Modification of Gene Expression, DNA Methylation and Small RNAs Expression in Rice Plants under In Vitro Culture
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Methylation-Sensitive Amplified Polymorphism (MSAP) Analysis
2.3. Bisulfite Sequencing
2.4. Analysis of SmRNA-Seq and Affymetrix GeneChip® Rice Genome Array
2.5. Gene Ontology (GO) Analysis
2.6. Real-Time qRT-PCR Analysis
3. Results
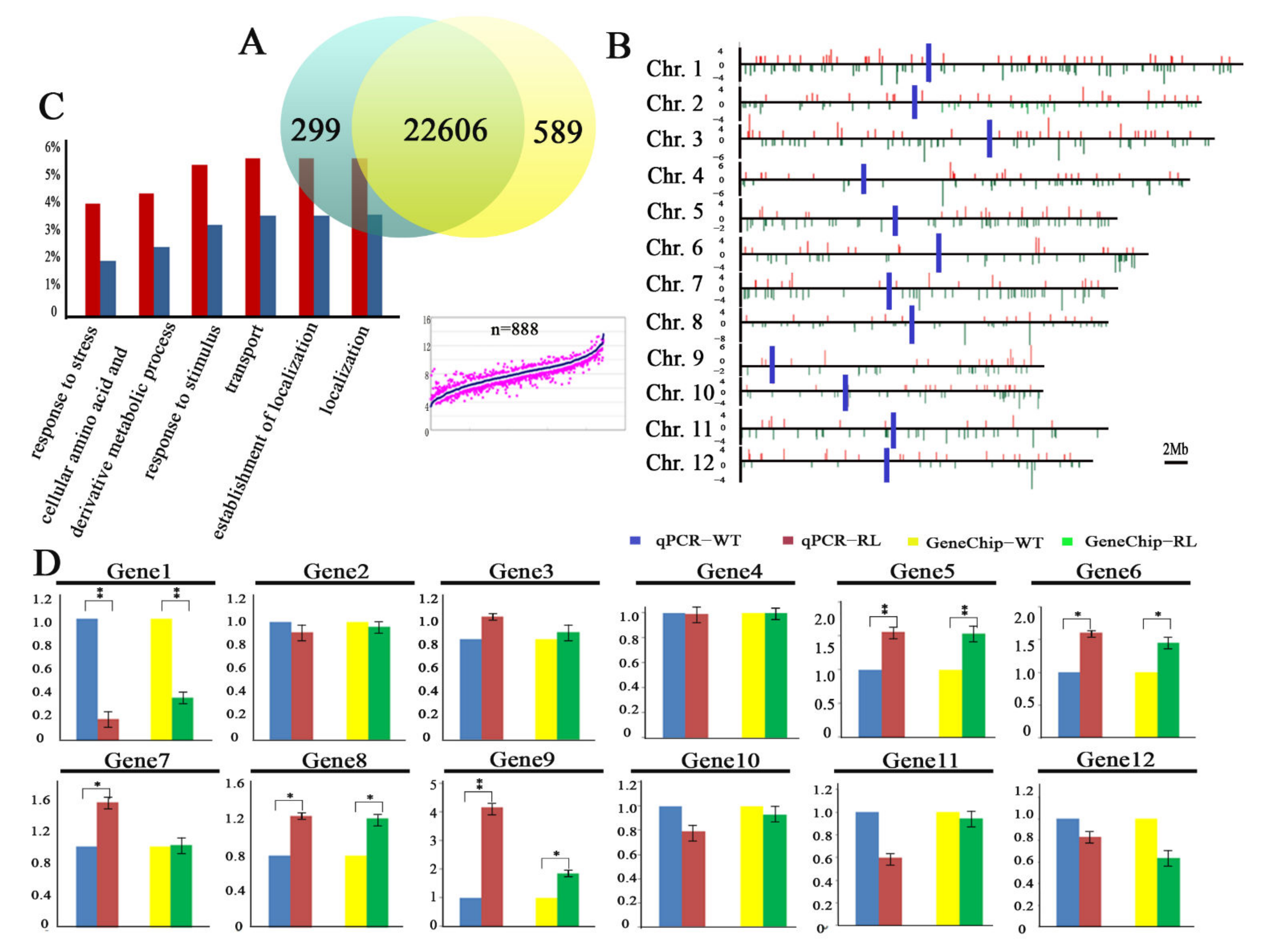
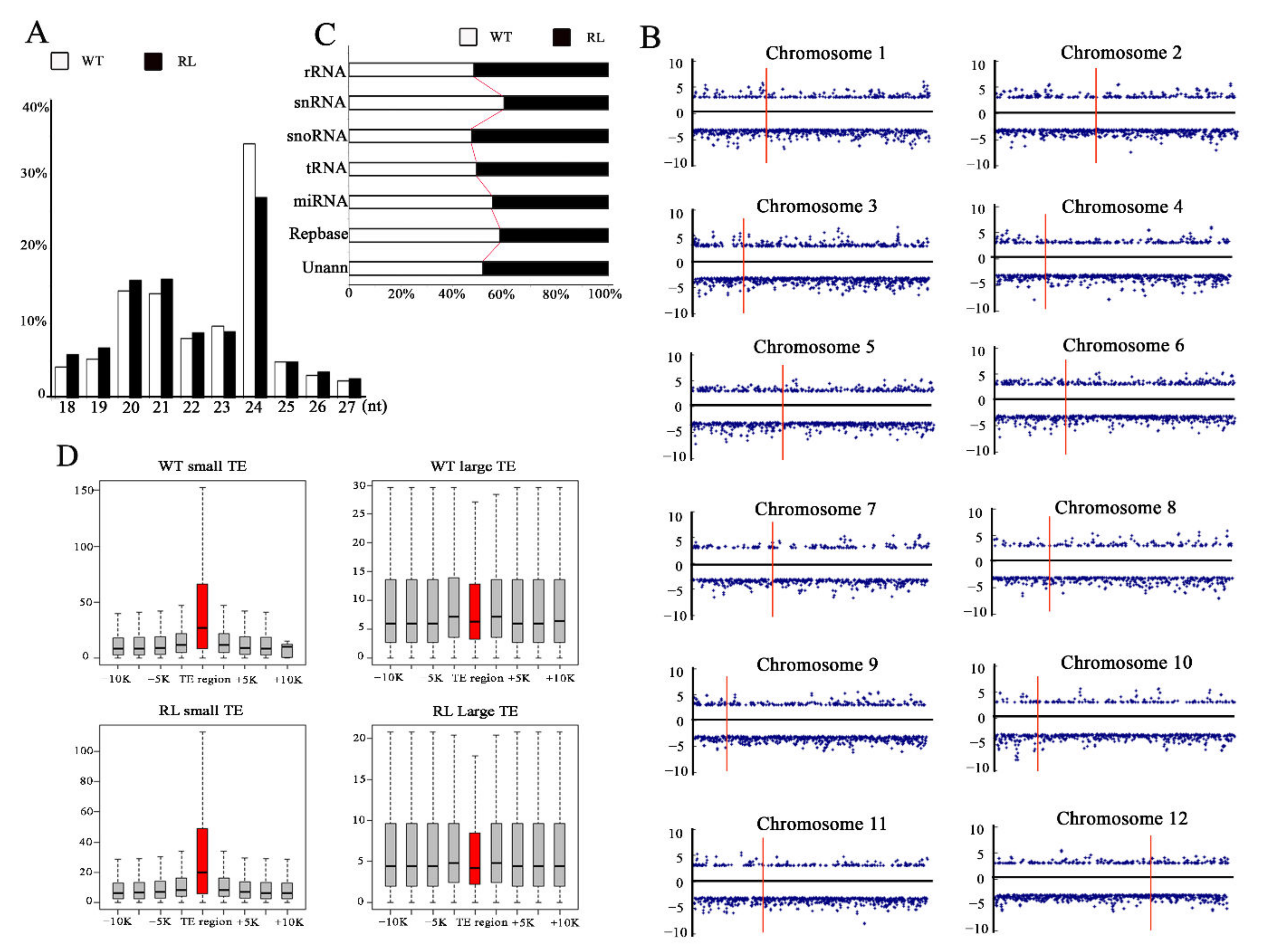
3.1. Tissue Culture Induces Gene Expression Variation and TE Activation in Regenerate Line of Rice cv. Hitomebore
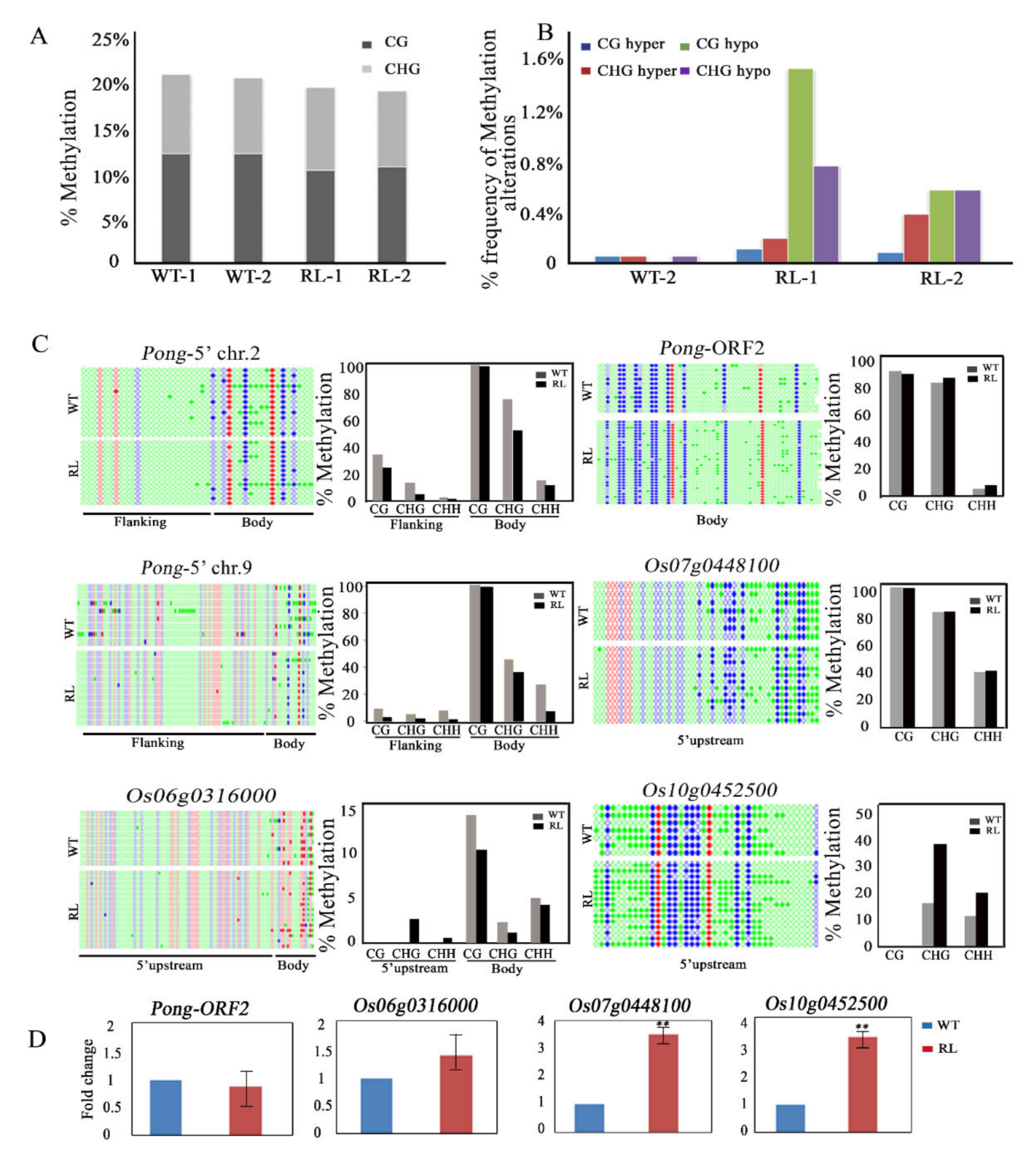
3.2. Tissue Culture Induced DNA Methylation Alteration in Regenerated Line of Rice cv. Hitomebore
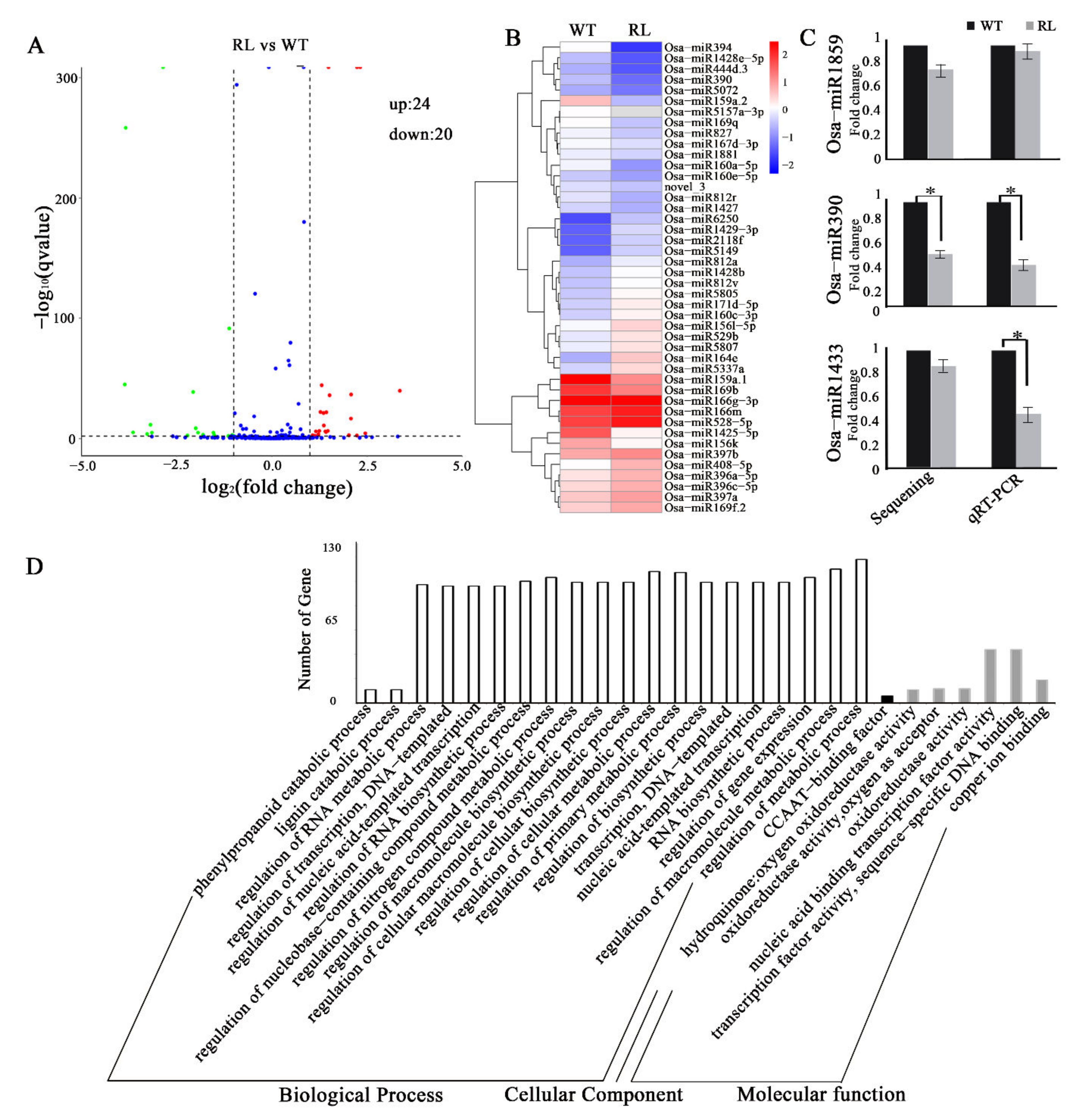
3.3. Tissue Culture Induced smRNAs Expression Variation in Regenerated Line of Rice cv. Hitomebore
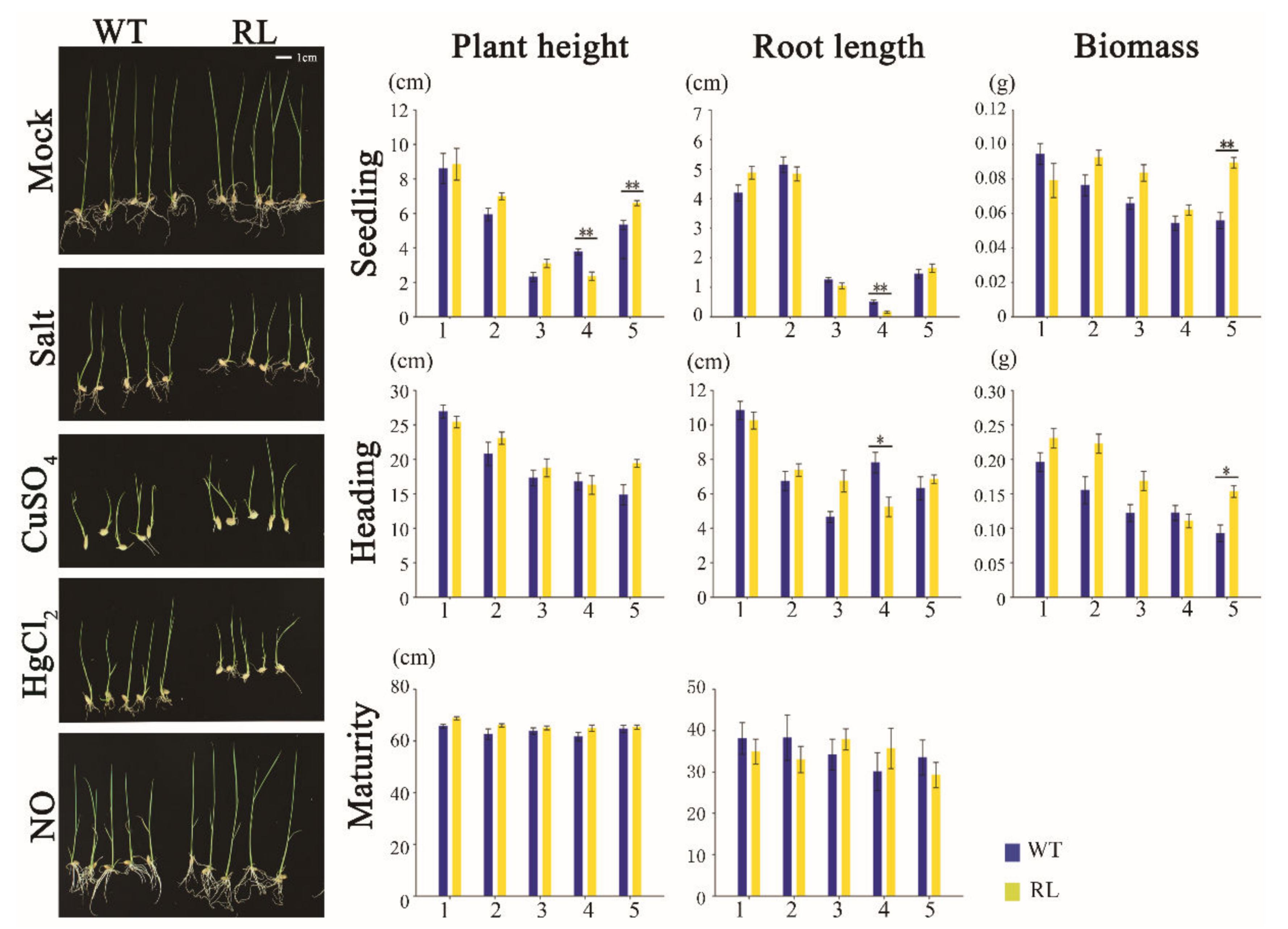
3.4. Regenerated Plants Display Phenotypic Variations Only under Stress Conditions
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grafi, G.; Avivi, Y. Stem cells: A lesson from dedifferentiation. Trends Biotechnol. 2004, 22, 388–389. [Google Scholar] [CrossRef] [PubMed]
- Grafi, G.; Florentin, A.; Ransbotyn, V.; Morgenstern, Y. The stem cell state in plant development and in response to stress. Front. Plant. Sci. 2011, 2, 53. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, T.A. History of plant tissue culture. Methods Mol. Biol. 2006, 318, 9–32. [Google Scholar] [CrossRef] [PubMed]
- Mcclintock, B. The significance of responses of the genome to challenge. Science 1984, 226, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Polanco, C.; Ruiz, M.L. AFLP analysis of somaclonal variation in Arabidopsis thaliana regenerated plants. Plant. Sci. 2002, 162, 817–824. [Google Scholar] [CrossRef]
- Larkin, P.J.; Scowcroft, W.R. Somaclonal variation-a novel source of variability from cell cultures for plant improvement. Theor. Appl. Genet. 1981, 60, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Johannes, F.; Porcher, E.; Teixeira, F.K.; Saliba-Colombani, V.; Simon, M.; Agier, N.; Bulski, A.; Albuisson, J.; Heredia, F.; Audigier, P.; et al. Assessing the impact of transgenerational epigenetic variation on complex traits. PLoS Genet. 2009, 5, e1000530. [Google Scholar] [CrossRef]
- Stroud, H.; Ding, B.; Simon, S.A.; Feng, S.; Bellizzi, M.; Pellegrini, M.; Wang, G.; Meyers, B.C.; Jacobsen, S.E. Plants regenerated from tissue culture contain stable epigenome changes in rice. eLife 2013, 2, e354. [Google Scholar] [CrossRef]
- Bednarek, P.T.; Orłowska, R.; Koebner, R.M.D.; Zimny, J. Quantification of the tissue-culture induced variation in barley (Hordeum vulgare L.). BMC Plant. Biol. 2007, 7, 10. [Google Scholar] [CrossRef][Green Version]
- Jiang, C.; Mithani, A.; Gan, X.; Belfield, E.J.; Klingler, J.P.; Zhu, J.K.; Ragoussis, J.; Mott, R.; Harberd, N.P. Regenerant Arabidopsis lineages display a distinct genome-wide spectrum of mutations conferring variant phenotypes. Curr. Biol. 2011, 21, 1385–1390. [Google Scholar] [CrossRef]
- Sabot, F.; Picault, N.; El-Baidouri, M.; Llauro, C.; Chaparro, C.; Piegu, B.; Roulin, A.; Guiderdoni, E.; Delabastide, M.; McCombie, R.; et al. Transpositional landscape of the rice genome revealed by paired-end mapping of high-throughput re-sequencing data. Plant. J. 2011, 66, 241–246. [Google Scholar] [CrossRef]
- Neelakandan, A.K.; Wang, K. Recent progress in the understanding of tissue culture-induced genome level changes in plants and potential applications. Plant. Cell Rep. 2012, 31, 597–620. [Google Scholar] [CrossRef]
- Lin, W.; Xiao, X.; Zhang, H.; Li, Y.; Liu, S.; Sun, W.; Zhang, X.; Wu, Q. Whole-Genome Bisulfite Sequencing Reveals a Role for DNA Methylation in Variants from Callus Culture of Pineapple (Ananas comosus L.). Genes 2019, 10, 877. [Google Scholar] [CrossRef]
- Smith, A.M.; Hansey, C.N.; Kaeppler, S.M. TCUP: A Novel hAT Transposon Active in Maize Tissue Culture. Front. Plant. Sci. 2012, 3, 6. [Google Scholar] [CrossRef]
- Kaeppler, S.M.; Phillips, R.L. Tissue culture-induced DNA methylation variation in maize. Proc. Natl. Acad. Sci. USA 1993, 90, 8773–8776. [Google Scholar] [CrossRef]
- Kaeppler, S.M.; Kaeppler, H.F.; Rhee, Y. Epigenetic aspects of somaclonal variation in plants. Plant. Mol. Biol. 2000, 43, 179–188. [Google Scholar] [CrossRef]
- Rhee, Y.; Sekhon, R.S.; Chopra, S.; Kaeppler, S. Tissue culture-induced novel epialleles of a Myb transcription factor encoded by pericarp color1 in maize. Genetics 2010, 186, 843–855. [Google Scholar] [CrossRef]
- Miguel, C.; Marum, L. An epigenetic view of plant cells cultured in vitro: Somaclonal variation and beyond. J. Exp. Bot. 2011, 62, 3713–3725. [Google Scholar] [CrossRef]
- Law, J.A.; Jacobsen, S.E. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat. Rev. Genet. 2010, 11, 204–220. [Google Scholar] [CrossRef]
- Zhang, H.; Lang, Z.; Zhu, J. Dynamics and function of DNA methylation in plants. Nat. Rev. Mol. Cell Biol. 2018, 19, 489–506. [Google Scholar] [CrossRef]
- Hu, L.; Li, N.; Xu, C.; Zhong, S.; Lin, X.; Yang, J.; Zhou, T.; Yuliang, A.; Wu, Y.; Chen, Y.; et al. Mutation of a major CG methylase in rice causes genome-wide hypomethylation, dysregulated genome expression, and seedling lethality. Proc. Natl. Acad. Sci. USA 2014, 111, 10642–10647. [Google Scholar] [CrossRef]
- Aguilar-Cruz, A.; Grimanelli, D.; Haseloff, J.; Arteaga-Vázquez, M.A. DNA methylation in Marchantia polymorpha. New Phytol. 2019, 223, 575–581. [Google Scholar] [CrossRef]
- Matzke, M.A.; Mosher, R.A. RNA-directed DNA methylation: An epigenetic pathway of increasing complexity. Nat. Rev. Genet. 2014, 15, 394–408. [Google Scholar] [CrossRef]
- Wei, L.; Gu, L.; Song, X.; Cui, X.; Lu, Z.; Zhou, M.; Wang, L.; Hu, F.; Zhai, J.; Meyers, B.C.; et al. Dicer-like 3 produces transposable element-associated 24-nt siRNAs that control agricultural traits in rice. Proc. Natl. Acad. Sci. USA 2014, 111, 3877–3882. [Google Scholar] [CrossRef]
- Stroud, H.; Greenberg, M.V.C.; Feng, S.; Bernatavichute, Y.V.; Jacobsen, S.E. Comprehensive analysis of silencing mutants reveals complex regulation of the Arabidopsis methylome. Cell 2013, 152, 352–364. [Google Scholar] [CrossRef]
- Jiang, N.; Bao, Z.; Zhang, X.; Hirochika, H.; Eddy, S.R.; Mccouch, S.R.; Wessler, S.R. An active DNA transposon family in rice. Nature 2003, 421, 163–167. [Google Scholar] [CrossRef]
- Nakazaki, T.; Okumoto, Y.; Horibata, A.; Yamahira, S.; Teraishi, M.; Nishida, H.; Inoue, H.; Tanisaka, T. Mobilization of a transposon in the rice genome. Nature 2003, 421, 170–172. [Google Scholar] [CrossRef]
- Hu, L.; Li, N.; Zhang, Z.; Meng, X.; Dong, Q.; Xu, C.; Gong, L.; Liu, B. CG hypomethylation leads to complex changes in DNA methylation and transpositional burst of diverse transposable elements in callus cultures of rice. Plant. J. 2020, 101, 188–203. [Google Scholar] [CrossRef]
- Tanurdzic, M.; Vaughn, M.W.; Jiang, H.; Lee, T.; Slotkin, R.K.; Sosinski, B.; Thompson, W.F.; Doerge, R.W.; Martienssen, R.A. Epigenomic consequences of immortalized plant cell suspension culture. PLoS Biol. 2008, 6, 2880–2895. [Google Scholar] [CrossRef]
- Ramakrishnan, M.; Satish, L.; Kalendar, R.; Narayanan, M.; Kandasamy, S.; Sharma, A.; Emamverdian, A.; Wei, Q.; Zhou, M. The Dynamism of Transposon Methylation for Plant Development and Stress Adaptation. Int. J. Mol. Sci. 2021, 22, 11387. [Google Scholar] [CrossRef]
- Eamens, A.; Wang, M.; Smith, N.A.; Waterhouse, P.M. RNA silencing in plants: Yesterday, today, and tomorrow. Plant. Physiol. 2008, 147, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Borges, F.; Martienssen, R.A. The expanding world of small RNAs in plants. Nat. Rev. Mol. Cell Biol. 2015, 16, 727–741. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Li, Y.; Cao, X.; Qi, Y. MicroRNAs and Their Regulatory Roles in Plant-Environment Interactions. Annu. Rev. Plant. Biol. 2019, 70, 489–525. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhou, H.; Li, Y.; Chen, J.; Yang, J.; Chen, Y.; Qu, L. Rice embryogenic calli express a unique set of microRNAs, suggesting regulatory roles of microRNAs in plant post-embryogenic development. FEBS Lett. 2006, 580, 5111–5116. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.; Huang, F.; Chang, L.; Ma, Y. MicroRNA expression profiles in conventional and micropropagated strawberry (Fragaria × ananassa Duch.) plants. Plant. Cell Rep. 2009, 28, 891–902. [Google Scholar] [CrossRef]
- Cokus, S.J.; Feng, S.; Zhang, X.; Chen, Z.; Merriman, B.; Haudenschild, C.D.; Pradhan, S.; Nelson, S.F.; Pellegrini, M.; Jacobsen, S.E. Shotgun bisulphite sequencing of the Arabidopsis genome reveals DNA methylation patterning. Nature 2008, 452, 215–219. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Z.; Wang, N.; Gao, Y.; Liu, Y.; Wu, Y.; Bai, Y.; Zhang, Z.; Lin, X.; Dong, Y.; et al. Tissue culture-induced heritable genomic variation in rice, and their phenotypic implications. PLoS ONE 2014, 9, e96879. [Google Scholar] [CrossRef]
- Beckmann, J.S.; Osborn, T.C. Simple plant DNA isolation procedures. In Plant Genomes: Methods for Genetic and Physical Mapping; Springer: Dordrecht, The Netherlands, 1992; pp. 1–13. [Google Scholar] [CrossRef]
- Dong, Z.Y.; Wang, Y.M.; Zhang, Z.J.; Shen, Y.; Lin, X.Y.; Ou, X.F.; Han, F.P.; Liu, B. Extent and pattern of DNA methylation alteration in rice lines derived from introgressive hybridization of rice and Zizania latifolia Griseb. Theor. Appl. Genet. 2006, 113, 196–205. [Google Scholar] [CrossRef]
- Wang, X.; Wu, R.; Lin, X.; Bai, Y.; Song, C.; Yu, X.; Xu, C.; Zhao, N.; Dong, Y.; Liu, B. Tissue culture-induced genetic and epigenetic alterations in rice pure-lines, F1 hybrids and polyploids. BMC Plant. Biol. 2013, 13, 77. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, D.; Wang, Z.; Xun, H.; Ma, J.; Wang, H.; Huang, W.; Liu, Y.; Lin, X.; Li, N.; et al. Mutation of the RDR1 gene caused genome-wide changes in gene expression, regional variation in small RNA clusters and localized alteration in DNA methylation in rice. BMC Plant. Biol. 2014, 14, 177. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Li, L.; Wang, X.; Xia, M.; Stolc, V.; Su, N.; Peng, Z.; Li, S.; Wang, J.; Wang, X.; Deng, X.W. Tiling microarray analysis of rice chromosome 10 to identify the transcriptome and relate its expression to chromosomal architecture. Genome Biol. 2005, 6, R52. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, M.; Jin, X.; Tao, H.; Wang, Y.; Peng, B.; Fu, C.; Yu, L. Transcriptional reprogramming strategies and miRNA-mediated regulation networks of Taxus media induced into callus cells from tissues. BMC Genom. 2020, 21, 168. [Google Scholar] [CrossRef]
- Zhao, N.; Zhang, K.; Wang, C.; Yan, H.; Liu, Y.; Xu, W.; Su, Z. Systematic Analysis of Differential H3K27me3 and H3K4me3 Deposition in Callus and Seedling Reveals the Epigenetic Regulatory Mechanisms Involved in Callus Formation in Rice. Front. Genet. 2020, 11, 766. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, F.; Qi, P.; Huang, Y.; Xie, Y.; Xu, L.; Han, N.; Xu, L.; Bian, H. OsHDA710-Mediated Histone Deacetylation Regulates Callus Formation of Rice Mature Embryo. Plant. Cell Physiol. 2020, 61, 1646–1660. [Google Scholar] [CrossRef]
- Orłowska, R.; Machczyńska, J.; Oleszczuk, S.; Zimny, J.; Bednarek, P.T. DNA methylation changes and TE activity induced in tissue cultures of barley (Hordeum vulgare L.). J. Biol. Res. 2016, 23, 19. [Google Scholar] [CrossRef]
- Gulzar, B.; Mujib, A.; Malik, M.Q.; Sayeed, R.; Mamgain, J.; Ejaz, B. Genes, proteins and other networks regulating somatic embryogenesis in plants. J. Genet. Eng. Biotechnol. 2020, 18, 31. [Google Scholar] [CrossRef]
- Li, J.; Li, N.; Zhu, L.; Zhang, Z.; Li, X.; Wang, J.; Xun, H.; Zhao, J.; Wang, X.; Wang, T.; et al. Mutation of a major CG methylase alters genome-wide lncRNA expression in rice. G3 (Bethesda Md.) 2021, 11, jkab049. [Google Scholar] [CrossRef]
- Ngezahayo, F.; Xu, C.; Wang, H.; Jiang, L.; Pang, J.; Liu, B. Tissue culture-induced transpositional activity of mPing is correlated with cytosine methylation in rice. BMC Plant. Biol. 2009, 9, 91. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, J.; Han, S.; Yang, W.; Li, W.; Wei, H.; Li, X.; Qi, L. Four abiotic stress-induced miRNA families differentially regulated in the embryogenic and non-embryogenic callus tissues of Larix leptolepis. Biochem. Biophys. Res. Commun. 2010, 398, 355–360. [Google Scholar] [CrossRef]
- Barrera-Rojas, C.H.; Rocha, G.H.B.; Polverari, L.; Pinheiro Brito, D.A.; Batista, D.S.; Notini, M.M.; Da Cruz, A.C.F.; Morea, E.G.O.; Sabatini, S.; Otoni, W.C.; et al. miR156-targeted SPL10 controls Arabidopsis root meristem activity and root-derived de novo shoot regeneration via cytokinin responses. J. Exp. Bot. 2020, 71, 934–950. [Google Scholar] [CrossRef]
- Wang, M.; Masuta, C.; Smith, N.A.; Shimura, H. RNA silencing and plant viral diseases. Mol. Plant.-Microbe Interact. MPMI 2012, 25, 1275–1285. [Google Scholar] [CrossRef]
- Yang, X.; Kang, Y.; Liu, Y.; Shi, M.; Zhang, W.; Fan, Y.; Yao, Y.; Li, H.; Qin, S. Integrated analysis of miRNA-mRNA regulatory networks of potato (Solanum tuberosum L.) in response to cadmium stress. Ecotox. Environ. Saf. 2021, 224, 112682. [Google Scholar] [CrossRef]
- Yan, W.; Cao, S.; Wu, Y.; Ye, Z.; Zhang, C.; Yao, G.; Yu, J.; Yang, D.; Zhang, J. Integrated Analysis of Physiological, mRNA Sequencing, and miRNA Sequencing Data Reveals a Specific Mechanism for the Response to Continuous Cropping Obstacles in Pogostemon cablin Roots. Front. Plant. Sci. 2022, 13, 853110. [Google Scholar] [CrossRef]
- Luján-Soto, E.; Juárez-González, V.T.; Reyes, J.L.; Dinkova, T.D. MicroRNA Zma-miR528 Versatile Regulation on Target mRNAs during Maize Somatic Embryogenesis. Int. J. Mol. Sci. 2021, 22, 5310. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, J.; Wu, Y.; Li, D.; Allan, A.C.; Yin, X. Genome-wide analysis of coding and non-coding RNA reveals a conserved miR164-NAC regulatory pathway for fruit ripening. New Phytol. 2020, 225, 1618–1634. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Enriquez, J.; Dickinson, H.G.; Grant-Downton, R.T. MicroRNA misregulation: An overlooked factor generating somaclonal variation? Trends Plant. Sci. 2011, 16, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, Q.; Zhou, H.; Ni, F.; Wu, X.; Qi, Y. Rice MicroRNA Effector Complexes and Targets. Plant. Cell. 2009, 21, 3421–3435. [Google Scholar] [CrossRef]
- Stelpflug, S.C.; Eichten, S.R.; Hermanson, P.J.; Springer, N.M.; Kaeppler, S.M. Consistent and heritable alterations of DNA methylation are induced by tissue culture in maize. Genetics 2014, 198, 209–218. [Google Scholar] [CrossRef]
| Source of sRNA | RL | WT | % Changes (RL vs. WT) |
|---|---|---|---|
| rRNA | 1,370,141 | 1,269,569 | +7.9% |
| snRNA | 1182 | 1759 | −32.8% |
| snoRNA | 6991 | 6242 | +12% |
| miRNA | 1,216,550 | 1,510,997 | −19.5% |
| Repeat DNA | 1,677,129 | 2,324,040 | −27.8% |
| Exon-sense | 892,763 | 794,805 | +12.3% |
| Exon-antisense | 29,751 | 35,949 | −17.2% |
| Intron-sense | 25,314 | 33,277 | −23.9% |
| Intron-antisense | 20,776 | 25,570 | −18.7% |
| unannotated | 3,379,729 | 3,395,299 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Yu, Y.; Zhang, D.; Zhang, Z.; Wang, Z.; Xun, H.; Li, G.; Liu, B.; Zhang, J. Modification of Gene Expression, DNA Methylation and Small RNAs Expression in Rice Plants under In Vitro Culture. Agronomy 2022, 12, 1675. https://doi.org/10.3390/agronomy12071675
Wang N, Yu Y, Zhang D, Zhang Z, Wang Z, Xun H, Li G, Liu B, Zhang J. Modification of Gene Expression, DNA Methylation and Small RNAs Expression in Rice Plants under In Vitro Culture. Agronomy. 2022; 12(7):1675. https://doi.org/10.3390/agronomy12071675
Chicago/Turabian StyleWang, Ningning, Yanan Yu, Di Zhang, Zhibin Zhang, Zhenhui Wang, Hongwei Xun, Guo Li, Bao Liu, and Jian Zhang. 2022. "Modification of Gene Expression, DNA Methylation and Small RNAs Expression in Rice Plants under In Vitro Culture" Agronomy 12, no. 7: 1675. https://doi.org/10.3390/agronomy12071675
APA StyleWang, N., Yu, Y., Zhang, D., Zhang, Z., Wang, Z., Xun, H., Li, G., Liu, B., & Zhang, J. (2022). Modification of Gene Expression, DNA Methylation and Small RNAs Expression in Rice Plants under In Vitro Culture. Agronomy, 12(7), 1675. https://doi.org/10.3390/agronomy12071675








