Application of Phenomics to Elucidate the Influence of Rootstocks on Drought Response of Tomato
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Grafting Conditions
2.3. Plant Growth Conditions
2.4. Field Capacity Estimation
2.5. Watering and Weighing
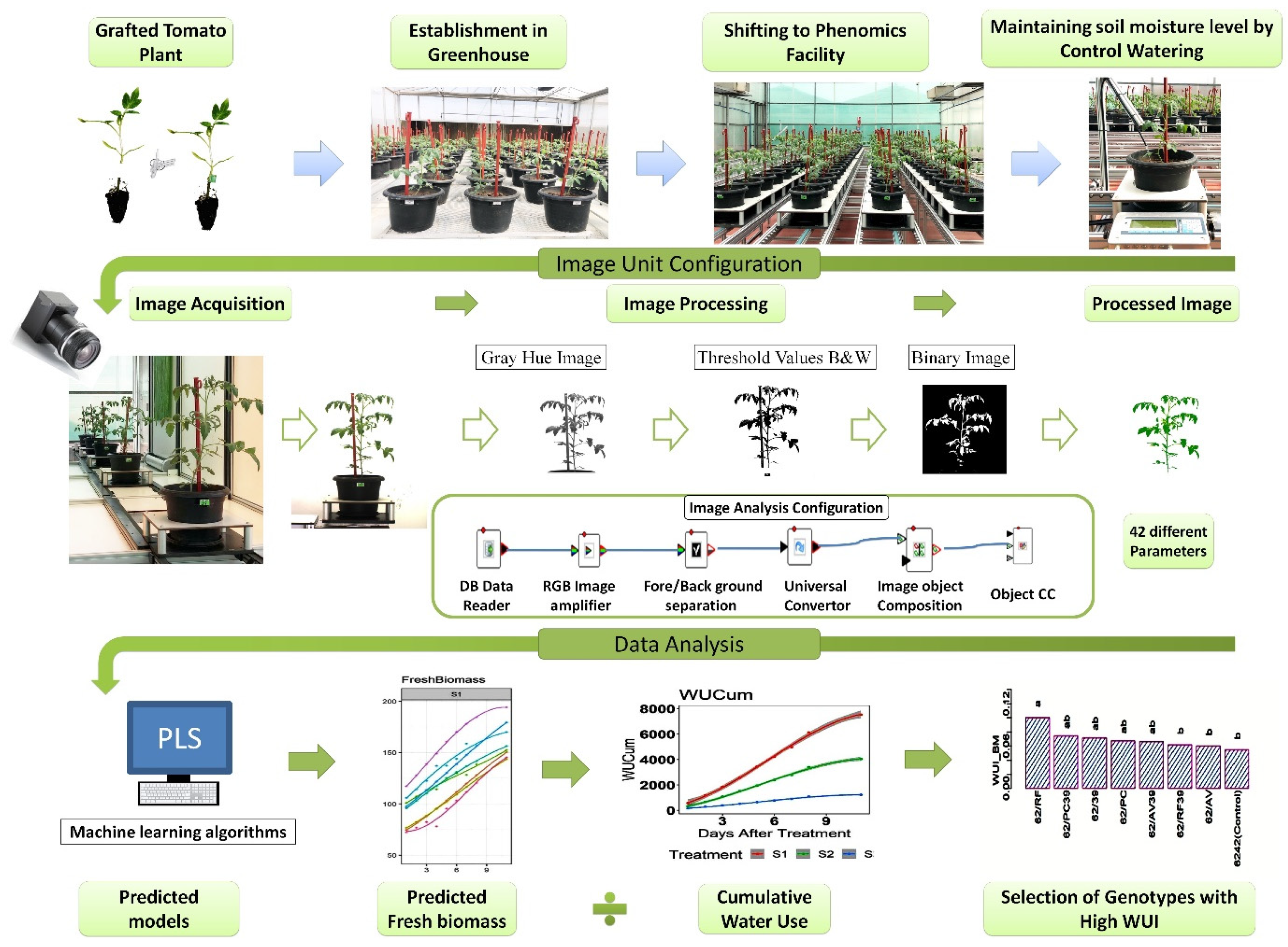
2.6. Image Acquisition
2.7. Image Analysis and Data Mining
2.8. Measure of the Actual Shoot and Root Parameters
2.9. Growth Rates and Water Use Index
2.10. Physiological Parameters
2.10.1. Chlorophyll Fluorescence (PSII)
2.10.2. Stomatal Conductance, Relative Water Content and Canopy Temperature
2.11. Statistical Analysis
3. Results
3.1. Soil Moisture Stress
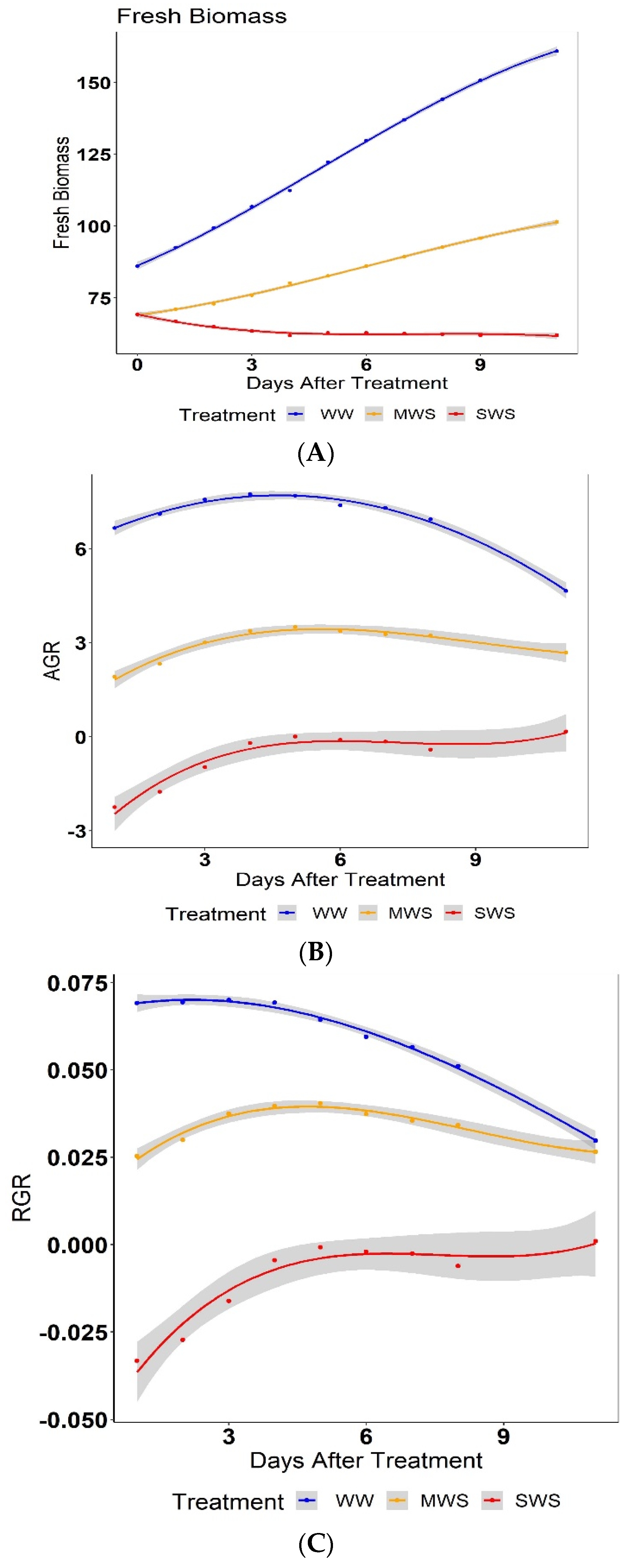
3.2. Effect of Treatment on Biomass and Growth Rate
3.3. Effect of Grafting on Biomass and Growth Rates
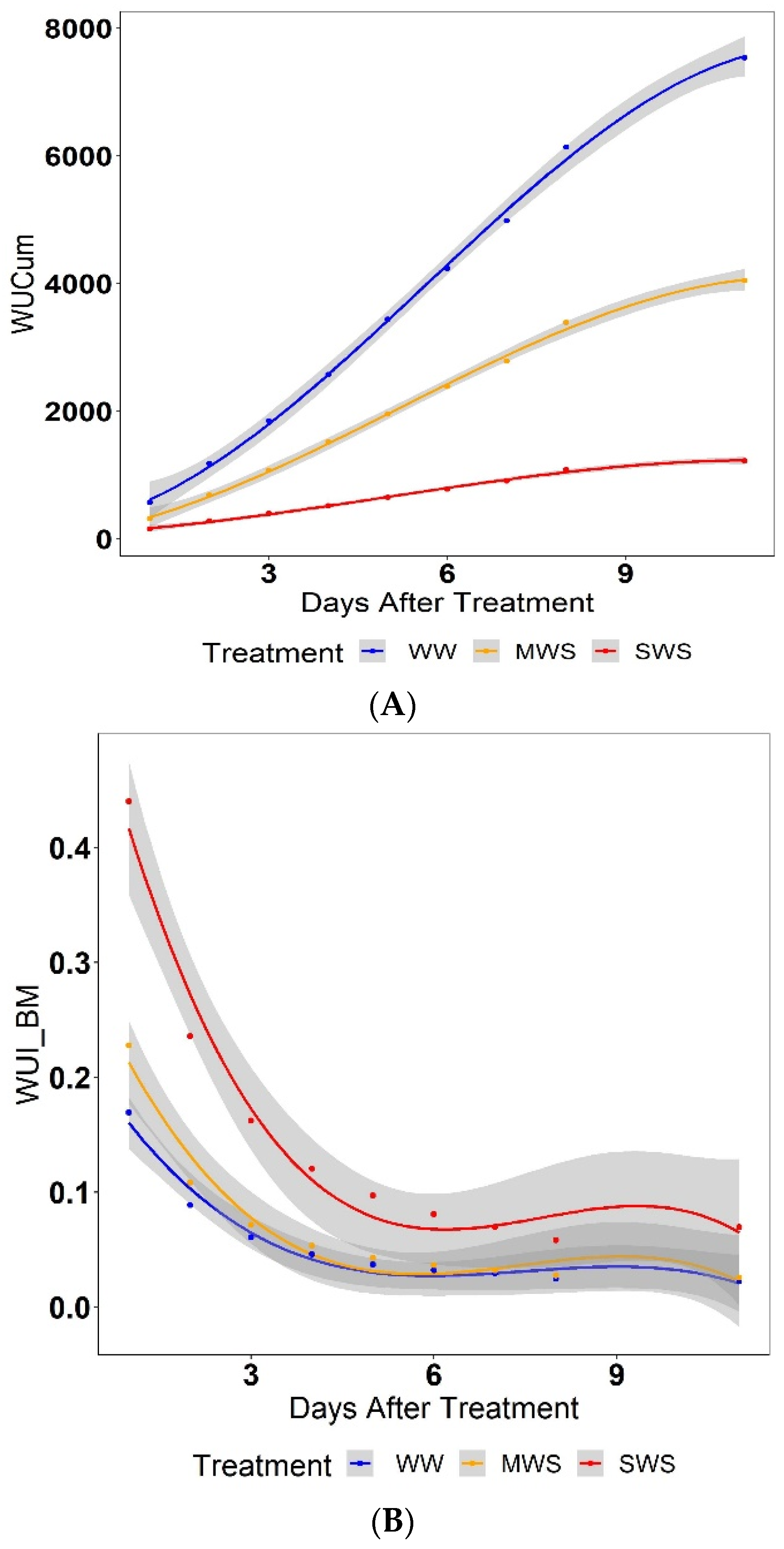
3.4. Water Use Index (WUI)
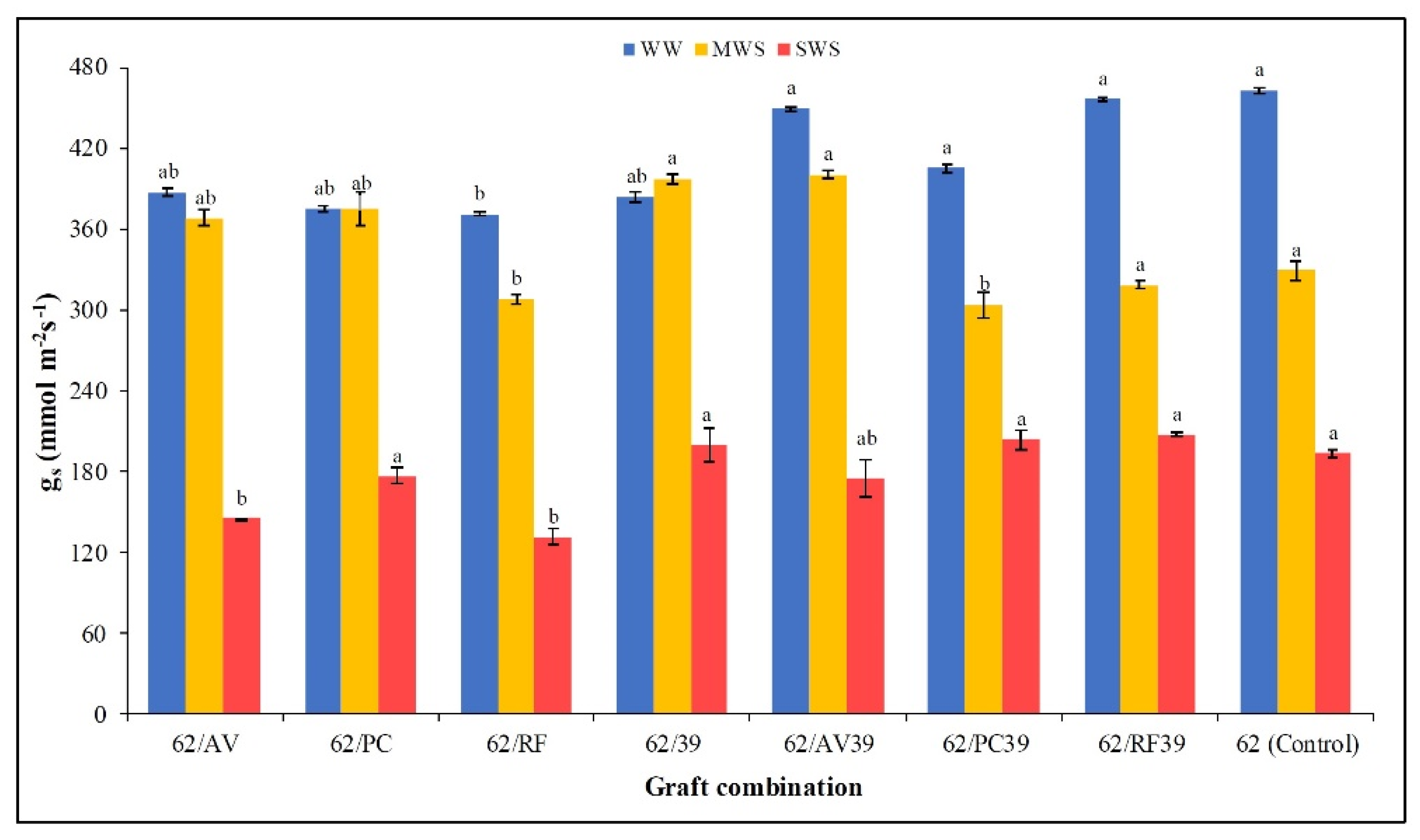
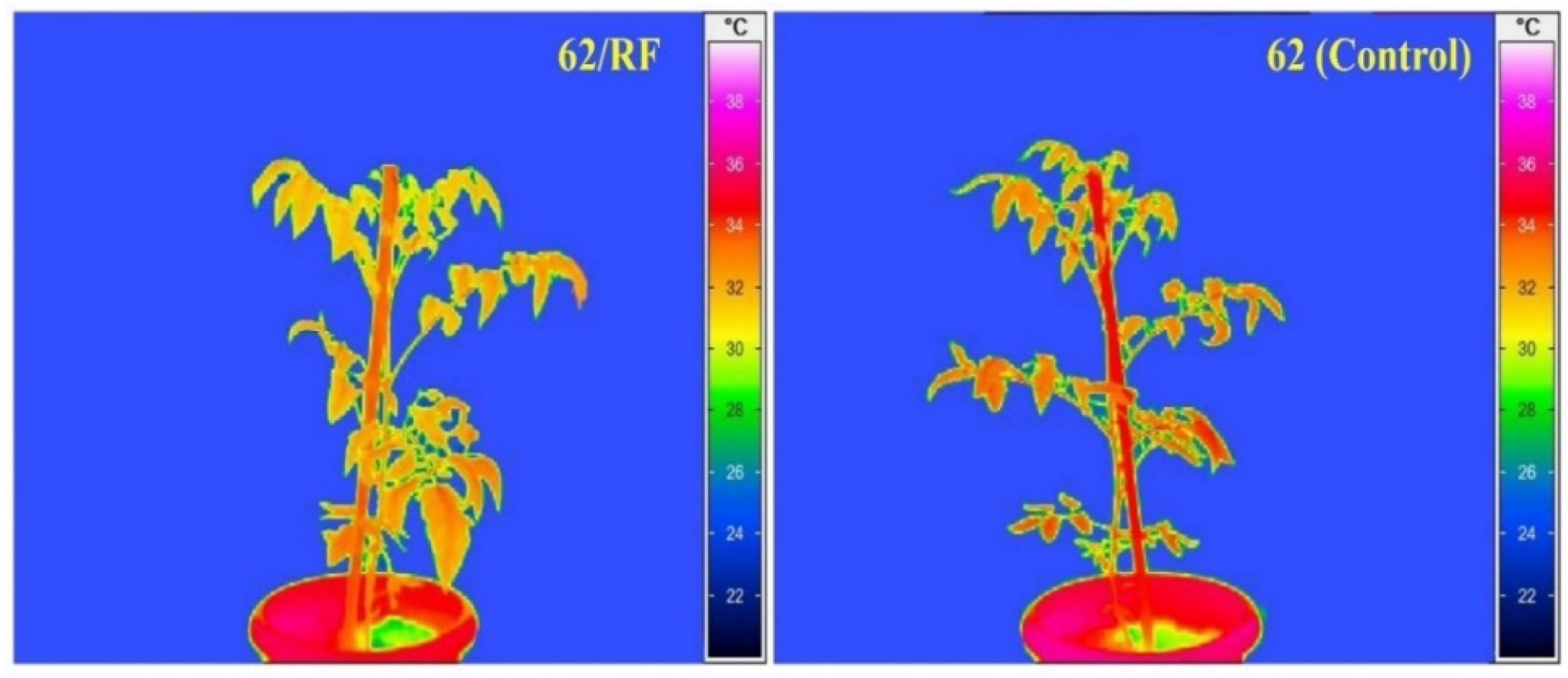
3.5. Stomatal Conductance, RWC, Canopy Temperature, and PSII Efficiency (Fv/Fm)
3.6. Shoot and Root Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khapte, P.S.; Jansirani, P.; Saraswathi, T. Heterosis in oblong fruited tomato (Solanum lycopersicum) hybrids for growth and yield traits. Indian J. Agric. Sci. 2019, 89, 1594–1598. [Google Scholar]
- Kumar, P.; Rouphael, Y.; Cardarelli, M.; Colla, G. Vegetable grafting as a tool to improve drought resistance and water use efficiency. Front. Plant Sci. 2017, 8, 1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ximénez-Embún, M.G.; González-Guzmán, M.; Arbona, V.; Gómez-Cadenas, A.; Ortego, F.; Castañera, P. Plant-mediated effects of water deficit on the performance of Tetranychus evansi on tomato drought-adapted accessions. Front. Plant Sci. 2018, 9, 1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayaz, M.; Ahmad, R.; Shahzad, M.; Khan, N.; Shah, M.M.; Khan, S.A. Drought stress stunt tomato plant growth and up-regulate expression of SlAREB, SlNCED3, and SlERF024 genes. Sci. Hortic. 2015, 195, 48–55. [Google Scholar] [CrossRef]
- Warschefsky, E.J.; Klein, L.L.; Frank, M.H.; Chitwood, D.H.; Londo, J.P.; Von Wettberg, E.J.B.; Miller, A.J. Rootstocks: Diversity, domestication, and impacts on shoot phenotypes. Trends Plant Sci. 2016, 21, 418–437. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Andújar, C.; Albacete, A.; Pérez-Alfoceaa, F. Rootstocks for increasing yield stability and sustainability in vegetable crops. Acta Hortic. 2020, 1273, 449–470. [Google Scholar] [CrossRef]
- Hodge, A.; Berta, G.; Doussan, C.; Merchan, F.; Crespi, M. Plant root growth, architecture and function. Plant Soil 2009, 321, 153–187. [Google Scholar] [CrossRef]
- Venema, J.H.; Dijk, B.E.; Bax, J.M.; van Hasselt, P.R.; Elzenga, J.T.M. Grafting tomato (Solanum lycopersicum) onto the rootstock of a high-altitude accession of Solanum habrochaites improves suboptimal-temperature tolerance. Environ. Exp. Bot. 2008, 63, 359–367. [Google Scholar] [CrossRef]
- Schwarz, D.; Rouphael, Y.; Colla, G.; Venema, J.H. Grafting as a tool to improve tolerance of vegetables to abiotic stresses: Thermal stress, water stress and organic pollutants. Sci. Hortic. 2010, 127, 162–171. [Google Scholar] [CrossRef]
- Gaion, L.A.; Braz, L.T.; Carvalho, R.F. Grafting in vegetable crops: A great technique for agriculture. Int. J. Veg. Sci. 2017, 24, 85–102. [Google Scholar] [CrossRef]
- Singh, H.; Kumar, P.; Chaudhari, S.; Edelstein, M. Tomato Grafting: A Global Perspective. Hort. Sci. 2017, 52, 1328–1336. [Google Scholar] [CrossRef] [Green Version]
- Singh, H.; Kumar, P.; Kumar, A.; Kyriacou, M.C.; Colla, G.; Rouphael, Y. Grafting tomato as a tool to improve salt tolerance. Agronomy 2020, 10, 263. [Google Scholar] [CrossRef] [Green Version]
- Bikdeloo, M.; Colla, G.; Rouphael, Y.; Hassandokht, M.R.; Soltani, F.; Salehi, R.; Kumar, P.; Cardarelli, M. Morphological and Physio-Biochemical Responses of Watermelon Grafted onto Rootstocks of Wild Watermelon [Citrullus colocynthis (L.) Schrad] and Commercial Interspecific Cucurbita Hybrid to Drought Stress. Horticulturae 2021, 7, 359. [Google Scholar] [CrossRef]
- Thompson, J.; Pico, M.B.; Yetişir, H.; Cohen, R.; Bebeli, P.J. Rootstock breeding: Current practices and future technologies. In Vegetable Grafting: Principles and Practices; Colla, G., Perez-Alfocea, F., Schwaz, D., Eds.; CAB International: Wallingford, UK, 2017; pp. 70–93. [Google Scholar]
- Foolad, M.R. Breeding for abiotic stress tolerances in tomato. In Abiotic Stresses: Plant Resistance through Breeding and Molecular Approaches; Ashraf, M., Harris, P.J.C., Eds.; The Haworth Press: New York, NY, USA, 2005; pp. 613–684. [Google Scholar]
- Ryder, P.; McKeown, P.C.; Fort, A.; Spillane, C. Epigenetics and heterosis in crop plants. In Epigenetics in Plants of Agronomic Importance: Fundamentals and Applications: Transcriptional Regulation and Chromatin Remodelling in Plants; Alvarez-Venegas, R., De La Pena, C., Casas-Mollano, J.A., Eds.; Springer: New York, NY, USA, 2014; pp. 13–31. [Google Scholar]
- King, S.R.; Davis, A.R.; Zhang, X.; Crosby, K. Genetics, breeding and selection of rootstocks for Solanaceae and Cucurbitaceae. Sci. Hortic. 2010, 127, 106–111. [Google Scholar] [CrossRef]
- Pico, B.; Thompson, A.; Gisbert, C.; Yetisir, H.; Bebeli, P. Genetic resources for rootstock breeding. In Vegetable Grafting: Principles and Practices; Colla, G., Pérez-Alfocea, F., Schwarz, D., Eds.; CABI International: Wallingford, UK, 2017; pp. 22–69. [Google Scholar]
- Cantero-Navarro, E.; Romero-Aranda, R.; Fernández-Munoz, R.; Martínez-Andújara, C.; Pérez-Alfocea, F.; Albacete, A. Improving agronomic water use efficiency in tomato by rootstock-mediated hormonal regulation of leaf biomass. Plant Sci. 2016, 251, 90–100. [Google Scholar] [CrossRef]
- Halperin, O.; Gebremedhin, A.; Wallach, R.; Moshelion, M. High-throughput physiological phenotyping and screening system for the characterization of plant–environment interactions. Plant J. 2017, 89, 839–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janni, M.; Coppede, N.; Bettelli, M.; Briglia, N.; Petrozza, A.; Summerer, S.; Vurro, F.; Danzi, D.; Cellini, F.; Marmiroli, N.; et al. In Vivo phenotyping for the early detection of drought stress in tomato. Plant Phenomics 2019, 2019, 6168209. [Google Scholar] [CrossRef] [Green Version]
- Das Choudhury, S.; Samal, A.; Awada, T. Leveraging image analysis for high-throughput plant phenotyping. Front. Plant Sci. 2019, 10, 508. [Google Scholar] [CrossRef]
- Tackenberg, O. A new method for non-destructive measurement of biomass, growth rates, vertical biomass distribution and dry matter content based on digital image analysis. Ann. Bot. 2007, 99, 777–783. [Google Scholar] [CrossRef]
- Rao, N.K.S.; Laxman, R.H. Phenotyping horticultural crops for abiotic stress tolerance. In Climate Resilient Horticulture: Adaptation and Mitigation Strategies; Singh, H.P., Rao, N.K.S., Shivashankara, K.S., Eds.; Springer: New Delhi, India, 2013; pp. 147–157. [Google Scholar]
- van Eeuwijk, F.A.; Bustos-Korts, D.; Millet, E.J.; Boer, M.P.; Kruijer, W.; Thompson, A.; Chapman, S.C. Modelling strategies for assessing and increasing the effectiveness of new phenotyping techniques in plant breeding. Plant Sci. 2019, 282, 23–39. [Google Scholar] [CrossRef]
- Joshi, S.; Thoday-Kennedy, E.; Daetwyler, H.D.; Hayden, M.; Spangenberg, G.; Kant, S. High-throughput phenotyping to dissect genotypic differences in safflower for drought tolerance. PLoS ONE 2021, 16, e0254908. [Google Scholar] [CrossRef] [PubMed]
- Golzarian, M.R.; Frick, R.A.; Rajendran, K.; Berger, B.; Roy, S.; Tester, M.; Lun, D.S. Accurate inference of shoot biomass from high-throughput images of cereal plants. Plant Methods 2011, 7, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, B.; Sadras, V.; Tester, M. A Water-centered framework to assess the effects of salinity on the growth and yield of wheat and barley. Plant Soil 2010, 336, 377–389. [Google Scholar] [CrossRef]
- Hairmansis, A.; Berger, B.; Tester, M.; Roy, S.J. Image based phenotyping for non-destructive screening of different salinity tolerance traits in rice. Rice 2014, 7, 16. [Google Scholar] [CrossRef] [Green Version]
- Rane, J.; Raina, S.K.; Govindasamy, V.; Bindumadhava, H.; Hanjagi, P.; Giri, R.; Jangid, K.K.; Kumar, M.; Nair, R.M. Use of phenomics for differentiation of mungbean (Vigna radiata L. Wilczek) genotypes varying in growth rates per unit of water. Front. Plant Sci. 2021, 12, 692564. [Google Scholar] [CrossRef]
- Laxman, R.H.; Hemamalini, P.; Bhatt, R.M.; Sadashiva, A.T. Non-invasive quantification of tomato (Solanum lycopersicum L.) plant biomass through digital imaging using phenomics platform. Indian J. Plant Physiol. 2018, 23, 369–375. [Google Scholar] [CrossRef]
- Rao, N.K.S.; Laxman, R.H.; Shivashankara, K.S. Physiological and morphological responses of horticultural crops to abiotic stresses. In Abiotic Stress Physiology of Horticultural Crops; Rao, N., Shivashankara, K., Laxman, R., Eds.; Springer: New Delhi, India, 2016; pp. 121–131. [Google Scholar]
- Canavar, O.; Gotz, K.P.; Ellmer, F.; Chmielewski, F.M.; Kaynak, M.A. Determination of the relationship between water use efficiency, carbon isotope discrimination and proline in sunflower genotypes under drought stress. Aust. J. Crop Sci. 2014, 8, 232–242. [Google Scholar]
- Nedbal, L.; Soukupová, J.; Kaftan, D.; Whitmarsh, J.; Trtilek, M. Kinetic imaging of chlorophyll fluorescence using modulated light. Photosyn. Res. 2000, 66, 3–12. [Google Scholar] [CrossRef]
- Khare, N.; Goyary, D.; Singh, N.K.; Shah, P.; Rathore, M.; Anandhan, S.; Sharma, D.; Arif, M.; Ahmed, Z. Transgenic tomato cv. Pusa Uphar expressing a bacterial mannitol-1-phosphate dehydrogenase gene confers abiotic stress tolerance. Plant Cell Tissue Organ Cult. 2010, 103, 267–277. [Google Scholar] [CrossRef]
- Brien, C. Package ‘growthPheno’. CRAN Repository. 2020. Available online: https://cran.r-project.org/web/packages/growthPheno/growthPheno.pdf (accessed on 22 September 2021).
- Bai, Y.; Lindhout, P. Domestication and breeding of tomatoes: What have we gained and what can we gain in the future? Ann. Bot. 2007, 100, 1085–1094. [Google Scholar] [CrossRef]
- Bolger, A.; Scossa, F.; Bolger, M.E.; Lanz, C.; Maumus, F.; Tohge, T.; Quesneville, H.; Alseekh, S.; Sørensen, I.; Lichtenstein, G.; et al. The genome of the stress-tolerant wild tomato species Solanum pennellii. Nat. Genet. 2014, 46, 1034–1038. [Google Scholar] [CrossRef]
- Tieman, D.; Zhu, G.T.; Resende, M.F.R.; Lin, T.; Taylor, M.; Zhang, B.; Ikeda, H.; Liu, Z.Y.; Fisher, J.; Zemach, I.; et al. Plant science a chemical genetic road map to improved tomato flavor. Science 2017, 355, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Sousaraei, N.; Mashayekhi, K.; Mousavizadeh, S.J.; Akbarpour, V.; Medina, J.; Aliniaeifard, S. Screening of tomato landraces for drought tolerance based on growth and chlorophyll fluorescence analyses. Hortic. Environ. Biotechnol. 2021, 62, 521–535. [Google Scholar] [CrossRef]
- Kadoglidou, K.; Xanthopoulou, A.; Kalyvas, A.; Mellidou, I. Utilization of Tomato Landraces to Improve Seedling Performance under Salt Stress. Stresses 2021, 1, 238–252. [Google Scholar] [CrossRef]
- Nilsen, E.T.; Freeman, J.; Grene, R.; Tokuhisa, J.A. rootstock provides water conservation for a grafted commercial tomato (Solanum lycopersicum L.) line in response to mild-drought conditions: A focus on vegetative growth and photosynthetic parameters. PLoS ONE 2014, 9, e115380. [Google Scholar] [CrossRef]
- Al-Harbi, A.; Hejazi, A.; Al-Omran, A. Responses of grafted tomato (Solanum lycopersiocon L.) to abiotic stresses in Saudi Arabia. Saudi J. Biol. Sci. 2016, 24, 1274–1280. [Google Scholar] [CrossRef] [Green Version]
- Bedinger, P.A.; Chetelat, R.T.; McClure, B.; Moyle, L.C.; Rose, J.K.; Stack, S.M.; van der Knaap, E.; Baek, Y.S.; Lopez-Casado, G.; Covey, P.A. Interspecific reproductive barriers in the tomato clade: Opportunities to decipher mechanisms of reproductive isolation. Sex. Plant Reprod. 2011, 24, 171–187. [Google Scholar] [CrossRef]
- Mahmoud, A.M.A. Tomato rootstock breeding: Evaluation of tomato interspecific hybrid rootstocks under greenhouse conditions. Hort. J. 2020, 89, 575–585. [Google Scholar] [CrossRef]
- Altunlu, H.; Gul, A. Increasing drought tolerance of tomato plants by grafting. Acta Hortic. 2012, 960, 183–190. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, E.; Leyva, R.; Constán-Aguilar, C.; Romero, L.; Ruiz, J.M. How does grafting affect the ionome of cherry tomato plants underwater stress? Soil Sci. Plant Nutri. 2014, 60, 145–155. [Google Scholar] [CrossRef]
- Khapte, P.S.; Kumar, P.; Burman, U.; Kumar, P. Deficit irrigation in tomato: Agronomical and physio-biochemical implications. Sci. Hortic. 2019, 248, 256–264. [Google Scholar] [CrossRef]
- Johansen, K.; Morton, M.; Malbeteau, Y.; Aragon, B.; Al-Mashharawi, S.; Ziliani, M.G.; Angel, Y.; Fiene, G.; Negrão, S.; Mousa, M.; et al. Predicting biomass and yield in a tomato phenotyping experiment using UAV imagery and random forest. Front. Artif. Intell. 2020, 3, 28. [Google Scholar] [CrossRef] [PubMed]
- Gur, A.; Semel, Y.; Osorio, S.; Friedmann, M.; Seekh, S.; Ghareeb, B.; Mohammad, A.; Pleban, T.; Gera, G.; Fernie, A.R.; et al. Yield quantitative trait loci from wild tomato are predominately expressed by the shoot. Theor. Appl. Genet. 2010, 122, 405–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poudyala, D.; Khatria, L.; Uptmoora, R. An introgression of Solanum habrochaites in the rootstock improves stomatal regulation and leaf area development of grafted tomatoes under drought and low root-zone temperatures. Adv. Crop Sci. Tech. 2015, 3, 175. [Google Scholar] [CrossRef] [Green Version]
- Holbrook, N.M.; Shashidhar, V.R.; James, R.A.; Munns, R. Stomatal control in tomato with ABA-deficient roots: Response of grafted plants to soil drying. J. Exp. Bot. 2002, 53, 1503–1514. [Google Scholar] [CrossRef]
- Pastenes, C.; Pimentel, P.; Lillo, J. Leaf movements and photoinhibition in relation to water stress in field-grown beans. J. Exp. Bot. 2005, 56, 425–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helyes, L.; Tuan, L.A.; Bakr, J.; Pék, Z. The simultaneous effect of water stress and biofertilizer on physiology and quality of processing tomato. Acta Hortic. 2019, 1233, 53–60. [Google Scholar] [CrossRef]
- Asins, M.J.; Albacete, A.; Martínez-Andújar, C.; Celiktopuz, E.; Solmaz, I.; Sarı, N.; Pérez-Alfocea, F.; Dodd, I.C.; Carbonell, E.A.; Topcu, S. Genetic analysis of root-to-shoot signalling and rootstock-mediated tolerance to water deficit in tomato. Genes 2021, 12, 10. [Google Scholar] [CrossRef]
- Laxman, R.H.; Hemamalini, P.; Namratha, M.R.; Bhatt, R.M.; Sadashiva, A.T. Phenotyping Deficit Moisture Stress Tolerance in Tomato Using Image Derived Digital Features. Int. J. Bio-Resour. Stress Manag. 2022, 13, 339–347. [Google Scholar] [CrossRef]
- He, Y.; Zhu, Z.J.; Yang, J.; Ni, X.L.; Zhu, B. Grafting increases the salt tolerance of tomato by improvement of photosynthesis and enhancement of antioxidant enzymes activity. Environ. Exp. Bot. 2009, 66, 270–278. [Google Scholar] [CrossRef]
- Critchley, C. Photoinhibition. In Photosynthesis: A Comprehensive Treatise; Raghavendra, A.S., Ed.; Cambridge University Press: Cambridge, UK, 1998; pp. 264–272. [Google Scholar]
- Dariva, F.D.; Copati, M.G.F.; Pessoa, H.P.; Alves, F.M.; Dias, F.O.; Picoli, E.A.T.; Da, C.F.F.; Nick, C. Evaluation of anatomical and physiological traits of Solanum pennellii Cor. associated with plant yield in tomato plants under water-limited conditions. Sci. Rep. 2020, 10, 16052. [Google Scholar] [CrossRef] [PubMed]
- Egea, I.; Albaladejo, I.; Meco, V.; Morales, B.; Sevilla, A.; Bolarin, M.C.; Flores, F.B. The drought-tolerant Solanum pennellii regulates leaf water loss and induces genes involved in amino acid and ethylene/jasmonate metabolism under dehydration. Sci. Rep. 2018, 8, 2791. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Yang, R.; Zhao, F.; Wang, S.; Li, C.; Zhao, W. An analysis of physiological index of differences in drought tolerance of tomato rootstock seedlings. J. Plant Biol. 2016, 59, 311–321. [Google Scholar] [CrossRef]
- Chetelat, R.T.; Petersen, J.P. Improved Maintenance of the Tomato-like Solanum spp. by Grafting. Rep. Tomato Genet. Coop. 2003, 53, 14–15. [Google Scholar]
- Giuffrida, F.; Cassaniti, C.; Leonardi, C. Tomato and eggplant scions influence the effect of rootstock under Na2SO4 salinity. Acta Agic. Scand. B. Soil Plant Sci. 2014, 64, 700–709. [Google Scholar]
- Albacete, A.; Andújar, C.; Pérez-Alfocea, F.; Lozano, J.; Asins, M. Rootstock mediated variation in tomato vegetative growth under low potassium or phosphorous supplies. Acta Hortic. 2015, 1086, 147–152. [Google Scholar] [CrossRef]
- Madrid-Espinoza, J.; Salinas-Cornejo, J.; Ruiz-Lara, S. The RabGAP Gene Family in Tomato (Solanum lycopersicum) and Wild Relatives: Identification, Interaction Networks, and Transcriptional Analysis during Plant Development and in Response to Salt Stress. Genes 2019, 10, 638. [Google Scholar] [CrossRef] [Green Version]

| Denomination | Species/Cross | Biological Status | Source |
|---|---|---|---|
| Rootstock | |||
| Arka Vikas (AV) | Solanum lycopersicum L. | Variety | IIHR, Bengaluru |
| P. Chhuhara (PC) | S. lycopersicum L. | Variety | PAU, Ludhiana |
| RF4A (RF) | S. lycopersicum × S. Pennellii | Derivative line | IIHR, Bengaluru |
| IIHR-1939 (39) | S. pimpinellifolium | Wild species | IIHR, Bengaluru |
| AV39 | Arka Vikas × IIHR-1939 | F1 hybrid | CAZRI, Jodhpur |
| PC39 | P. Chhuhara × IIHR-1939 | F1 hybrid | CAZRI, Jodhpur |
| RF39 | RF4A × IIHR-1939 | F1 hybrid | CAZRI, Jodhpur |
| Scion | |||
| 6242 (62) | S. lycopersicum L. | F1 hybrid | Syngenta Seeds |
| Stomatal Conductance (gs) (mmol m−2s−1) | RWC (%) | Canopy temperature (°C) | PSII (Fv/Fm) | |
|---|---|---|---|---|
| Graft combination (G) | ||||
| 62/AV | 300.54 ab | 84.08 abc | 30.91 bc | 0.817 abc |
| 62/PC | 308.57 ab | 84.68 ab | 31.18 ab | 0.819 abc |
| 62/RF | 270.42 b | 84.34 abc | 30.16 d | 0.823 a |
| 62/39 | 326.85 a | 81.95 de | 30.44 d | 0.814 c |
| 62/AV39 | 341.65 a | 82.43 cd | 30.56 cd | 0.816 bc |
| 62/PC39 | 304.51 ab | 83.15 bcd | 30.34 d | 0.820 abc |
| 62/RF39 | 327.40 a | 85.76 a | 30.26 d | 0.822 ab |
| 6242 (Control) | 328.43 a | 80.10 e | 31.48 a | 0.813 c |
| Irrigation regime (S) | ||||
| WW | 411.38 a | 89.58 a | 29.91 b | 0.825 a |
| MWS | 350.02 b | 83.80 b | 30.18 b | 0.820 b |
| SWS | 179.24 c | 76.56 c | 31.85 a | 0.809 c |
| Significance | ||||
| G | * | *** | *** | * |
| S | *** | *** | *** | *** |
| G × S | * | *** | *** | NS |
| Fresh Biomass (g) | Dry Biomass (g) | Leaf Area (m2) | Root Length (cm) | Root Dry Mass (g) | |
|---|---|---|---|---|---|
| Graft combination (G) | |||||
| 62/AV | 120.22 bc | 16.07 bcd | 0.14 d | 37.06 bc | 33.68 b |
| 62/PC | 143.61 b | 17.78 bcd | 0.19 abc | 36.67 bc | 32.22 c |
| 62/RF | 136.49 bc | 21.42 ab | 0.18 bcd | 56.41 ab | 36.54 a |
| 62/39 | 106.44 c | 12.33 d | 0.15 cd | 52.06 ab | 34.41 b |
| 62/AV39 | 154.43 ab | 21.81 ab | 0.20 ab | 51.50 ab | 36.58 a |
| 62/PC39 | 150.49 ab | 20.13 bc | 0.22 a | 44.21 bc | 36.62 a |
| 62/RF39 | 183.05 a | 25.85 a | 0.23 a | 66.43 a | 36.47 a |
| 6242 (Control) | 105.85 c | 15.48 cd | 0.17 bcd | 48.18 bc | 33.80 b |
| Irrigation regime (S) | |||||
| WW | 217.13 a | 27.95 a | 0.30 a | 47.77 | 36.08 a |
| MWS | 128.06 b | 19.08 b | 0.17 b | 49.47 | 34.97 b |
| SWS | 67.53 c | 9.55 c | 0.09 c | 49.96 | 34.06 c |
| Significance | |||||
| G | *** | *** | *** | ** | *** |
| S | *** | *** | *** | NS | *** |
| G × S | NS | NS | NS | NS | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khapte, P.S.; Kumar, P.; Wakchaure, G.C.; Jangid, K.K.; Colla, G.; Cardarelli, M.; Rane, J. Application of Phenomics to Elucidate the Influence of Rootstocks on Drought Response of Tomato. Agronomy 2022, 12, 1529. https://doi.org/10.3390/agronomy12071529
Khapte PS, Kumar P, Wakchaure GC, Jangid KK, Colla G, Cardarelli M, Rane J. Application of Phenomics to Elucidate the Influence of Rootstocks on Drought Response of Tomato. Agronomy. 2022; 12(7):1529. https://doi.org/10.3390/agronomy12071529
Chicago/Turabian StyleKhapte, Pratapsingh S., Pradeep Kumar, Goraksha C. Wakchaure, Krishna Kumar Jangid, Giuseppe Colla, Mariateresa Cardarelli, and Jagadish Rane. 2022. "Application of Phenomics to Elucidate the Influence of Rootstocks on Drought Response of Tomato" Agronomy 12, no. 7: 1529. https://doi.org/10.3390/agronomy12071529
APA StyleKhapte, P. S., Kumar, P., Wakchaure, G. C., Jangid, K. K., Colla, G., Cardarelli, M., & Rane, J. (2022). Application of Phenomics to Elucidate the Influence of Rootstocks on Drought Response of Tomato. Agronomy, 12(7), 1529. https://doi.org/10.3390/agronomy12071529










