Essential Oil of Citrus aurantium L. Leaves: Composition, Antioxidant Activity, Elastase and Collagenase Inhibition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Essential Oil Extraction and Yield Estimation
2.3. Chemical Characterization of EO by Coupling Gas Chromatography/Mass Spectrometry
2.4. Assessement of Total Phenolic Content
2.5. DPPH Radical Scavenging Activity
2.6. ABTS [2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonate)] Radical Scavenging Activity
2.7. Determination of Collagenase and Elastase Inhibition
2.8. Statistical Analyses
3. Results and Discussion
3.1. Essential Oil Yield and Composition
3.2. Phenolic and Antioxidant Activity
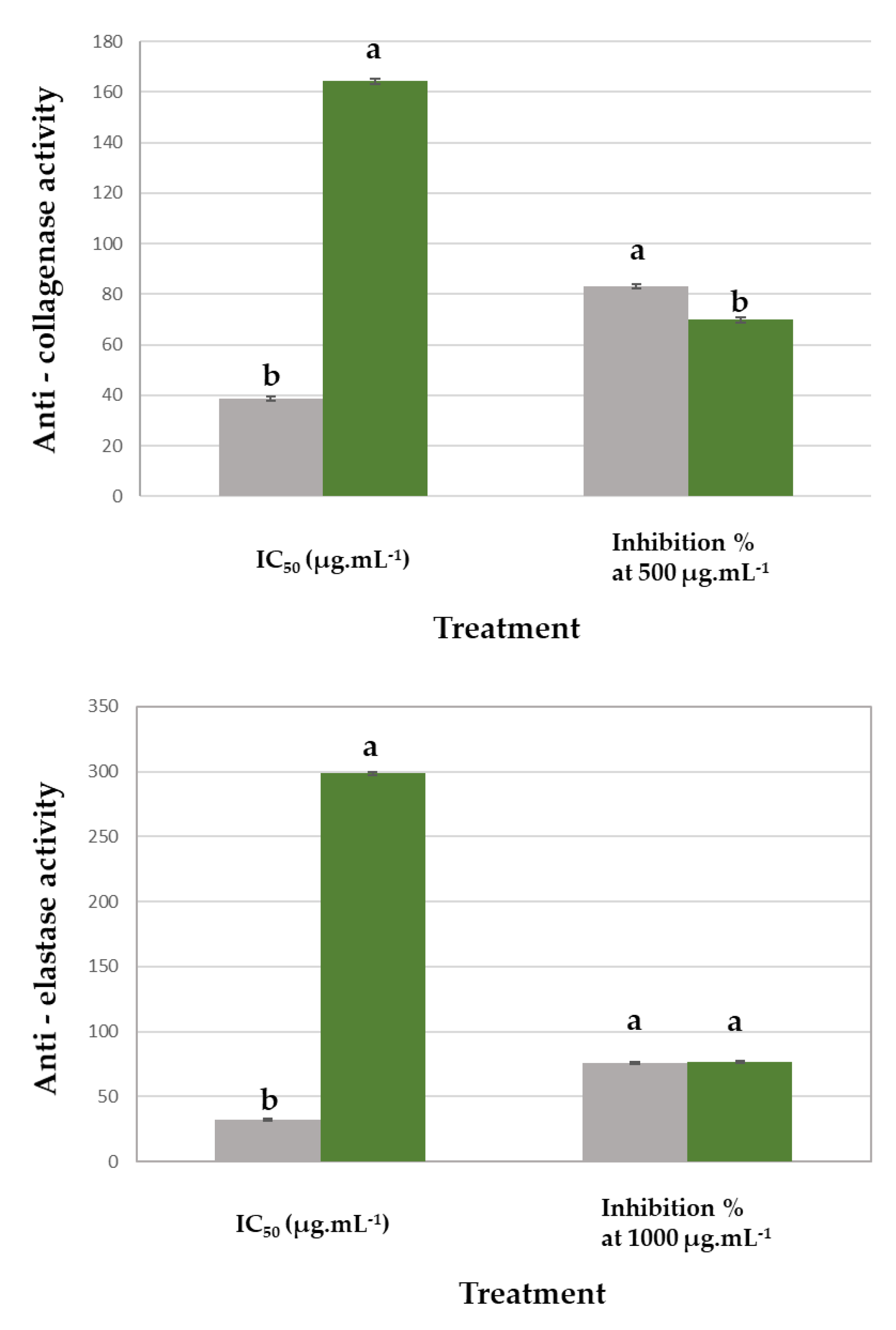
3.3. Collagenase and Elastase Inhibition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thiviya, P.; Gamage, A.; Piumali, D.; Merah, O.; Madhujith, T. Apiaceae as an Important Source of Antioxidants and Their Applications. Cosmetics 2021, 8, 111. [Google Scholar] [CrossRef]
- Sayed Ahmad, B.; Talou, T.; Saad, Z.; Hijazi, H.; Merah, O. The Apiaceae: Ethnomedicinal family as source for industrial uses. Ind. Crops Prod. 2017, 109, 661–671. [Google Scholar] [CrossRef] [Green Version]
- FAO. Citrus Fruits: Fresh and Processed. Statistical Bulletin; FAO: Rome, Italy, 2017; 77p. [Google Scholar]
- Hwang, S.-L.; Shih, P.-H.; Yen, G.-C. Neuroprotective effects of Citrus flavonoids. J. Agric. Food Chem. 2012, 60, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Othman, M.; Atiqah, S.N.; Hassan, M.A.; Nahar, L.; Basar, N.; Jamil, S.; Sarker, S.D. Essential oils from the Malaysian Citrus (Rutaceae) medicinal plants. Medicines 2016, 3, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bora, H.; Kamle, M.; Mahato, D.K.; Tiwari, P.; Kumar, P. Citrus essential oils (CEOs) and their applications in food: An overview. Plants 2020, 9, 357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tranchida, P.Q.; Bonaccorsi, I.; Dugo, P.; Mondello, L.; Dugo, G. Analysis of Citrus essential oils: State of the art and future perspectives. A review. Flavour Fragr. J. 2012, 27, 98–123. [Google Scholar] [CrossRef]
- De Pasquale, F.; Siragusa, M.; Abbate, L.; Tusa, N.; De Pasquale, C.; Alonzo, G. Characterization of five sour orange clones through molecular markers and leaf essential oils analysis. Sci. Hortic. 2006, 109, 54–59. [Google Scholar] [CrossRef]
- Elshafie, H.S. Plant Essential Oil with Biological Activity. Plants 2022, 11, 980. [Google Scholar] [CrossRef]
- Hosni, K.; Zahed, N.; Chrif, R.; Abid, I.; Medfei, W.; Kallel, K.; Ben Brahim, N.; Sebei, H. Composition of peel essential oils from four selected Tunisian Citrus species: Evidence for the genotypic influence. Food Chem. 2010, 123, 1098–1104. [Google Scholar] [CrossRef]
- Djenane, D. Chemical profile, antibacterial and antioxidant activity of Algerian citrus essential oils and their application in Sardina pilchardus. Foods 2015, 4, 208–228. [Google Scholar] [CrossRef] [Green Version]
- Radan, M.; Parcina, A.; Burcul, F. Chemical composition and antioxidant activity of essential oil obtained from bitter orange peel (Citrus aurantium L.) using two methods. Croatica Chem. Acta 2018, 91, 125–128. [Google Scholar] [CrossRef]
- Sarrou, E.; Chatzopoulou, P.; Dimassi-Theriou, K.; Therios, I. Volatile constituents and antioxidant activity of peel, flowers and leaf oils of Citrus aurantium L. growing in Greece. Molecules 2013, 18, 10639–10647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohagheghniapoura, A.; Saharkhiza, M.J.; Golmakanic, M.T.; Niakousari, M. Variations in chemical compositions of essential oil from sour orange (Citrus aurantium L.) blossoms by different isolation methods. Sustain. Chem. Pharm. 2018, 10, 118–124. [Google Scholar] [CrossRef]
- Sanei-Dehkordi, A.; Sedaghat, M.M.; Vatandoost, H.; Abai, M.R. Chemical compositions of the peel essential oil of Citrus aurantium and its natural larvicidal activity against the malaria vector Anopheles stephensi (Diptera: Culicidae) in comparison with Citrus paradisi. J. Arthropod-Borne Dis. 2016, 10, 577. [Google Scholar]
- Azanchi, T.; Shafaroodi, H.; Asgarpanah, J. Anticonvulsant activity of Citrus aurantium blossom essential oil (neroli): Involvment of the GABAergic system. Nat. Prod. Commun. 2014, 9, 1615–1618. [Google Scholar]
- Khodabakhsh, P.; Shafaroodi, H.; Asgarpanah, J. Analgesic and anti-inflammatory activities of Citrus aurantium L. blossoms essential oil (neroli): Involvement of the nitric oxide/cyclic-guanosine monophosphate pathway. J. Nat. Med. 2015, 69, 324–331. [Google Scholar] [CrossRef]
- Hsouna, A.B.; Hamdi, N.; Halima, N.B.; Abdelkafi, S. Characterization of essential oil from Citrus aurantium L. flowers: Antimicrobial and antioxidant activities. J. Oleo Sci. 2013, 62, 763–772. [Google Scholar] [CrossRef] [Green Version]
- Bnina, E.B.; Hajlaoui, H.; Chaieb, I.; Said, M.B.; Jannet, H.B. Chemical composition, antimicrobial and insecticidal activities of the tunisian Citrus aurantium essential oils. Czech J. Food Sci. 2019, 37, 81–92. [Google Scholar] [CrossRef]
- Hosni, K.; Hassen, I.; M’rabet, Y.; Sebei, H.; Casabianca, H. Genetic relationships between some Tunisian Citrus species based on their leaf volatile oil constituents. Biochem. Syst. Ecol. 2013, 50, 65–71. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Gargouri, M.; Dhifi, W.; Ben Saad, R.; Sayahi, N.; Mnif, W.; Saibi, W. Potential anti-inflammatory and antioxidant effects of Citrus aurantium essential oil against carbon tetrachloride-mediated hepatotoxicity: A biochemical, molecular and histopathological changes in adult rats. Environ. Toxicol. 2019, 34, 388–400. [Google Scholar] [CrossRef]
- Zarrad, K.; Ben Hamouda, A.; Chaiebb, I.; Laarif, A.; Mediouni-Ben Jemâa, J. Chemical composition, fumigant and anti-acetylcholinesterase activity of the Tunisian Citrus aurantium L. essential oils. Ind. Crop Prod. 2015, 76, 121–127. [Google Scholar] [CrossRef]
- Degirmenci, H.; Erkurt, H. Chemical profile and antioxidant potency of Citrus aurantium L. flower extracts with antibacterial effect against foodborne pathogens in rice pudding. LWT Food Sci. Technol. 2020, 126, 109273. [Google Scholar] [CrossRef]
- Hamdani, F.Z.; Allem, R. Propriétés antifongiques des huiles essentielles des feuilles de Citrus vis-à-vis d’Alternaria alternata et Penicillium sp in vitro. Phytothérapie 2017, 15, 263–266. [Google Scholar] [CrossRef]
- Lin, X.; Cao, S.; Sun, J.; Lu, D.; Zhong, B.; Chun, J. The chemical compositions, and antibacterial and antioxidant activities of four types of citrus essential oils. Molecules 2021, 26, 3412. [Google Scholar] [CrossRef]
- Proestos, C.; Sereli, D.; Komaitis, M. Determination of phenolic compounds in aromatic plants by RP-HPLC and GC-MS. Food Chem. 2006, 95, 44–52. [Google Scholar] [CrossRef]
- NIST/EPA/NIH. Mass Spectral Library Gaithersburg; National Institute of Standard and Technology: Palmer, MA, USA, 2022.
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corp.: Carol Stream, IL, USA, 2007; pp. 102–133. [Google Scholar]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retentions indices for frequently reported compound of plant essential oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef] [Green Version]
- Davies, N.W. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicon and Carbowax 20M phases. J. Chromatogr. A 1990, 503, 1–24. [Google Scholar] [CrossRef]
- Dwivedy, A.K.; Prakash, B.; Chanotiya, C.S.; Bisht, D.; Dubey, N.K. Chemically characterized Mentha cardiaca L. essential oil as plant based preservative in view of e_cacy against biodeteriorating fungi of dry fruits, aflatoxin secretion, lipid peroxidation and safety profile assessment. Food Chem. Toxicol. 2017, 106, 175–184. [Google Scholar] [CrossRef]
- Morshedloo, M.R.; Maggi, F.; Neko, H.T.; Aghdam, M.S. Sumac (Rhus coriaria L.) fruit: Essential oil variability in Iranian populations. Ind. Crops Prod. 2018, 111, 1–7. [Google Scholar] [CrossRef]
- Araújo, F.M.; Dantas, M.C.S.M.; Silva, L.S.; Aona, L.Y.S.; de Souza-Neta, L.C. Antibacterial activity and chemical composition of the essential oil of Croton heliotropiifolius Kunth from Amargosa, Bahia, Brazil. Ind. Crops Prod. 2017, 105, 203–206. [Google Scholar] [CrossRef]
- Salachna, P.; Łopusiewicz, Ł.; Wesołowska, A.; Meller, E.; Piechocki, R. Mushroom waste biomass alters the yield, total phenolic content, antioxidant activity and essential oil composition of Tagetes patula L. Ind. Crop Prod. 2021, 171, 113961. [Google Scholar] [CrossRef]
- Popovici, C.; Saykova, I.; Tylkowski, B. Evaluation de l’activité antioxydant des composés phénoliques par la réactivité avec le radical libre DPPH. Rev. Génie Ind. 2009, 4, 25–39. [Google Scholar]
- Barragan Ferrer, D.; Venskutonis, P.R.; Talou, T.; Zebib, B.; Barragan Ferrer, M.J.; Merah, O. Bioactive compounds and antioxidant properties of Myrrhis odorata deodorized residue leaves extracts from Lithuania and France origins. Pharm. Chem. J. 2016, 3, 43–48. [Google Scholar]
- Wittenauer, J.; Mäckle, S.; Sußmann, D.; Schweiggert-Weisz, U.; Carle, R. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia 2015, 101, 179–187. [Google Scholar] [CrossRef]
- Zemour, K.; Labdelli, A.; Adda, A.; Dellal, A.; Talou, T.; Merah, O. Phenol content, antioxidant and antiaging activities of safflower seed oil (Carthamus tinctorius L.). Cosmetics 2019, 6, 55. [Google Scholar] [CrossRef] [Green Version]
- Almeida, L.A.H.; Santos, J.Z.; Soares Filho, W.S.; Bizzo, H.R.; Silva, J.P.; Vieira, R.F. Chemical Characterization of Leaf Essential Oil from Seven Accessions of Sour Orange (Citrus aurantium L.). J. Essent. Oil Bearing Plant 2015, 18, 426–435. [Google Scholar] [CrossRef]
- Ferrer, V.; Costantino, G.; Paoli, M.; Paymal, N.; Quinton, C.; Ollitrault, P.; Tomi, F.; Luro, F. Intercultivar Diversity of Sour Orange (Citrus aurantium L.) Based on Genetic Markers, Phenotypic Characteristics, Aromatic Compounds and Sensorial Analysis. Agronomy 2021, 11, 1084. [Google Scholar] [CrossRef]
- Guo, L.; Liu, Y.; Luo, L.; Hussain, S.B.; Bai, Y.; Alam, S.M. Comparative Metabolites and Citrate-Degrading Enzymes Activities in Citrus Fruits Reveal the Role of Balance between ACL and Cyt-ACO in Metabolite Conversions. Plants 2020, 9, 350. [Google Scholar] [CrossRef] [Green Version]
- Dugo, G.; Bonaccorsi, I.; Sciarrone, D.; Costa, R.; Dugo, P.; Mondello, L.; Santi, L.; Fakhry, H.A. Characterization of oils from the fruits leaves and flowers of the bitter orange tree. J. Essent. Oil Res. 2011, 23, 45–59. [Google Scholar] [CrossRef]
- Aazza, S.; Lyoussi, B.; Miguel, M.G. Antioxidant and antiacetylcholinesterase activities of some commercial essential oils and their major compounds. Molecules 2011, 16, 7672–7690. [Google Scholar] [CrossRef] [Green Version]
- Boussaada, O.; Skoula, M.; Kokkalou, E.; Chemli, R. Chemical Variability of Flowers, Leaves, and Peels Oils of Four Sour Orange Provenances. Essent. Oil Bearing Plant 2013, 10, 453–464. [Google Scholar] [CrossRef]
- Mahdi, A.A.; Al-Maqtari, Q.A.; Mohammed, J.K.; Al-Ansi, W.; Cui, H.; Lin, L. Enhancement of antioxidant activity, antifungal activity, and oxidation stability of Citrus reticulata essential oil nanocapsules by clove and cinnamon essential oils. Food Biosci. 2021, 43, 101226. [Google Scholar] [CrossRef]
- Rădulescu, M.; Jianu, C.; Lukinich-Gruia, A.T.; Mioc, M.; Mioc, A.; Șoica, C.; Stana, L.G. Chemical composition, in vitro and in silico antioxidant potential of Melissa officinalis subsp. officinalis essential oil. Antioxidants 2021, 10, 1081. [Google Scholar] [CrossRef] [PubMed]
- Barragan Ferrer, D.; Venskutonis, P.R.; Talou, T.; Barragan Ferrer, J.M.; Zebib, B.; Merah, O. Identification and in vitro activity of bioactive compounds extracted from Tussilago farfara (L.) plant grown in Lithuania and France. Free. Radic. Antioxid. 2018, 8, 40–47. [Google Scholar] [CrossRef]
- Raeis Abad, M.K.; Besheli, B.A. Insecticidal potential of essential oil from the leaves of Citrus aurantium L. against Oryzaephilus surinamensis (F.), Lasioderma serricorne (L.) and Sitophilus oryzae (L.). J. Entomol. Zool. Stud. 2016, 4, 865–869. [Google Scholar]
- Perera, S.; Silva, A.B.G.; Amarathunga, Y.; De Silva, S.; Jayatissa, R.; Gamage, A.; Merah, O.; Madhujith, T. Nutritional Composition and Antioxidant Activity of Selected Underutilized Fruits Grown in Sri Lanka. Agronomy 2022, 12, 1073. [Google Scholar] [CrossRef]
- Merah, O.; Sayed-Ahmad, B.; Talou, T.; Saad, Z.; Cerny, M.; Grivot, S.; Evon, P.; Hijazi, A. Biochemical Composition of Cumin Seeds, and Biorefining Study. Biomolecules 2020, 10, 1054. [Google Scholar] [CrossRef]
- Garg, C.; Khurana, P.; Garg, M. Molecular mechanisms of skin photoaging and plant inhibitors. Inter. J. Green Pharm. 2017, 11, 217–232. [Google Scholar] [CrossRef]
- Zhang, S.; Duan, E. Fighting against skin aging: The way from bench to bedside. Cell Transplan. 2018, 27, 729–738. [Google Scholar] [CrossRef]
- El Khetabi, A.; Ezrari, S.; El Ghadraoui, L.; Tahiri, A.; Ait Haddou, L.; Belabess, Z.; Merah, O.; Lahlali, R. In Vitro and In Vivo Antifungal Activities of Nine Commercial Essential Oils against Brown Rot in Apples. Horticulturae 2021, 7, 545. [Google Scholar] [CrossRef]
- El-Akhal, F.; El Ouali Lalami, A.; Guemmouh, R. Larvicidal activity of essential oils of Citrus sinensis and Citrus aurantium (Rutaceae) cultivated in Morocco against the malaria vector Anopheles labranchiae (Diptera: Culicidae). Asian Pac. J. Trop. Dis. 2015, 5, 458–462. [Google Scholar] [CrossRef]
- Costa, C.A.R.A.; Cury, T.C.; Cassettari, B.O.; Takahira, R.K.; Flório, J.C.; Costa, M. Citrus aurantium L. essential oil exhibits anxiolyticlike activity mediated by 5-HT1A-receptors and reduces cholesterol after repeated oral treatment. BMC Complementary Altern. Med. 2013, 13, 42. [Google Scholar] [CrossRef] [PubMed]
- Fernandes Pimenta, F.C.; Alves, M.F.; Fernandes Pimenta, M.B.; Melo, S.A.L.; Figueirêdo de Almeida, A.A.; Leite, J.R.; de Morais Pordeus, L.C.; Melo Diniz, M.F.F.; de Almeida, R.N. Anxiolytic Effect of Citrus aurantium L. on Patients with Chronic Myeloid Leukemia. Phytother. Res. 2016, 30, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Rozza, A.L.; Pellizzon, C.H. Essential oils from medicinal and aromatic plants: A review of the gastroprotective and ulcer-healing activities. Fundam. Clin. Pharm. 2013, 27, 51–63. [Google Scholar] [CrossRef]
- Moraes, T.M.; Kushima, H.; Moleiro, F.C.; Santos, R.C.; Rocha, L.R.; Marques, M.O.; Vilegas, W.; Hiruma-Lima, C.A. Effects of limonene and essential oil from Citrus aurantium on gastric mucosa: Role of prostaglandins and gastric mucus secretion. Chem.-Biol. Inter. 2009, 180, 499–505. [Google Scholar] [CrossRef]
| Pics | TR (Min) | Constituants | % | RIliterature | RIexp | Group |
|---|---|---|---|---|---|---|
| 1 | 5.4 | 2-Ethyl furan | 0.01 ± 0.00 | 689 | 692 | Fur |
| 2 | 6.9 | 1-Hexanol | 0.02 ± 0.00 | 799 | 803 | Alc |
| 3 | 7 | α-Pinene | 0.20 ± 0.01 | 925 | 923 | M |
| 4 | 7.8 | α-Thuyene | 0.01 ± 0.00 | 926 | 925 | M |
| 5 | 8.9 | Camphene | 0.01 ± 0.00 | 943 | 945 | M |
| 6 | 9.2 | β-Pinene | 3.20 ± 0.02 | 945 | 947 | M |
| 7 | 10.1 | Sabinene | 0.40 ± 0.01 | 973 | 974 | M |
| 8 | 10.4 | δ3−Χαρενε | 0.01 ± 0.00 | 986 | 989 | M |
| 9 | 11.1 | β-Myrcene | 2.25 ± 0.01 | 989 | 992 | M |
| 10 | 11.8 | α-Terpinene | 0.03 ± 0.00 | 1008 | 1016 | M |
| 11 | 12.2 | Limonene | 0.71 ± 0.01 | 1023 | 1024 | M |
| 12 | 12.5 | β-Phellandrene | 0.05 ± 0.00 | 1023 | 1025 | M |
| 13 | 13 | 2-Hexanal | 0.11 ± 0.01 | 1024 | 1028 | A |
| 14 | 13.7 | Cis-β-Ocimene | 0.81 ± 0.02 | 1027 | 1030 | M |
| 15 | 13.8 | γ−Τερπινενε | 0.05 ± 0.00 | 1028 | 1031 | M |
| 16 | 14.8 | Trans-β-Ocimene | 2.40 ± 0.02 | 1028 | 1032 | M |
| 17 | 15.4 | p-Cymene | 0.05 ± 0.00 | 1029 | 1033 | M |
| 18 | 18.3 | Cis-Oxide linalool | 0.10 ± 0.00 | 1059 | 1065 | O M |
| 19 | 23.5 | Trans-Oxide linalool | 0.05 ± 0.00 | 1072 | 1073 | O M |
| 20 | 25.1 | Terpinolene | 0.45 ± 0.01 | 1078 | 1079 | M |
| 21 | 29.8 | Linalool | 30.62 ± 0.04 | 1083 | 1084 | O M |
| 22 | 30.5 | Terpinen-4-ol | 0.15 ± 0.00 | 1124 | 1129 | O M |
| 23 | 32.9 | α-Terpineol | 9.57 ± 0.05 | 1173 | 1175 | O M |
| 24 | 33 | Citronellol | 0.05 ± 0.00 | 1212 | 1213 | M |
| 25 | 33.5 | Nerol | 2.01 ± 0.00 | 1214 | 1216 | O M |
| 26 | 36.5 | Neral | 0.02 ± 0.00 | 1226 | 1227 | O M |
| 27 | 36.7 | Geraniol | 5.53 ± 0.05 | 1234 | 1235 | O M |
| 28 | 37 | Linalyl Acetate | 33.01 ± 0.07 | 1239 | 1239 | O M |
| 29 | 37.6 | Linalyl propionate | 0.08 ± 0.00 | 1318 | 1319 | O M |
| 30 | 38.5 | Terpenyl acetate | 0.10 ± 0.00 | 1334 | 1338 | O M |
| 31 | 38.6 | Citronellyl acetate | 0.02 ± 0.00 | 1335 | 1336 | O M |
| 32 | 40.2 | Neryl acetate | 2.43 ± 0.02 | 1342 | 1345 | O M |
| 33 | 40.5 | Geranyl acetate | 4.51 ± 0.03 | 1359 | 1361 | O M |
| 34 | 40.8 | β-Caryophyllene | 0.40 ± 0.01 | 1417 | 1425 | S |
| 35 | 42 | α-Humulene | 0.05 ± 0.00 | 1437 | 1440 | S |
| 36 | 42.1 | E-β-Farnesene | 0.02 ± 0.00 | 1443 | 1444 | S |
| 37 | 44,3 | B-Germacrene | 0.10 ± 0.00 | 1475 | 1477 | S |
| 38 | 46.9 | β-Bisabolene | 0.02 ± 0.00 | 1496 | 1499 | S |
| 39 | 57.2 | Nerolidol | 0.15 ± 0.01 | 1547 | 1550 | O S |
| 40 | 57,8 | Germacra-1,5-dien-4-ol | 0.02 ± 0.00 | 1568 | 1569 | S |
| 41 | 61.5 | Spathulenol | 0.01 ± 0.00 | 1576 | 1573 | O S |
| 42 | 63.8 | T-Cadinol | 0.02 ± 0.00 | 1626 | 1624 | S |
| 43 | 66.8 | α-Cadinol | 0.05 ± 0.00 | 1652 | 1653 | S |
| Compound group (%) | ||||||
| Oxygenated Monoterpenes | 86.02 | |||||
| Monoterpenes | 7.6 | |||||
| Sesquiterpenes | 0.47 | |||||
| Oxygenated Sesquiterpenes | 0.25 | |||||
| Other | 5.29 | |||||
| Total | 99,63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oulebsir, C.; Mefti-Korteby, H.; Djazouli, Z.-E.; Zebib, B.; Merah, O. Essential Oil of Citrus aurantium L. Leaves: Composition, Antioxidant Activity, Elastase and Collagenase Inhibition. Agronomy 2022, 12, 1466. https://doi.org/10.3390/agronomy12061466
Oulebsir C, Mefti-Korteby H, Djazouli Z-E, Zebib B, Merah O. Essential Oil of Citrus aurantium L. Leaves: Composition, Antioxidant Activity, Elastase and Collagenase Inhibition. Agronomy. 2022; 12(6):1466. https://doi.org/10.3390/agronomy12061466
Chicago/Turabian StyleOulebsir, Chahinez, Hakima Mefti-Korteby, Zahr-Eddine Djazouli, Bachar Zebib, and Othmane Merah. 2022. "Essential Oil of Citrus aurantium L. Leaves: Composition, Antioxidant Activity, Elastase and Collagenase Inhibition" Agronomy 12, no. 6: 1466. https://doi.org/10.3390/agronomy12061466
APA StyleOulebsir, C., Mefti-Korteby, H., Djazouli, Z.-E., Zebib, B., & Merah, O. (2022). Essential Oil of Citrus aurantium L. Leaves: Composition, Antioxidant Activity, Elastase and Collagenase Inhibition. Agronomy, 12(6), 1466. https://doi.org/10.3390/agronomy12061466








