Effects of Combined Application of Solid Pyrolysis Products and Digestate on Selected Soil Properties of Arenosol and Plant Growth and Composition in Laboratory Experiments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sampling and Soil Properties
2.2. Biochars
2.3. Liquid Digestate
2.4. Experimental Design
2.5. Soil and Plant Analysis
2.6. Statistical Analysis
3. Results
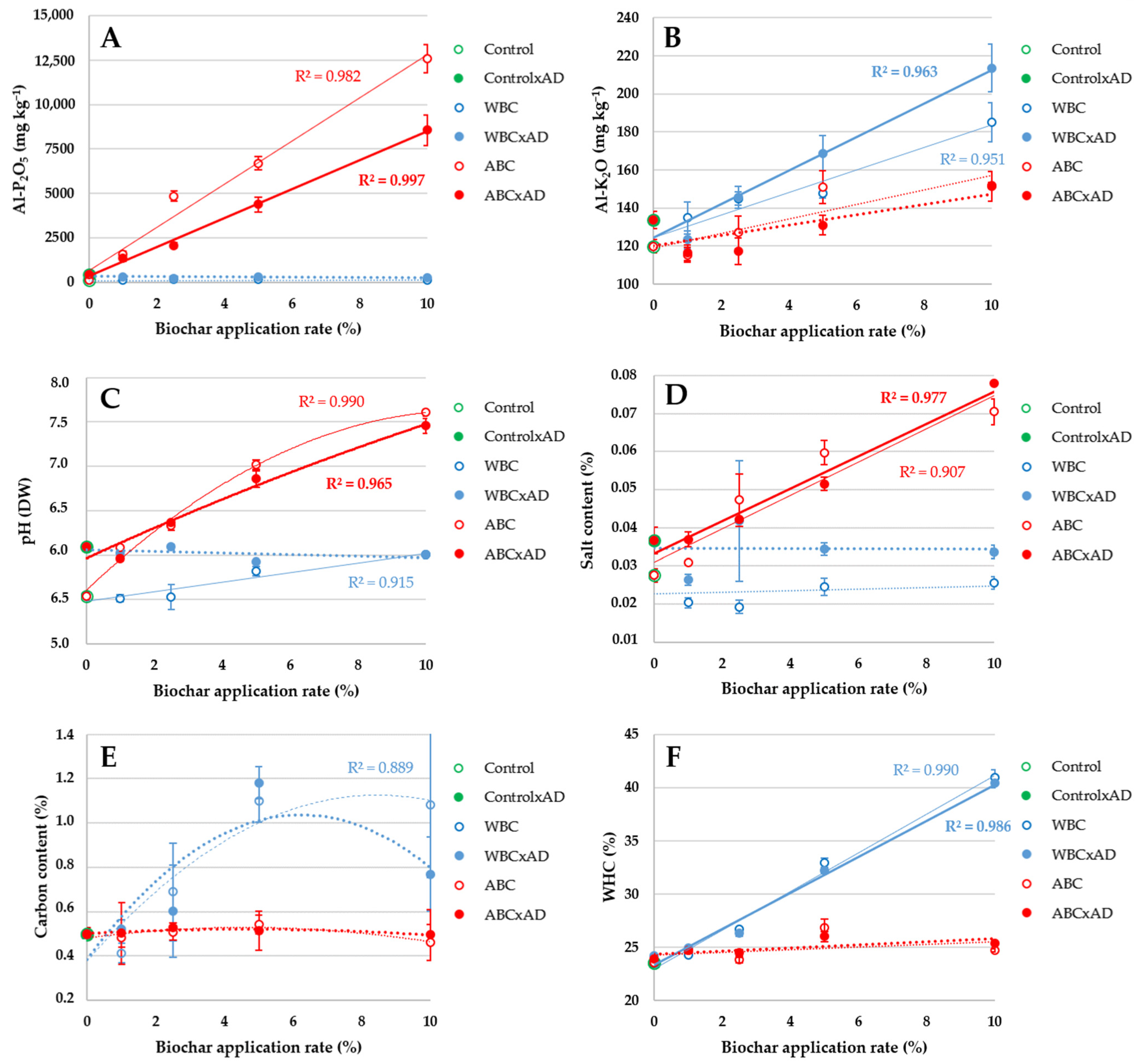
3.1. Soil Physical and Chemical Characteristics
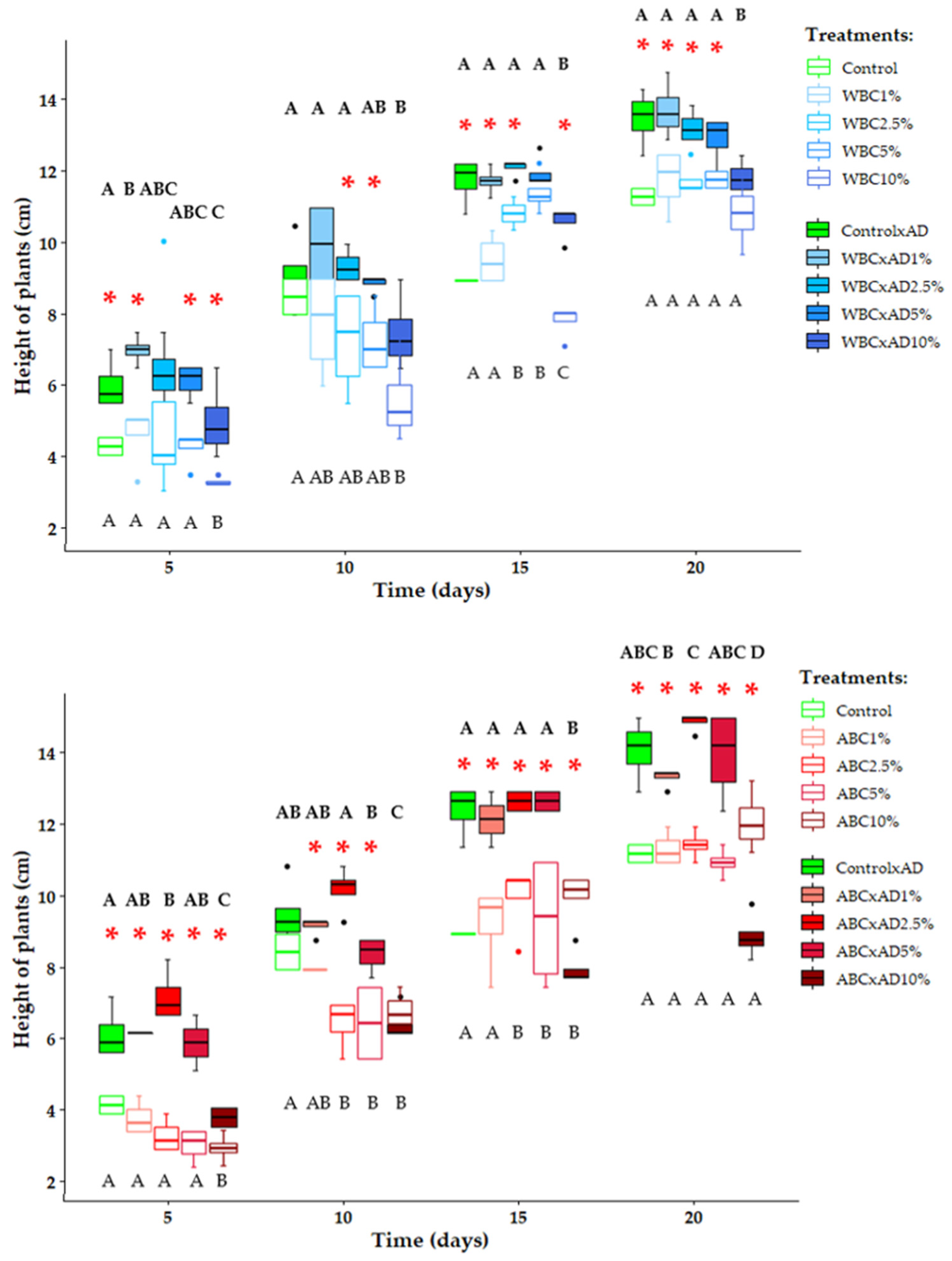
3.2. Plant Growth and Yields
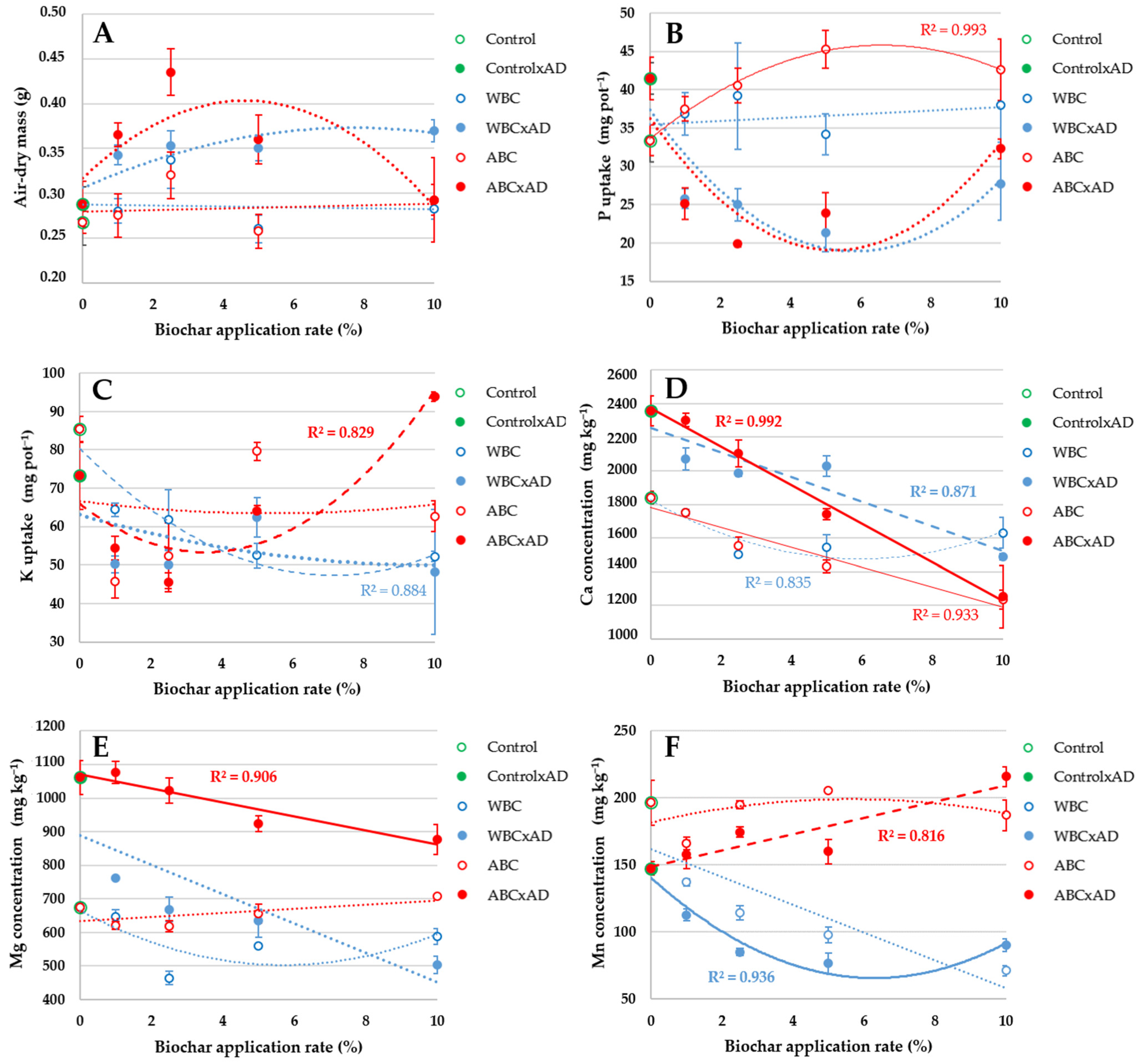
3.3. Plant Tissue Analyses
4. Discussion
4.1. Soil Physical and Chemical Characteristics
4.2. Plant Growth and Yield
4.3. Plant Tissue Analyses
4.4. Limitations and Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lehmann, J.; da Silva, J.P., Jr.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: Fertilizer, manure and charcoal amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-char sequestration in terrestrial Ecosystems—A review. Mitig. Adapt. Strateg. Glob. Chang. 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Hardy, B.; Sleutel, S.; Dufey, J.E.; Cornelis, J.-T. The long-term effect of biochar on soil microbial abundance, activity and community structure is overwritten by land management. Front. Environ. Sci. 2019, 7, 110. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.; Rossi, L.; Zotarelli, L.; Gao, B.; Shahid, M.A.; Sarkhosh, A. Biochar improves soil physical characteristics and strengthens root architecture in Muscadine grape (Vitis rotundifolia L.). Chem. Biol. Technol. Agric. 2021, 8, 7. [Google Scholar] [CrossRef]
- Ndede, E.O.; Kurebito, S.; Idowu, O.; Tokunari, T.; Jindo, K. The potential of biochar to enhance the water retention properties of sandy agricultural soils. Agronomy 2022, 12, 311. [Google Scholar] [CrossRef]
- Haider, F.U.; Coulter, J.A.; Cai, L.Q.; Hussain, S.; Cheema, S.A.; Wu, J.; Zhang, R.Z. An overview on biochar production, its implications, and mechanisms of biochar-induced amelioration of soil and plant characteristics. Pedosphere 2022, 32, 107–130. [Google Scholar] [CrossRef]
- Wang, L.; Ok, Y.S.; Tsang, D.C.W.; Alessi, D.S.; Rinklebe, J.; Mašek, O.; Bolan, N.S.; Hou, D. Biochar composites: Emerging trends, field successes and sustainability implications. Soil Use Manag. 2022, 38, 14–38. [Google Scholar] [CrossRef]
- Nath, H. Biochar from biomass: A review on biochar preparation its modification and impact on soil including soil microbiology. Geomicrobiol. J. 2022, 39, 373–388. [Google Scholar] [CrossRef]
- Hu, J.; Tang, H.; Wang, Y.Z.; Yang, C.; Gao, M.; Tsang, Y.F.; Lib, J. Effect of dissolved solids released from biochar on soil microbial metabolism. Environ. Sci. 2022, 22, 598–608. [Google Scholar] [CrossRef]
- Someus, E. REFERTIL: Reducing mineral fertilizers and chemicals use in agriculture by recycling treated organic waste as compost and bio-char products. In Terra Preta Sanitation; Bettendorf, T., Wendland, C., Otterpohl, R., Eds.; Deutsche Bundesstiftung Umwelt: Berlin, Germany, 2014; pp. 1–12. ISBN 978-3-00-046586-4. [Google Scholar]
- Verheijen, F.; Jeffery, S.; Bastos, A.C.; van der Velde, M.; Diafas, I. Biochar Application to Soils. In A Critical Scientific Review of Effects on Soil Properties, Processes and Functions; EUR 24099 EN; Office for the Official Publications of the European Communities: Luxembourg, 2010; pp. 1–149. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC55799 (accessed on 15 June 2022). [CrossRef]
- Spokas, K.A.; Koskinen, W.C.; Baker, J.M.; Reicosky, D.C. Impacts of woodchip biochar additions on greenhouse gas production and sorption/degradation of two herbicides in a Minnesota soil. Chemosphere 2009, 77, 574–581. [Google Scholar] [CrossRef]
- Woofl, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable biochar to mitigate global climate change. Nature Comm. 2010, 1, 56–64. [Google Scholar] [CrossRef]
- Smith, P. Soil carbon sequestration and biochar as negative emission technologies. Glob. Chang. Biol. 2016, 22, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhou, X.; Jiang, L.; Li, M.; Du, Z.; Zhou, G.; Shao, J.; Wang, X.; Xu, Z.; Hosseini Bai, S.; et al. Effects of biochar application on soil greenhouse gas fluxes: A meta-analysis. GCB Bioenergy 2017, 9, 743–755. [Google Scholar] [CrossRef]
- Liu, X.; Mao, P.; Li, L.; Ma, J. Impact of biochar application on yield-scaled greenhouse gas intensity: A meta-analysis. Sci. Total Environ. 2019, 656, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Borchard, N.; Schirrmann, M.; Cayuela, M.L.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Fuertes-Mendizábal, T.; Sigua, G.; Spokas, K.; Ippolito, J.A.; et al. Biochar, soil and land-use interactions that reduce nitrate leaching and N2O emissions: A meta-analysis. Sci. Total Environ. 2019, 651, 2354–2364. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhou, D.; Zhu, L. Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperature. Environ. Sci. Technol. 2008, 42, 5137–5143. [Google Scholar] [CrossRef]
- Cao, X.; Ma, L.; Gao, B.; Harris, W. Dairy-manure derived biochar effectively sorbs lead and atrazine. Environ. Sci. Technol. 2009, 43, 3285–3291. [Google Scholar] [CrossRef]
- Yu, X.Y.; Ying, G.G.; Kookana, R.S. Reduced plant uptake of pesticides with biochar additions to soil. Chemosphere 2009, 76, 665–671. [Google Scholar] [CrossRef]
- Zheng, W.; Guo, M.; Chow, T.; Bennett, D.N.; Rajagopalan, N. Sorption properties of greenwaste biochar from two trizaine pesticides. J. Hazard. Mater. 2010, 181, 121–126. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Wang, L.; Guo, J.; Wang, H.; Mašek, O.; Wang, H.; Bolan, N.S.; Alessi, D.S.; Hou, D. Aging features of metal(loid)s in biochar-amended soil: Effects of biochar type and aging method. Sci. Total Environ. 2022, 815, 152922. [Google Scholar] [CrossRef]
- Zheng, W.; Sharma, B.K.; Rajagopalan, N. Using Biochar as a Soil Amendment for Sustainable Agriculture. In Illinois Department of Agriculture Sustainable Agriculture Grant’s Research Report Series; Illinois Department of Agriculture: Springfield, IL, USA, 2010; pp. 1–36. [Google Scholar]
- Kapoor, A.; Sharma, R.; Kumar, A.; Sepehya, S. Biochar as a means to improve soil fertility and crop productivity: A review. J. Plant Nutr. 2022, 45. online first. [Google Scholar] [CrossRef]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—A review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Yamato, M.; Okimori, Y.; Wibowo, I.F.; Anshori, S.; Ogawa, M. Effects of the application of charred bark of Acacia mangium on the yield of maize, cowpea and peanut, and soil chemical properties in South Sumatra. Indonesia. Soil Sci. Plant Nutr. 2006, 52, 489–495. [Google Scholar] [CrossRef]
- Chan, K.Y.; Zwieten, L.V.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic values of greenwaste biochar as a soil amendment. Aust. J. Soil Res. 2007, 45, 629–634. [Google Scholar] [CrossRef]
- Steiner, C.; Teixeira, W.G.; Lehmann, J.; Nehls, T.; Vasconcelos de Macędo, J.L.; Blum, W.E.H.; Zech, W. Long term effects of manure. charcoal and mineral fertilization on crop production and fertility on a highly weathered Central Amazonian upland soil. Plant Soil 2007, 291, 275–290. [Google Scholar] [CrossRef] [Green Version]
- Major, J.; Rondon, M.; Riha, D.M.S.J.; Lehmann, J. Maize yield and nutrition during 4 years after biochar application to a Colombian savanna oxisol. Plant Soil 2010, 333, 117–128. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Xu, Y.; Lu, X. Biochar phosphorus fertilizer effects on soil phosphorus availability. Chemosphere 2020, 244, 25471. [Google Scholar] [CrossRef]
- Dai, Y.; Zheng, H.; Jiang, Z.; Xing, B. Combined effects of biochar properties and soil conditions on plant growth: A meta-analysis. Sci. Total Environ. 2020, 713, 136635. [Google Scholar] [CrossRef]
- Zeeshan, M.; Ahmad, W.; Hussain, F.; Numan, M.; Shah, M.; Ahmad, I. Phytostabilization of the heavy metals in the soil with biochar applications, the impact on chlorophyll, carotene, soil fertility and tomato crop yield. J. Clean. Prod. 2020, 255, 120318. [Google Scholar] [CrossRef]
- Laird, D.A. The charcoal vision: A win-win-win scenario for simultaneously producing bioenergy. permanently sequestering carbon, while improving soil and water quality. Agron. J. 2008, 100, 178–181. [Google Scholar] [CrossRef]
- Kloss, S.; Zehetner, F.; Wimmer, B.; Buecker, J.; Rempt, F.; Soja, G. Biochar application to temperate soils: Effects on soil fertility and crop growth under greenhouse conditions. J. Plant. Nutr. Soil Sci. 2014, 177, 3–15. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, B.; Chen, Z.; Zhu, L.; Schnoor, J.L. Insight into multiple and multilevel structures of biochars and their potential environmental applications: A critical review. Environ. Sci. Technol. 2018, 52, 5027–5047. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.L.; Rousk, J.; Edwards-Jones, G.; deLuca, T.H.; Murphy, D.V. Biochar-mediated changes in soil quality and plant growth in a three year field trial. Soil Biol. Biochem. 2012, 45, 113–124. [Google Scholar] [CrossRef]
- Jeffery, S.; Abalos, D.; Prodana, M.; Bastos, A.C.; Van Groenigen, J.W.; Hungate, B.A.; Verheijen, F. Biochar boosts tropical but not temperate crop yields. Environ. Res. Lett. 2017, 12, 053001. [Google Scholar] [CrossRef]
- Güereña, D.; Lehmann, J.; Hanley, K.; Enders, A.; Hyland, C.; Riha, S. Nitrogen dynamics following field application of biochar in a temperate North American maize-based production system. Plant Soil 2013, 365, 239–254. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.; van der Velde, M.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Karer, J.; Wimmer, B.; Zehetne, F.; Kloss, S.; Soja, G. Biochar application to temperate soils: Effects on nutrient uptake and crop yield under field conditions. Agric. Food Sci. 2013, 22, 390–403. [Google Scholar] [CrossRef] [Green Version]
- Gomez, J.D.; Denef, K.; Stewart, C.E.; Zheng, J.; Cotrufo, M.F. Biochar addition rate influences soil microbial abundance and activity in temperate soils. Eur. J. Soil Sci. 2014, 65, 28–39. [Google Scholar] [CrossRef]
- Liu, J.; Schulz, H.; Brandl, S.; Miehtke, H.; Huwe, B.; Glaser, B. Short-term effect of biochar and compost on soil fertility and water status of a Dystric Cambisol in NE Germany under field conditions. J. Plant Nutr. Soil Sci. 2012, 175, 698–707. [Google Scholar] [CrossRef]
- Moragues-Saitua, L.; Arias-González, A.; Gartzia-Bengoetxea, N. Effects of biochar and wood ash on soil hydraulic properties: A field experiment involving contrasting temperate soils. Geoderma 2017, 305, 144–152. [Google Scholar] [CrossRef]
- Smider, B.; Singh, B. Agronomic performance of a high ash biochar in two contrasting soils. Agric. Ecosyst. Environ. 2014, 191, 99–107. [Google Scholar] [CrossRef]
- Revell, K.T.; Maguire, R.O.; Agblevor, F.A. Influence of poultry litter biochar on soil properties and plant growth. Soil Sci. 2012, 177, 402–408. [Google Scholar] [CrossRef]
- Buss, W.; Wurzer, C.; Manning, D.A.C.; Rohling, E.J.; Borevitz, J.; Mašek, O. Mineral-enriched biochar delivers enhanced nutrient recovery and carbon dioxide removal. Commun. Earth Environ. 2022, 3, 67. [Google Scholar] [CrossRef]
- Lehmann, J.; da Silva, J.P., Jr.; Rondon, M.; Cravo, M.S.; Greenwood, J.; Nehls, T.; Steiner, C.; Glaser, B. Slash-and-char—A feasible alternative for soil fertility management in the central Amazon? In Proceedings of the 17th World Congress of Soil Science, Symposium No. 13, Bangkok, Thailand, 14–21 August 2002. [Google Scholar]
- Hu, X.; Zhang, X.; Ngo, H.H.; Guo, W.; Wen, H.; Li, C.; Ma, C. Comparison study on the ammonium adsorption of the biochars derived from different kinds of fruit peel. Sci. Total Environ. 2020, 707, 135544. [Google Scholar] [CrossRef]
- Mizuta, K.; Matsumoto, T.; Hatate, Y.; Nishihara, K.; Nakanishi, T. Removal of nitrate-nitrogen from drinking water using bamboo powder charcoal. Bioresour. Technol. 2004, 95, 255–257. [Google Scholar] [CrossRef]
- Wang, H.; Xiao, K.; Yang, J.; Yu, Z.; Yu, W.; Xu, Q.; Liu, B. Phosphorus recovery from the liquid phase of anaerobic digestate using biochar derived from iron-rich sludge: A potential phosphorus fertilizer. Water Res. 2020, 174, 115629. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Wang, Y.; Zhao, X.; Chen, H.; Chen, G.; Wang, S. Seven years of biochar amendment has a negligible effect on soil available P and a progressive effect on organic C in paddy soils. Biochar 2022, 4, 1. [Google Scholar] [CrossRef]
- Liu, D.; Ding, Z.; Ali, E.F.; Kheir, A.M.S.; Eissa, M.A.; Ibrahim, O.H.M. Biochar and compost enhance soil quality and growth of roselle (Hibiscus sabdariffa L.) under saline conditions. Sci. Rep. 2021, 11, 8739. [Google Scholar] [CrossRef]
- Rombel, A.; Krasucka, P.; Oleszczuk, P. Sustainable biochar-based soil fertilizers and amendments as a new trend in biochar research. Sci. Total Environ. 2022, 816, 151588. [Google Scholar] [CrossRef]
- Xu, H.; Cai, A.; Wu, D.; Liang, G.; Xiao, J.; Xu, M.; Colinet, G.; Zhang, W. Effects of biochar application on crop productivity, soil carbon sequestration, and global warming potential controlled by biochar C:N ratio and soil pH: A global meta-analysis. Soil Tillage Res. 2021, 213, 105125. [Google Scholar] [CrossRef]
- Drosg, B.; Fuchs, W.; Al Seadi, T.; Madsen, M.; Linke, B. Nutrient Recovery by Biogas Digestate Processing; IEA Bioenergy: Paris, France, 2015; pp. 1–37. ISBN 978-910154-16-8. Available online: http://task37.ieabioenergy.com/files/daten-redaktion/download/Technical%20Brochures/NUTRIENT_RECOVERY_RZ_web1.pdf (accessed on 15 June 2022).
- Makádi, M.; Tomócsik, A.; Lengyel, J.; Márton, Á. Problems and successes of digestate utilization on crops. In Proceedings of the Internationale Conference ORBIT 2008, Wageningen, The Netherlands, 13–16 October 2008; CD-ROM. ISBN 3-935974-19-1. [Google Scholar]
- Qiao, Y.T.; Zhang, S.M.; Quan, C.; Gao, N.B.; Josnston, C.; Wu, C.F. One-pot synthesis of digestate-derived biochar for carbon dioxide capture. Fuel 2020, 279, 118525. [Google Scholar] [CrossRef]
- Song, S.; Lim, J.W.; Lee, J.T.E.; Cheong, J.C.; Hoy, S.H.; Hu, Q.; Tan, J.K.N.; Chiam, Z.; Arora, S.; Lum, T.Q.H.; et al. Food-waste anaerobic digestate as a fertilizer: The agronomic properties of untreated digestate and biochar-filtered digestate residue. Waste Manag. 2021, 136, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Möller, K.; Müller, T. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Checchi, F.; Traverso, P.G.; Mata-Alvarez, J.; Clancy, J.; Zaror, C. State-of-the-art of R&D in the anaerobic digestion process of municipal solid waste in Europe. Biomass 1988, 16, 257–284. [Google Scholar] [CrossRef]
- Gissén, C.; Prade, T.; Kreuger, E.; Nges, I.A.; Rosenqvist, H.; Svensson, S.E.; Lantz, M.; Mattsson, J.E.; Börjesson, P.; Björnsson, L. Comparing energy crops for biogas production—Yields, energy input and costs in cultivation using digestate and mineral fertilization. Biomass Bioenerg. 2014, 64, 199–210. [Google Scholar] [CrossRef]
- Panyadee, S.; Petiraksakul, A.; Phalakornkule, C. Biogas production from co-digestion of Phyllanthus emblica residues and food waste. Energy Sustain. Dev. 2013, 17, 515–520. [Google Scholar] [CrossRef]
- Zhang, C.; Su, H.; Baeyens, J.; Tan, T. Reviewing the anaerobic digestion of food waste for biogas production. Renew. Sust. Energ. Rev. 2014, 38, 383–392. [Google Scholar] [CrossRef]
- Risberg, K.; Sun, L.; Levén, L.; Horn, S.J.; Schnürer, A. Biogas production from wheat straw and manure—Impact of pretreatment and process operating parameters. Bioresour. Technol. 2013, 149, 232–237. [Google Scholar] [CrossRef]
- Luostarinen, S.; Luste, S.; Sillanpää, M. Increased biogas production at wastewater treatment plants through co-digestion of sewage sludge with grease trap sludge from a meat processing plant. Bioresour. Technol. 2009, 100, 79–85. [Google Scholar] [CrossRef]
- Kafle, G.K.; Kim, S.H. Effects of chemical compositions and ensiling on the biogas productivity and degradation rates of agricultural and food processing by-products. Bioresour. Technol. 2013, 14, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Zeeman, G.; Wiegant, W.M.; Koster-Treffers, M.E.; Lettinga, G. The influence of the total-ammonia concentration on the thermophilic digestion of cow manure. Agric. Sci. 1985, 14, 19–35. [Google Scholar] [CrossRef]
- Gómez, X.; Cuetos, M.J.; García, A.I.; Morán, A. An evaluation of stability by thermogravimetric analysis of digestate obtained from different biowastes. J. Hazard. Mater. 2007, 149, 97–105. [Google Scholar] [CrossRef]
- Kirchmann, H.; Witter, E. Composition of fresh, aerobic and anaerobic farm animal dungs. Bioresour. Technol. 1992, 40, 137–142. [Google Scholar] [CrossRef]
- Makádi, M.; Tomócsik, A.; Kátai, J.; Eichler-Loebermann, B.; Schiemenz, K. Nutrient Cycling by Using Residues of Bioenergy Production—Effects of Biogas-Digestate on Plant and Soil Parameters. Cereal Res. Commun. 2008, 36, 1807–1810. Available online: https://www.jstor.org/stable/90003077 (accessed on 15 June 2022).
- Makádi, M.; Tomócsik, A.; Orosz, V. Digestate: A new nutrient source—Review. In Biogas; Kumar, S., Ed.; InTech: Rijeka, Croatia, 2012; pp. 259–310. [Google Scholar] [CrossRef]
- Möller, K.; Stinner, W.; Deuker, A.; Leithold, G. Effects of different manuring systems with and without biogas digestion on nitrogen cycle and crop yield in mixed organic dairy farming systems. Nutr. Cycl. Agroecosys. 2008, 82, 209–232. [Google Scholar] [CrossRef]
- Gulyás, M.; Tomócsik, A.; Orosz, V.; Makádi, M.; Füleky, G. Risk of agricultural use of sewage sludge compost and anaerobic digestate. Acta Phytopathol. Entomol. Hung. 2012, 47, 213–221. [Google Scholar] [CrossRef]
- Vágó, I.; Kátai, J.; Makádi, M.; Balla Kovács, A. Effects of biogas fermentation residues on the easily soluble macro- and microelement content of soil. In Trace Elements in the Food Chain. Deficiency or Excess of Trace Elements in the Environment as a Risk of Health; Szilágyi, M., Szentmihályi, K., Eds.; Working Committee on Trace Elements and Institute of Materials and Environmental Chemistry of the Hungarian Academy of Sciences: Budapest, Hungary, 2009; Volume 3, pp. 252–256. [Google Scholar]
- Odlare, M.; Pell, M.; Svensson, K. Changes in soil chemical and microbiological properties during 4 years of application of various organic residues. Waste Manag. 2008, 28, 1246–1253. [Google Scholar] [CrossRef]
- Fuchs, J.G.; Schleiss, K. Effects of compost and digestate on environment and plant production—Results of two research project. In Proceedings of the International Conference ORBIT 2008, Wageningen, The Netherlands, 13–16 October 2008; CD-ROM. ISBN 3-935974-19-1. [Google Scholar]
- Levine, R.B.; Costanza-Robinson, M.S.; Spatafora, G.A. Neochloris oleoabundans grown on anaerobically digested dairy manure for concomitant nutrient removal and biodiesel feedstock production. Biomass Bioenerg. 2011, 35, 40–49. [Google Scholar] [CrossRef]
- Mukherjee, S.; Weihermueller, L.; Tappe, W. Microbial respiration of biochar- and digestate-based mixtures. Biol. Fertil. Soils 2016, 52, 151–164. [Google Scholar] [CrossRef]
- Hammerschmiedt, T.; Holatko, J.; Sudoma, M.; Kintl, A.; Vopravil, J.; Ryant, P.; Skarpa, P.; Radziemska, M.; Latal, O.; Brtnicky, M. Biochar and sulphur enriched digestate: Utilization of agriculture associated waste products for improved soil carbon and nitrogen content, microbial activity, and plant growth. Agronomy 2021, 11, 2041. [Google Scholar] [CrossRef]
- Ronga, D.; Caradonia, F.; Parisi, M.; Bezzi, G.; Parisi, B.; Allesina, G.; Pedrazzi, S.; Francia, E. Using digestate and biochar as fertilizers to improve processing tomato production sustainability. Agronomy 2020, 10, 138. [Google Scholar] [CrossRef] [Green Version]
- Calamai, A.; Palchetti, E.; Masoni, A.; Marini, L.; Chiaramonti, D.; Dibari, C.; Brilli, L. The influence of biochar and solid digestate on rose-scented geranium (Pelargonium graveolens L’Hér.) productivity and essential oil quality. Agronomy 2019, 9, 260. [Google Scholar] [CrossRef] [Green Version]
- Karami, N.; Clemente, R.; Moreno-Jiménez, E.; Lepp, N.W.; Beesley, L. Efficiency of green waste compost and biochar soil amendments for reducing lead and copper mobility and uptake to ryegrass. J. Hazard. Mater. 2011, 191, 41–48. [Google Scholar] [CrossRef]
- Bidar, G.; Garçon, G.; Pruvot, C.; Cazier, F.; Douay, F.; Shirali, P. Behavior of Trifolium repens and Lolium perenne growing in a heavy metal contaminated field: Plant metal concentration and phytotoxicity. Environ. Pollut. 2007, 147, 546–553. [Google Scholar] [CrossRef]
- Bernard, J.M. Forest floor moisture capacity of the New Jersey Pine Barrens. Ecology 1963, 44, 574–576. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization of the United Nations). Standard Operating Procedure for Soil Organic Carbon. Tyurin Spectrophotometric Method; FAO: Rome, Italy, 2021; Available online: https://www.fao.org/publications/card/en/c/CB4757EN (accessed on 15 June 2022).
- Luo, Y.; Durenkamp, M.; De Nobili, M.; Lin, Q.; Brookes, P.C. Short term soil priming effects and the mineralisation of biochar following its incorporation to soils of different pH. Soil Biol. Biochem. 2011, 43, 2304–2314. [Google Scholar] [CrossRef]
- Rogoskova, N.; Laird, D.A.; Rathke, S.J.; Karlen, D.L. Biochar impact on Midwestern Mollisols and maize nutrient availability. Geoderma 2014, 230–231, 340–347. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science and Technology; Earthscan: London, UK, 2009; pp. 1–404. ISBN 978-1-84407-658-1. [Google Scholar]
| Parameter | WBC * | ABC * | Parameter | WBC * | ABC * |
|---|---|---|---|---|---|
| Bulk density (g cm−3) | 0.36 | 0.31 | Potassium (mg kg−1) | 4450 | 2000 |
| Dry matter (%) | 93.87 | 99.95 | Potassium (AL) (mg kg−1) | 1450 | 1500 |
| Ignition residue (ash) of dry matter (%) | 11.61 | 100 | Magnesium (mg kg−1) | 1200 | 6000 |
| Total carbon (%) | 79.8 | 9.9 | Manganese (mg kg−1) | 1140 | 1 |
| Total nitrogen (%) | 0.7 | 1.8 | Sodium (mg kg−1) | 170 | 7000 |
| C/N ratio (%) | 99.4 | 5.1 | Phosphorus (mg kg−1) | 780 | 133,000 |
| pH | 8.32 | 7.58 | Phosphorus (AL) (mg kg−1) | 214 | 24,600 |
| CEC (cmol kg−1) | 14.7 | n.d. ** | Zinc (mg kg−1) | 41 | 152 |
| Calcium (mg kg−1) | 30,200 | 300,000 | Sum of PAH (mg kg−1) *** | 4.82 | 0.37 |
| Chromium (mg kg−1) | 4 | 4 | Sum of PCB *** | - | - |
| Copper (mg kg−1) | 9 | 5 | Nitrite (KCl) (mg kg−1) | 0.4 | 0.6 |
| Iron (mg kg−1) | 2280 | 63 | Nitrate (KCl) (mg kg−1) | <10 | <10 |
| Parameter | SSD * | Parameter | SSD * |
|---|---|---|---|
| pH | 7.51 | Cr (VI) (mg kg−1) | 1.03 |
| Density (g cm−3) | 0.995 | Cu (mg kg−1) | 166.9 |
| Dry matter (m m−1 %) | 5.04 | Fe (mg kg−1) | 10,853 |
| Organic matter (m m−1 %) | 3.28 | Hg (mg kg−1) | 0.12 |
| Total N (mg kg−1) | 104,683 | Mg (mg kg−1) | 8948 |
| Total P (mg kg−1) | 28,175 | Mn (mg kg−1) | 291.7 |
| Total K (mg kg−1) | 13,036 | Mo (mg kg−1) | 4.62 |
| As (mg kg−1) | 3.43 | Na (mg kg−1) | 11,706 |
| Ca (mg kg−1) | 57,937 | Ni (mg kg−1) | 19.5 |
| Cd (mg kg−1) | 0.69 | Pb (mg kg−1) | 20.0 |
| Co (mg kg−1) | 8.23 | Se (mg kg−1) | 3.51 |
| Total Cr (mg kg−1) | 21.2 | Zn (mg kg−1) | 748.0 |
| Investigated Factor/Parameter | AL-P2O5 | AL-K2O | pH | Salt | Carbon | WHC |
|---|---|---|---|---|---|---|
| p Values * | ||||||
| Type:Rate:Digestate | 3.55 × 10−7 | 1.77 × 10−4 | 1.15 × 10−5 | 0.002 | 0.124 | 0.003 |
| Type:Rate | <2 × 10−16 | 0.001 | 2.10 × 10−10 | 1.27 × 10−11 | 0.001 | <2 × 10−16 |
| Biochar type | <2 × 10−16 | 2.33 × 10−7 | <2 × 10−16 | 1.15 × 10−6 | 6.25 × 10−5 | <2 × 10−16 |
| Biochar rate | 5.21 × 10−16 | 6.00 × 10−9 | 8.43 × 10−16 | 0.002 | 9.39 × 10−4 | <2 × 10−16 |
| Type:Rate (xAD) | 7.47 × 10−13 | 3.68 × 10−5 | 8.41 × 10−16 | 0.002 | 5.01 × 10−4 | <2 × 10−16 |
| Biochar type | 5.00 × 10−16 | 3.51 × 10−9 | <2 × 10−16 | 3.56 × 10−5 | 7.95 × 10−5 | <2 × 10−16 |
| Biochar rate | 8.03 × 10−13 | 7.72 × 10−11 | 3.76 × 10−15 | 0.002 | 5.40 × 10−4 | <2 × 10−16 |
| Type:Digestate (1%) | 0.017 | 0.091 | 4.77 × 10−8 | 0.901 | 0.355 | 8.15 × 10−4 |
| Biochar type | 9.43 × 10−8 | 0.004 | 2.07 × 10−7 | 2.81 × 10−4 | 0.601 | 0.071 |
| Digestate | 0.672 | 0.158 | 1.59 × 10−6 | 0.003 | 0.216 | 0.002 |
| Type:Digestate (2.5%) | 3.32 × 10−7 | 2.79 × 10−4 | 4.06 × 10−4 | 0.003 | 0.540 | 0.005 |
| Biochar type | 4.57 × 10−10 | 2.79 × 10−4 | 2.47 × 10−6 | 0.006 | 0.191 | 2.15 × 10−7 |
| Digestate | 4.94 × 10−7 | 0.257 | 1.83 × 10−4 | 0.026 | 0.716 | 0.332 |
| Type:Digestate (5%) | 6.05 × 10−5 | 9.19 × 10−4 | 0.006 | 0.011 | 0.237 | 0.823 |
| Biochar type | 8.55 × 10−10 | 0.003 | 1.36 × 10−9 | 3.30 × 10−5 | 7.26 × 10−7 | 3.11 × 10−8 |
| Digestate | 1.64 × 10−4 | 0.895 | 0.437 | 0.299 | 0.565 | 0.027 |
| Type:Digestate (10%) | 1.26 × 10−9 | 1.52 × 10−5 | 0.043 | 0.572 | 0.169 | 0.038 |
| Biochar type | 3.97 × 10−4 | 0.027 | 2.78 × 10−11 | 3.46 × 10−6 | 0.006 | 4.47 × 10−12 |
| Digestate | 2.67 × 10−4 | 0.023 | 0.036 | 0.059 | 0.267 | 0.745 |
| Rate:Digestate (WBC) | 8.02 × 10−5 | 0.002 | 3.76 × 10−7 | 0.180 | 0.188 | 0.021 |
| Biochar rate | 1.44 × 10−4 | 3.70 × 10−10 | 2.15 × 10−5 | 0.180 | 3.01 × 10−5 | <2 × 10−16 |
| Digestate | 5.04 × 10−13 | 0.008 | 2.36 × 10−9 | 2.19 × 10−4 | 0.476 | 0.098 |
| Rate:Digestate (ABC) | 4.12 × 10−5 | 1.01 × 10−7 | 0.030 | 1.96 × 10−7 | 0.900 | 0.005 |
| Biochar rate | 2.75 × 10−15 | 0.013 | <2 × 10−16 | 1.92 × 10−14 | 0.726 | 7.25 × 10−8 |
| Digestate | 2.06 × 10−9 | 0.037 | 5.83 × 10−4 | 0.243 | 0.729 | 0.412 |
| Investigated Factor/Parameter | Air-Dry Mass | P Uptake | K Uptake | Ca Uptake | Mg Uptake | Mn Uptake |
|---|---|---|---|---|---|---|
| p Value * | ||||||
| Type:Rate:Digestate | 5.94 × 10−5 | 0.299 | 2.09 × 10−6 | 0.005 | 0.011 | 0.006 |
| Type:Rate | 0.579 | 0.091 | 1.73 × 10−6 | 2.49 × 10−6 | 3.46 × 10−7 | 3.96 × 10−8 |
| Biochar type | 0.334 | 0.004 | 2.64 × 10−7 | 1.60 × 10−8 | 4.11 × 10−10 | 8.67 × 10−16 |
| Biochar rate | 1.11 × 10−4 | 0.460 | 0.002 | 1.65 × 10−8 | 2.56 × 10−8 | 4.87 × 10−5 |
| Type:Rate (xAD) | 1.22 × 10−4 | 0.020 | 1.51 × 10−5 | 4.05 × 10−5 | 0.203 | 6.27 × 10−8 |
| Biochar type | 2.38 × 10−4 | 1.74 × 10−8 | 1.24 × 10−4 | 6.04 × 10−8 | 1.01 × 10−14 | <2 × 10−16 |
| Biochar rate | 1.44 × 10−3 | 1.57 × 10−4 | 4.42 × 10−5 | 6.77 × 10−12 | 2.16 × 10−8 | 4.47 × 10−7 |
| Type:Digestate (1%) | 0.150 | 0.586 | 6.11 × 10−4 | 0.001 | 4.38 × 10−7 | 0.058 |
| Biochar type | 0.251 | 0.970 | 0.005 | 0.001 | 1.58 × 10−6 | 6.95 × 10−6 |
| Digestate | 4.55 × 10−5 | 8.19 × 10−6 | 0.096 | 6.78 × 10−8 | 7.54 × 10−9 | 0.002 |
| Type:Digestate (2.5%) | 6.95 × 10−3 | 0.173 | 0.493 | 0.261 | 3.47 × 10−4 | 0.093 |
| Biochar type | 0.058 | 0.418 | 0.086 | 0.016 | 3.55 × 10−7 | 2.27 × 10−10 |
| Digestate | 0.002 | 4.60 × 10−5 | 0.030 | 8.74 × 10−8 | 9.11 × 10−8 | 3.56 × 10−6 |
| Type:Digestate (5%) | 0.694 | 0.021 | 1.60 × 10−4 | 0.024 | 6.90 × 10−4 | 0.015 |
| Biochar type | 0.694 | 0.002 | 6.88 × 10−5 | 2.31 × 10−4 | 4.96 x10−6 | 8.48 × 10−9 |
| Digestate | 5.45 × 10−5 | 3.12 × 10−6 | 0.177 | 1.46 × 10−6 | 1.19 × 10−5 | 2.81 × 10−5 |
| Type:Digestate (10%) | 0.014 | 0.992 | 0.007 | 0.245 | 5.30 × 10−5 | 0.289 |
| Biochar type | 0.058 | 0.086 | 4.22 × 10−4 | 9.76 × 10−4 | 3.63 × 10−7 | 2.74 × 10−9 |
| Digestate | 0.014 | 0.002 | 0.024 | 0.339 | 0.028 | 5.01 × 10−4 |
| Rate:Digestate (WBC) | 0.001 | 0.823 | 0.048 | 4.92 × 10−8 | 8.70 × 10−7 | 6.98 × 10−7 |
| Biochar rate | 0.001 | 0.157 | 0.290 | 6.41 × 10−8 | 9.03 × 10−8 | 2.12 × 10−10 |
| Digestate | 1.95 × 10−8 | 1.17 × 10−6 | 0.207 | 6.23 × 10−10 | 2.62 × 10−6 | 3.57 × 10−6 |
| Rate:Digestate (ABC) | 0.009 | 9.94 × 10−4 | 1.54 × 10−7 | 6.24 × 10−5 | 5.12 × 10−7 | 1.72 × 10−6 |
| Biochar rate | 3.74 × 10−4 | 2.32 × 10−4 | 6.08 × 10−10 | 9.17 × 10−11 | 0.007 | 9.16 × 10−7 |
| Digestate | 3.72 × 10−6 | 1.03 × 10−11 | 0.018 | 8.27 × 10−9 | 3.98 × 10−15 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulyás, M.; Someus, E.; Klátyik, S.; Fuchs, M.; Varga, Z.I.; Dér, S.; Fekete, G.; Czinkota, I.; Székács, A.; Gyuricza, C.; et al. Effects of Combined Application of Solid Pyrolysis Products and Digestate on Selected Soil Properties of Arenosol and Plant Growth and Composition in Laboratory Experiments. Agronomy 2022, 12, 1440. https://doi.org/10.3390/agronomy12061440
Gulyás M, Someus E, Klátyik S, Fuchs M, Varga ZI, Dér S, Fekete G, Czinkota I, Székács A, Gyuricza C, et al. Effects of Combined Application of Solid Pyrolysis Products and Digestate on Selected Soil Properties of Arenosol and Plant Growth and Composition in Laboratory Experiments. Agronomy. 2022; 12(6):1440. https://doi.org/10.3390/agronomy12061440
Chicago/Turabian StyleGulyás, Miklós, Edward Someus, Szandra Klátyik, Márta Fuchs, Zsolt István Varga, Sándor Dér, György Fekete, Imre Czinkota, András Székács, Csaba Gyuricza, and et al. 2022. "Effects of Combined Application of Solid Pyrolysis Products and Digestate on Selected Soil Properties of Arenosol and Plant Growth and Composition in Laboratory Experiments" Agronomy 12, no. 6: 1440. https://doi.org/10.3390/agronomy12061440
APA StyleGulyás, M., Someus, E., Klátyik, S., Fuchs, M., Varga, Z. I., Dér, S., Fekete, G., Czinkota, I., Székács, A., Gyuricza, C., & Aleksza, L. (2022). Effects of Combined Application of Solid Pyrolysis Products and Digestate on Selected Soil Properties of Arenosol and Plant Growth and Composition in Laboratory Experiments. Agronomy, 12(6), 1440. https://doi.org/10.3390/agronomy12061440








