Arbuscular Mycorrhizal Fungi Improve Growth, Photosynthetic Activity, and Chlorophyll Fluorescence of Vitis vinifera L. cv. Ecolly under Drought Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Materials
2.2. AMF Colonization Measurement and Biomass
2.3. Relative Water Content (RWC)
2.4. Chlorophyll Concentration
2.5. Photosynthesis Measurements
2.6. Chlorophyll Fluorescence Imaging
2.7. Data Analysis
3. Results
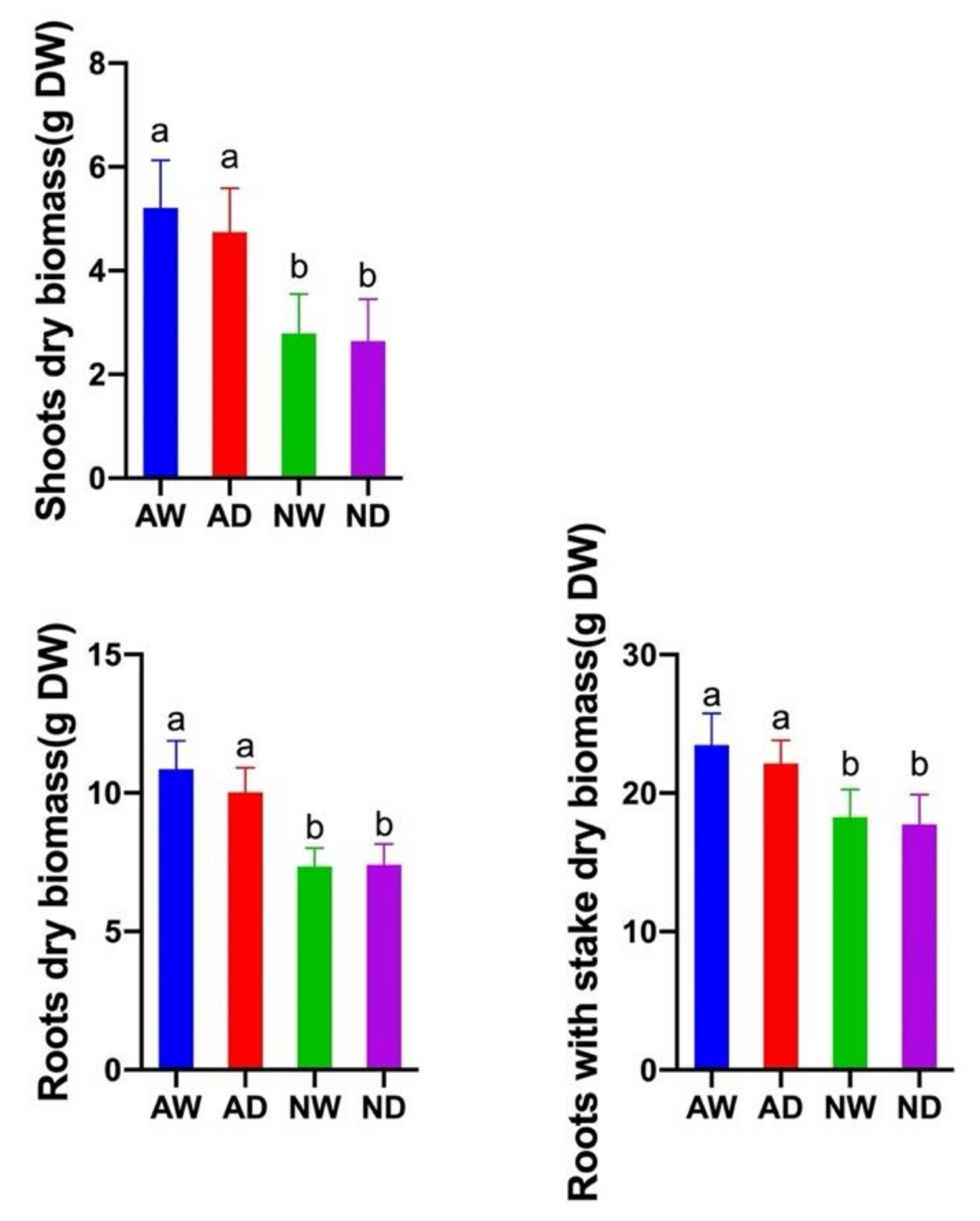
3.1. AMF Colonization and Plant Growth
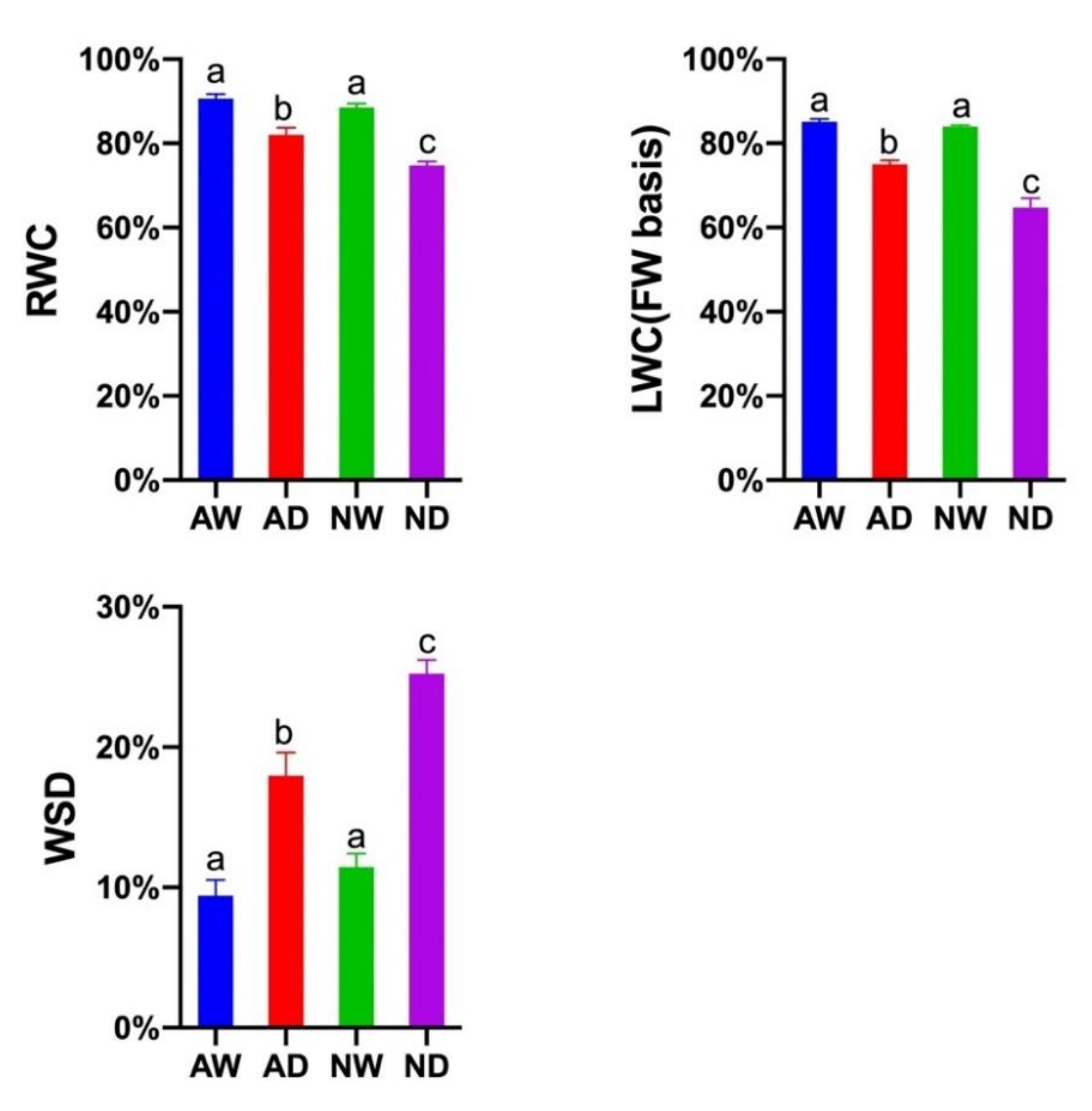
3.2. Leaf Water Content
3.3. Chlorophyll Content
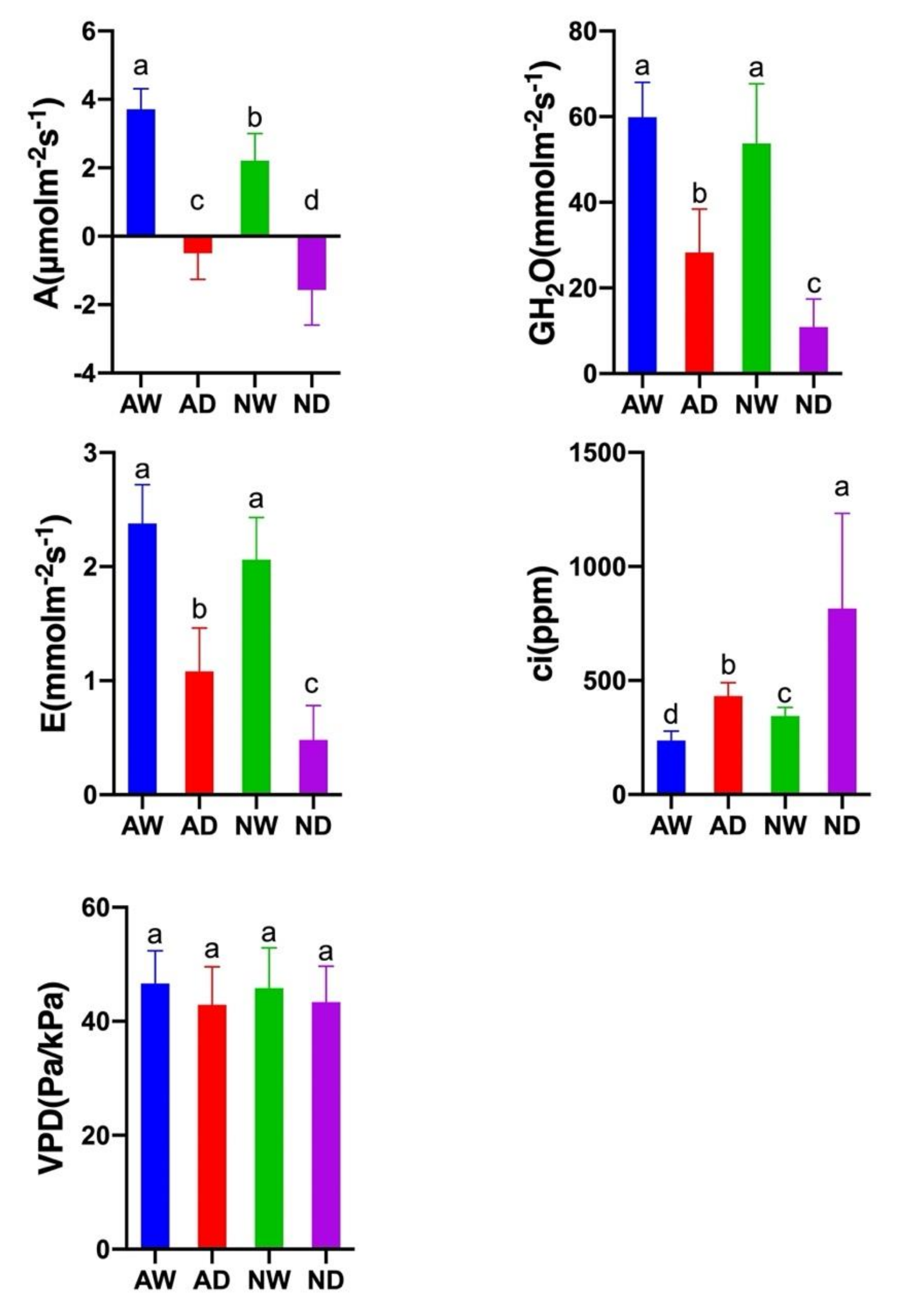
3.4. Photosynthesis Activity
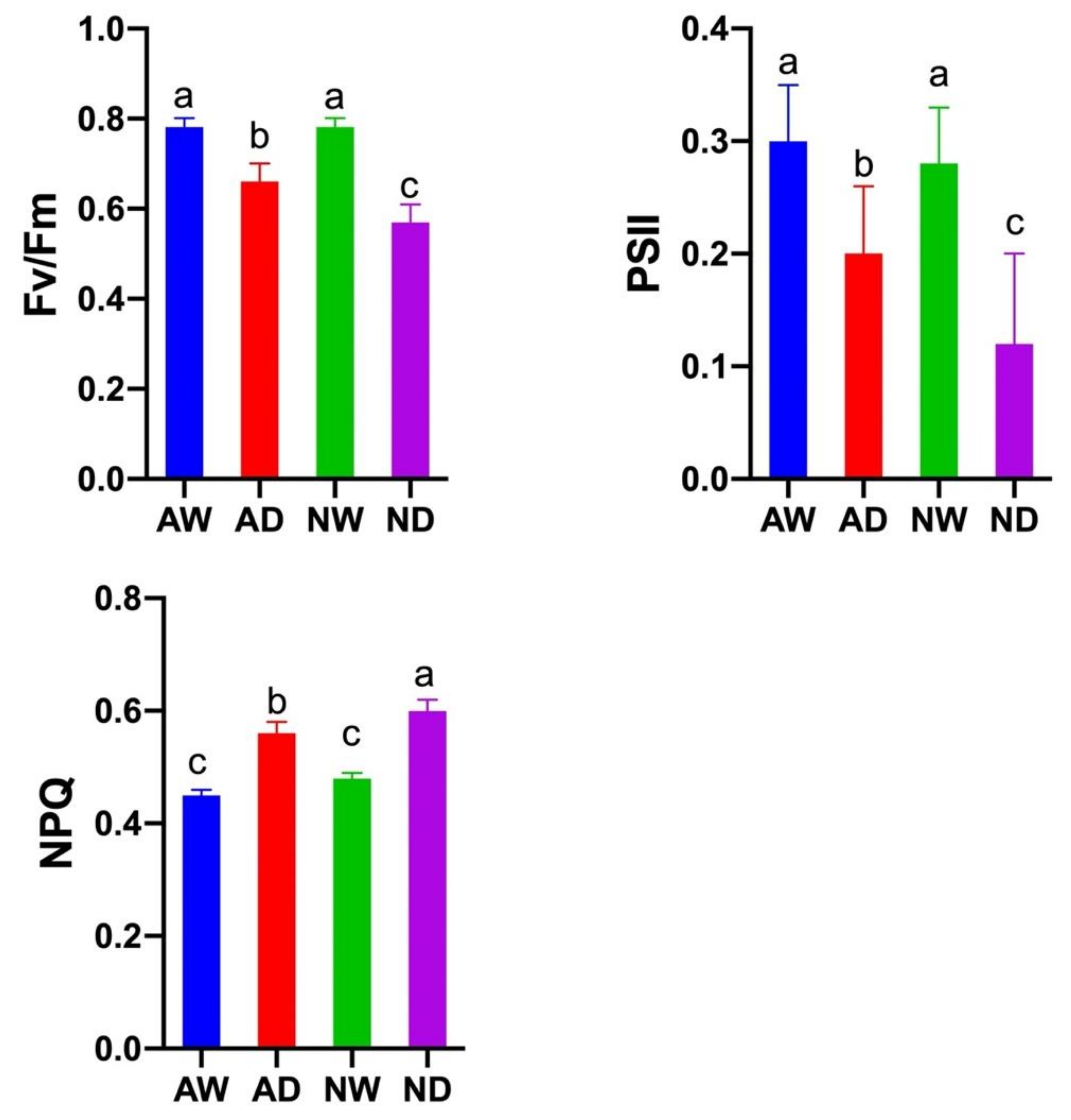
3.5. Chlorophyll Fluorescence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, S.; Li, H.; Ye, Q.; Wang, H. Winter Chill Protection of Grapevines by Burial: Evaluation of the Crawled Cordon Training System. Vitis 2016, 55, 45–51. [Google Scholar] [CrossRef]
- Trouvelot, S.; Bonneau, L.; Redecker, D.; van Tuinen, D.; Adrian, M.; Wipf, D. Arbuscular Mycorrhiza Symbiosis in Viticulture: A Review. Agron. Sustain. Dev. 2015, 35, 1449–1467. [Google Scholar] [CrossRef] [Green Version]
- Pillet, J.; Berdeja Aramayo, M.; Guan, L.; Delrot, S. Berry Response to Water, Light and Heat Stresses: A Molecular and Ecophysiological Perspective. In Grapevine in a Changing Environment: A Molecular and Ecophysiological Perspective; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 223–257. ISBN 978-1-118-73605-0. [Google Scholar]
- Soudzilovskaia, N.A.; van Bodegom, P.M.; Terrer, C.; Zelfde, M.V.; McCallum, I.; Luke McCormack, M.; Fisher, J.B.; Brundrett, M.C.; de Sá, N.C.; Tedersoo, L. Global Mycorrhizal Plant Distribution Linked to Terrestrial Carbon Stocks. Nat. Commun. 2019, 10, 5077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.E.; Read, D. Mycorrhizal Symbiosis, 3rd ed.; Smith, S.E., Read, D., Eds.; Academic Press: London, UK, 2008; ISBN 978-0-12-370526-6. [Google Scholar]
- Huang, D.; Ma, M.; Wang, Q.; Zhang, M.; Jing, G.; Li, C.; Ma, F. Arbuscular Mycorrhizal Fungi Enhanced Drought Resistance in Apple by Regulating Genes in the MAPK Pathway. Plant Physiol. Biochem. 2020, 149, 245–255. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Srivastava, A.K.; Zou, Y.-N. AMF-Induced Tolerance to Drought Stress in Citrus: A Review. Sci. Hortic. 2013, 164, 77–87. [Google Scholar] [CrossRef]
- Basile, B.; Rouphael, Y.; Colla, G.; Soppelsa, S.; Andreotti, C. Appraisal of Emerging Crop Management Opportunities in Fruit Trees, Grapevines and Berry Crops Facilitated by the Application of Biostimulants. Sci. Hortic. 2020, 267, 109330. [Google Scholar] [CrossRef]
- Begum, N.; Ahanger, M.A.; Su, Y.; Lei, Y.; Mustafa, N.S.A.; Ahmad, P.; Zhang, L. Improved Drought Tolerance by AMF Inoculation in Maize (Zea Mays) Involves Physiological and Biochemical Implications. Plants 2019, 8, 579. [Google Scholar] [CrossRef] [Green Version]
- Tuo, X.-Q.; He, L.; Zou, Y.-N. Alleviation of Drought Stress in White Clover after Inoculation with Arbuscular Mycorrhizal Fungi. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 45, 220–224. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zhang, J.; Xu, G.; Zhou, L.; Li, Y. Arbuscular Mycorrhizal Fungi Improve the Growth and Drought Tolerance of Zenia Insignis Seedlings under Drought Stress. New For. 2019, 50, 593–604. [Google Scholar] [CrossRef]
- Doubková, P.; Vlasáková, E.; Sudová, R. Arbuscular Mycorrhizal Symbiosis Alleviates Drought Stress Imposed on Knautia Arvensis Plants in Serpentine Soil. Plant Soil 2013, 370, 149–161. [Google Scholar] [CrossRef]
- Al-Karaki, G.N. Benefit, Cost and Water-Use Efficiency of Arbuscular Mycorrhizal Durum Wheat Grown under Drought Stress. Mycorrhiza 1998, 8, 41–45. [Google Scholar] [CrossRef]
- Boutasknit, A.; Baslam, M.; Ait-El-Mokhtar, M.; Anli, M.; Ben-Laouane, R.; Douira, A.; El Modafar, C.; Mitsui, T.; Wahbi, S.; Meddich, A. Arbuscular Mycorrhizal Fungi Mediate Drought Tolerance and Recovery in Two Contrasting Carob (Ceratonia Siliqua L.) Ecotypes by Regulating Stomatal, Water Relations, and (In)Organic Adjustments. Plants 2020, 9, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chareesri, A.; De Deyn, G.B.; Sergeeva, L.; Polthanee, A.; Kuyper, T.W. Increased Arbuscular Mycorrhizal Fungal Colonization Reduces Yield Loss of Rice (Oryza Sativa L.) under Drought. Mycorrhiza 2020, 30, 315–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Salam, E.; Alatar, A.; El-Sheikh, M.A. Inoculation with Arbuscular Mycorrhizal Fungi Alleviates Harmful Effects of Drought Stress on Damask Rose. Saudi J. Biol. Sci. 2018, 25, 1772–1780. [Google Scholar] [CrossRef]
- Gui, L.-X.; Lu, S.-S.; Chen, Q.; Yang, L.; Xiao, J.-X. ITRAQ-Based Proteomic Analysis Reveals Positive Impacts of Arbuscular Mycorrhizal Fungi Inoculation on Photosynthesis and Drought Tolerance in Blueberry. Trees-Struct. Funct. 2021, 35, 81–92. [Google Scholar] [CrossRef]
- Torres, N.; Yu, R.; Kurtural, S.K. Arbuscular Mycrorrhizal Fungi Inoculation and Applied Water Amounts Modulate the Response of Young Grapevines to Mild Water Stress in a Hyper-Arid Season. Front. Plant Sci. 2021, 11, 2189. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 5th ed.; Sinauer Associates, Inc.: Sunderland, UK, 2010. [Google Scholar]
- Sheng, M.; Tang, M.; Chen, H.; Yang, B.; Zhang, F.; Huang, Y. Influence of Arbuscular Mycorrhizae on Photosynthesis and Water Status of Maize Plants under Salt Stress. Mycorrhiza 2008, 18, 287–296. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.; Zhang, X.; Tang, M. Arbuscular Mycorrhizal Symbiosis Alleviates Salt Stress in Black Locust through Improved Photosynthesis, Water Status, and K+/Na+ Homeostasis. Front. Plant Sci. 2017, 8, 1739. [Google Scholar] [CrossRef]
- Khalvandi, M.; Amerian, M.; Pirdashti, H.; Keramati, S. Does Co-Inoculation of Mycorrhiza and Piriformospora Indica Fungi Enhance the Efficiency of Chlorophyll Fluorescence and Essential Oil Composition in Peppermint under Irrigation with Saline Water from the Caspian Sea? PLoS ONE 2021, 16, e0254076. [Google Scholar] [CrossRef]
- Khalil, H.A.; Eissa, A.M.; El-Shazly, S.M.; Aboul Nasr, A.M. Improved Growth of Salinity-Stressed Citrus after Inoculation with Mycorrhizal Fungi. Sci. Hortic. 2011, 130, 624–632. [Google Scholar] [CrossRef]
- Nikolaou, N.; Angelopoulos, K.; Karagiannidis, N. Effects of Drought Stress on Mycorrhizal and Non-Mycorrhizal Cabernet Sauvignon Grapevine, Grafted onto Various Rootstocks. Exp. Agric. 2003, 39, 241–252. [Google Scholar] [CrossRef] [Green Version]
- Karoglan, M.; Radić, T.; Anić, M.; Andabaka, Ž.; Stupić, D.; Tomaz, I.; Mesić, J.; Karažija, T.; Petek, M.; Lazarević, B.; et al. Mycorrhizal Fungi Enhance Yield and Berry Chemical Composition of in Field Grown “Cabernet Sauvignon” Grapevines (V. Vinifera L.). Agriculture 2021, 11, 615. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Sitko, K.; Rusinowski, S.; Pogrzeba, M.; Daszkowska-Golec, A.; Gieron, Z.; Kalaji, H.M.; Malkowski, E. Development and Aging of Photosynthetic Apparatus of Vitis Vinifera L. during Growing Season. Photosynthetica 2020, 58, 186–193. [Google Scholar] [CrossRef] [Green Version]
- Bao, S. Soil Agricultural Chemistry Analysis, 3rd ed.; Chinese Agriculture Press: Beijing, China, 2015; ISBN 978-7-109-06644-1. [Google Scholar]
- Li, H.; Zhang, Z.; Wang, H.; Liu, Y. A New Grape Variety—‘Ecolly’. Acta Hortic. Sin. 2000, 27, 75. [Google Scholar]
- Justine, V.H.; Mariam, B.; Qiuhong, Y. Enhancing Vine Health with Commercial Arbuscular Mycorrhizal Inoculants; Appellation Cornell; Cornell University: Ithaca, NY, USA, 2020; Available online: https://grapesandwine.cals.cornell.edu/sites/grapesandwine.cals.cornell.edu/files/shared/2019Research%20Focus2020-4.pdf. (accessed on 1 February 2022).
- Koske, R.E.; Gemma, J.N. A Modified Procedure for Staining Roots to Detect VA Mycorrhizas. Mycol. Res. 1989, 92, 486–488. [Google Scholar] [CrossRef]
- Barrs, H.; Weatherley, P. A Re-Examination of the Relative Turgidity Technique for Estimating Water Deficits in Leaves. Aust. Jnl. Bio. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef] [Green Version]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A New Method Which Gives an Objective Measure of Colonization of Roots by Vesicular-Arbuscular Mycorrhizal Fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- Turner, N.C. Techniques and Experimental Approaches for the Measurement of Plant Water Status. Plant Soil 1981, 58, 339–366. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Torres, N.; Antolín, M.C.; Goicoechea, N. Arbuscular Mycorrhizal Symbiosis as a Promising Resource for Improving Berry Quality in Grapevines Under Changing Environments. Front. Plant Sci. 2018, 9, 897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berruti, A.; Lumini, E.; Balestrini, R.; Bianciotto, V. Arbuscular Mycorrhizal Fungi as Natural Biofertilizers: Let’s Benefit from Past Successes. Front. Microbiol. 2016, 6, 1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begum, N.; Ahanger, M.A.; Zhang, L. AMF Inoculation and Phosphorus Supplementation Alleviates Drought Induced Growth and Photosynthetic Decline in Nicotiana Tabacum by up Regulating Antioxidant Metabolism and Osmolyte Accumulation. Environ. Exp. Bot. 2020, 176, 104088. [Google Scholar] [CrossRef]
- Armada, E.; Azcón, R.; López-Castillo, O.M.; Calvo-Polanco, M.; Ruiz-Lozano, J.M. Autochthonous Arbuscular Mycorrhizal Fungi and Bacillus Thuringiensis from a Degraded Mediterranean Area Can Be Used to Improve Physiological Traits and Performance of a Plant of Agronomic Interest under Drought Conditions. Plant Physiol. Biochem. 2015, 90, 64–74. [Google Scholar] [CrossRef]
- Kong, J.; Pei, Z.; Du, M.; Sun, G.; Zhang, X. Effects of Arbuscular Mycorrhizal Fungi on the Drought Resistance of the Mining Area Repair Plant Sainfoin. Int. J. Min. Sci. Technol. 2014, 24, 485–489. [Google Scholar] [CrossRef]
- Hao, Z.; Fayolle, L.; van Tuinen, D.; Chatagnier, O.; Li, X.; Gianinazzi, S.; Gianinazzi-Pearson, V. Local and Systemic Mycorrhiza-Induced Protection against the Ectoparasitic Nematode Xiphinema Index Involves Priming of Defence Gene Responses in Grapevine. J. Exp. Bot. 2012, 63, 3657–3672. [Google Scholar] [CrossRef]
- Quiroga, G.; Erice, G.; Aroca, R.; Chaumont, F.; Ruiz-Lozano, J.M. Contribution of the Arbuscular Mycorrhizal Symbiosis to the Regulation of Radial Root Water Transport in Maize Plants under Water Deficit. Environ. Exp. Bot. 2019, 167, 103821. [Google Scholar] [CrossRef]
- Eroğlu, Ç.G.; Cabral, C.; Ravnskov, S.; Bak Topbjerg, H.; Wollenweber, B. Arbuscular Mycorrhiza Influences Carbon-Use Efficiency and Grain Yield of Wheat Grown under Pre- and Post-Anthesis Salinity Stress. Plant Biol. 2020, 22, 863–871. [Google Scholar] [CrossRef]
- Kavroulakis, N.; Tsiknia, M.; Ipsilantis, I.; Kavadia, A.; Stedel, C.; Psarras, G.; Tzerakis, C.; Doupis, G.; Karpouzas, D.G.; Papadopoulou, K.K.; et al. Arbuscular Mycorrhizal Fungus Inocula from Coastal Sand Dunes Arrest Olive Cutting Growth under Salinity Stress. Mycorrhiza 2020, 30, 475–489. [Google Scholar] [CrossRef]
- Dalal, V.K.; Tripathy, B.C. Modulation of Chlorophyll Biosynthesis by Water Stress in Rice Seedlings during Chloroplast Biogenesis. Plant Cell Environ. 2012, 35, 1685–1703. [Google Scholar] [CrossRef]
- Yooyongwech, S.; Phaukinsang, N.; Cha-um, S.; Supaibulwatana, K. Arbuscular Mycorrhiza Improved Growth Performance in Macadamia Tetraphylla L. Grown under Water Deficit Stress Involves Soluble Sugar and Proline Accumulation. Plant Growth Regul. 2013, 69, 285–293. [Google Scholar] [CrossRef]
- Zhu, X.Q.; Tang, M.; Zhang, H.Q. Arbuscular Mycorrhizal Fungi Enhanced the Growth, Photosynthesis, and Calorific Value of Black Locust under Salt Stress. Photosynthetica 2017, 55, 378–385. [Google Scholar] [CrossRef]
- de Andrade, S.A.L.; Domingues, A.P.; Mazzafera, P. Photosynthesis Is Induced in Rice Plants That Associate with Arbuscular Mycorrhizal Fungi and Are Grown under Arsenate and Arsenite Stress. Chemosphere 2015, 134, 141–149. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Xia, R.-X. Arbuscular Mycorrhizal Fungi Influence Growth, Osmotic Adjustment and Photosynthesis of Citrus under Well-Watered and Water Stress Conditions. J. Plant Physiol. 2006, 163, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, M.; Alizadeh, M.; Mashayekhi, K.; Asghari, H.R. In Vitro Propagation of Four Iranian Grape Varieties: Influence of Genotype and Pretreatment with Arbuscular Mycorrhiza. Vitis 2012, 51, 175–182. [Google Scholar]
- Nicolás, E.; Maestre-Valero, J.F.; Alarcón, J.J.; Pedrero, F.; Vicente-Sánchez, J.; Bernabé, A.; Gómez-Montiel, J.; Hernández, J.A.; Fernández, F. Effectiveness and Persistence of Arbuscular Mycorrhizal Fungi on the Physiology, Nutrient Uptake and Yield of Crimson Seedless Grapevine. J. Agric. Sci. 2015, 153, 1084–1096. [Google Scholar] [CrossRef] [Green Version]
- Betancur-Agudelo, M.; Meyer, E.; Lovato, P.E. Growth, Heavy Metal Uptake, and Photosynthesis in “Paulsen 1103” (Vitis Berlandieri × Rupestris) Grapevine Rootstocks Inoculated with Arbuscular Mycorrhizal Fungi from Vineyard Soils with High Copper Contents. Vitis 2020, 59, 169–180. [Google Scholar] [CrossRef]
- Valentine, A.J.; Mortimer, P.E.; Lintnaar, A.; Borgo, R. Drought Responses of Arbuscular Mycorrhizal Grapevines. Symbiosis 2006, 41, 127–133. [Google Scholar]
- Mitra, D.; Djebaili, R.; Pellegrini, M.; Mahakur, B.; Sarker, A.; Chaudhary, P.; Khoshru, B.; Gallo, M.D.; Kitouni, M.; Barik, D.P.; et al. Arbuscular Mycorrhizal Symbiosis: Plant Growth Improvement and Induction of Resistance under Stressful Conditions. J. Plant Nutr. 2021, 44, 1993–2028. [Google Scholar] [CrossRef]
- Krause, G.H.; Weis, E. Chlorophyll Fluorescence and Photosynthesis: The Basics. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 1991, 42, 313–349. [Google Scholar] [CrossRef]
- Subrahmanyam, D. Effects of Chromium Toxicity on Leaf Photosynthetic Characteristics and Oxidative Changes in Wheat (Triticum Aestivum L.). Photosynthetica 2008, 46, 339. [Google Scholar] [CrossRef]
- Nieva, F.J.J.; Castellanos, E.M.; Figueroa, M.E.; Gil, F. Gas Exchange and Chlorophyll Fluorescence of C3 and C4 Saltmarsh Species. Photosynthetica 1999, 36, 397–406. [Google Scholar] [CrossRef]
- He, L.; Li, C.; Liu, R. Indirect Interactions between Arbuscular Mycorrhizal Fungi and Spodoptera Exigua Alter Photosynthesis and Plant Endogenous Hormones. Mycorrhiza 2017, 27, 525–535. [Google Scholar] [CrossRef]
- Mo, Y.; Wang, Y.; Yang, R.; Zheng, J.; Liu, C.; Li, H.; Ma, J.; Zhang, Y.; Wei, C.; Zhang, X. Regulation of Plant Growth, Photosynthesis, Antioxidation and Osmosis by an Arbuscular Mycorrhizal Fungus in Watermelon Seedlings under Well-Watered and Drought Conditions. Front. Plant Sci. 2016, 7, 644. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Treatment | Mycorrhizal Colonization % | Arbuscules Colonization % | ||
|---|---|---|---|---|
| A | 89.2 ± 2.9 | a | 76.8 ± 4.7 | a |
| N | 0 | b | 0 | b |
| Treatment | Chlorophyll a (mg/g FW) | Chlorophyll b (mg/g FW) | Total Chlorophyll (mg/g FW) | |||
|---|---|---|---|---|---|---|
| AD | 0.670 ± 0.08 | b | 0.344 ± 0.05 | a | 1.014 ± 0.13 | a |
| AW | 0.899 ± 0.20 | a | 0.433 ± 0.08 | a | 1.332 ± 0.27 | a |
| ND | 0.330 ± 0.04 | c | 0.220 ± 0.04 | b | 0.550 ± 0.07 | b |
| NW | 0.689 ± 0.08 | b | 0.365 ± 0.03 | a | 1.054 ± 0.08 | a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Q.; Wang, H.; Li, H. Arbuscular Mycorrhizal Fungi Improve Growth, Photosynthetic Activity, and Chlorophyll Fluorescence of Vitis vinifera L. cv. Ecolly under Drought Stress. Agronomy 2022, 12, 1563. https://doi.org/10.3390/agronomy12071563
Ye Q, Wang H, Li H. Arbuscular Mycorrhizal Fungi Improve Growth, Photosynthetic Activity, and Chlorophyll Fluorescence of Vitis vinifera L. cv. Ecolly under Drought Stress. Agronomy. 2022; 12(7):1563. https://doi.org/10.3390/agronomy12071563
Chicago/Turabian StyleYe, Qiuhong, Hua Wang, and Hua Li. 2022. "Arbuscular Mycorrhizal Fungi Improve Growth, Photosynthetic Activity, and Chlorophyll Fluorescence of Vitis vinifera L. cv. Ecolly under Drought Stress" Agronomy 12, no. 7: 1563. https://doi.org/10.3390/agronomy12071563
APA StyleYe, Q., Wang, H., & Li, H. (2022). Arbuscular Mycorrhizal Fungi Improve Growth, Photosynthetic Activity, and Chlorophyll Fluorescence of Vitis vinifera L. cv. Ecolly under Drought Stress. Agronomy, 12(7), 1563. https://doi.org/10.3390/agronomy12071563




