects plant architecture in rice (Oryza sativa L.). Front. Plant Sci. 2015, 6, 833. [Google Scholar] [CrossRef]
- Gao, J.; Liang, H.; Huang, J.; Qing, D.; Wu, H.; Zhou, W.; Chen, W.; Pan, Y.; Dai, G.; Gao, L.; et al. Development of the PARMS marker of the TAC1 gene and its utilization in rice plant architecture breeding. Euphytica 2021, 217, 49. [Google Scholar] [CrossRef]
- Liu, H.; Zhan, J.; Li, J.; Lu, X.; Liu, J.; Wang, Y.; Zhao, Q.; Ye, G. Genome-wide association study (GWAS) for mesocotyl elongation in rice (Oryza sativa L.) under multiple culture conditions. Genes 2019, 11, 49. [Google Scholar] [CrossRef]
- Zhang, J.; Tong, T.; Potcho, P.M.; Huang, S.; Ma, L.; Tang, X. Nitrogen effects on yield, quality and physiological characteristics of giant rice. Agronomy 2020, 10, 1816. [Google Scholar] [CrossRef]
- Huang, R.; Jiang, L.; Zheng, J.; Wang, T.; Wang, H.; Huang, Y.; Hong, Z. Genetic bases of rice grain shape: So many genes, so little known. Trends Plant Sci. 2013, 18, 218–226. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Li, H.; Rono, J.K.; Wang, M.Q.; Yang, Z.M. A Metal Chaperone Gene Regulates Rice Growth and Seed Development by Manganese Acquisition and Homeostasis. Agronomy 2022, 12, 1676. https://doi.org/10.3390/agronomy12071676
Li C, Li H, Rono JK, Wang MQ, Yang ZM. A Metal Chaperone Gene Regulates Rice Growth and Seed Development by Manganese Acquisition and Homeostasis. Agronomy. 2022; 12(7):1676. https://doi.org/10.3390/agronomy12071676
Chicago/Turabian StyleLi, Chao, He Li, Justice Kipkorir Rono, Mong Qi Wang, and Zhi Min Yang. 2022. "A Metal Chaperone Gene Regulates Rice Growth and Seed Development by Manganese Acquisition and Homeostasis" Agronomy 12, no. 7: 1676. https://doi.org/10.3390/agronomy12071676
APA StyleLi, C., Li, H., Rono, J. K., Wang, M. Q., & Yang, Z. M. (2022). A Metal Chaperone Gene Regulates Rice Growth and Seed Development by Manganese Acquisition and Homeostasis. Agronomy, 12(7), 1676. https://doi.org/10.3390/agronomy12071676





