Impacts of Protein from High-Protein Rice on Gelatinization and Retrogradation Properties in High- and Low-Amylose Reconstituted Rice Flour
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Rice Protein and Starch
2.3. Preparation of High-Amylose and Low-Amylose Reconstituted Rice Flour (RRF) with Different Protein Fractions
2.4. Determination of RVA Spectrum of Reconstituted Rice Flour
2.5. Differential Scanning Calorimetry (DSC)
2.6. Determination of Gel Hardness of Reconstituted Rice Gel
2.7. Scanning Electron Microscopy (SEM)
2.8. Statistical Analysis
3. Results
3.1. RVA Parameters of the Reconstituted Rice Flour (RRF)
3.2. Thermal Properties of Reconstituted Rice Flour
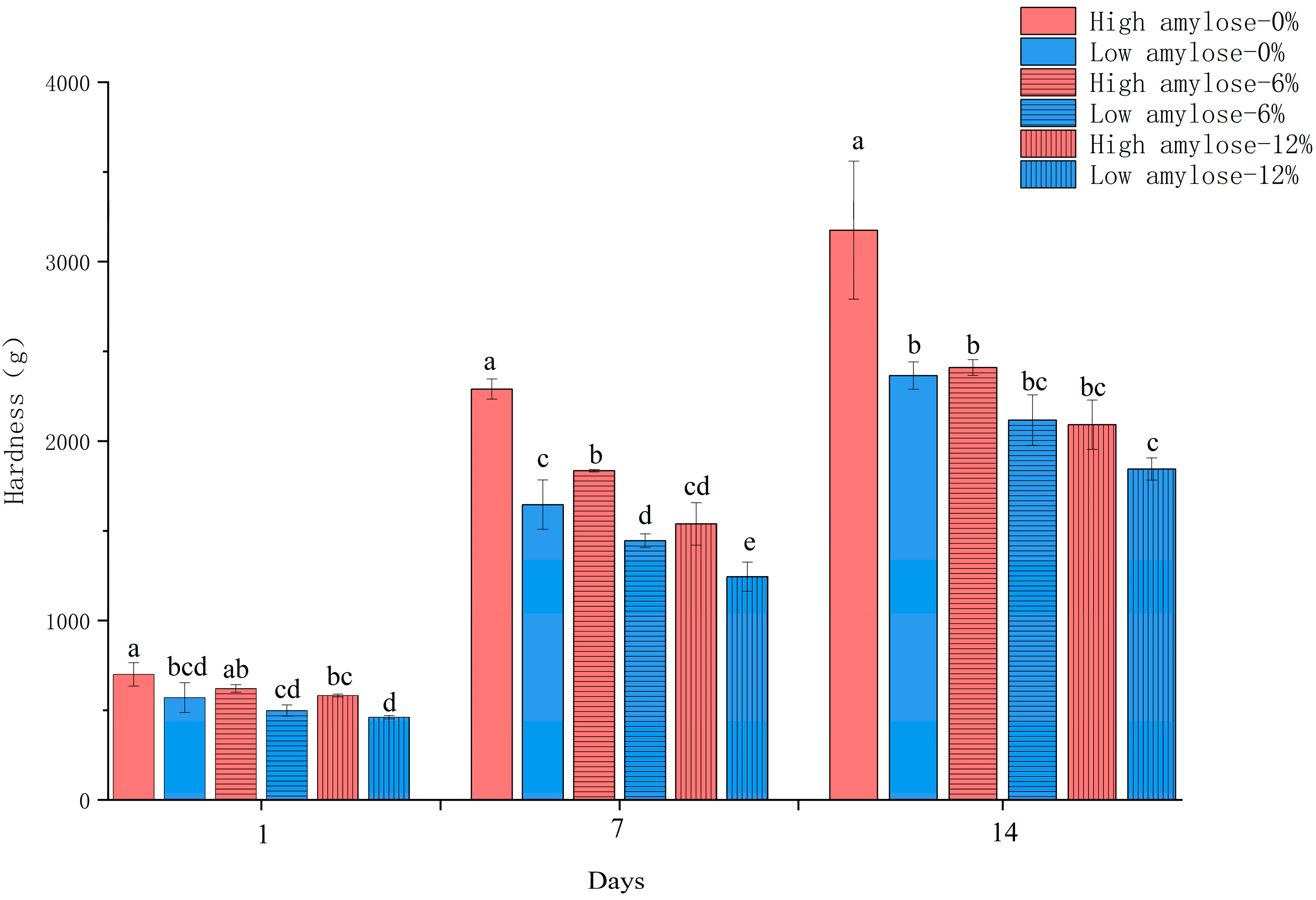
3.3. Texture of Reconstituted Rice Flour Gel
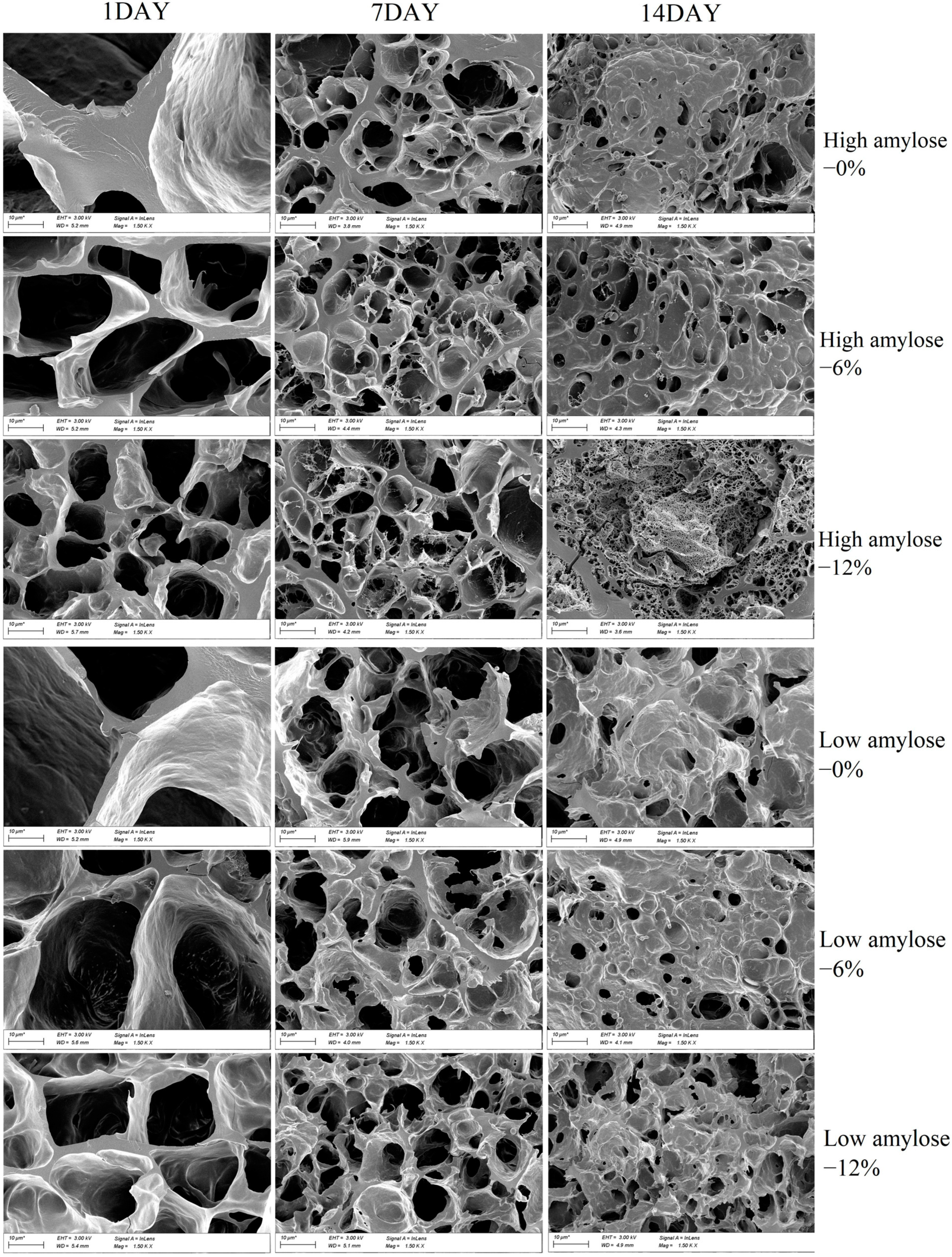
3.4. Scanning Electron Microscope (SEM)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Description |
| AC | Amylose Content |
| BD | Breakdown |
| DSC | Differential scanning calorimetry |
| FV | Final Viscosity |
| PaT | Pasting Temperature |
| PV | Peak Viscosity |
| RRF | Reconstituted Rice Flour |
| RVA | Rapid Viscosity Analyzer |
| SB | Set Back |
| SEM | Scanning electron microscope |
| ZGA | Zhenguiai |
References
- Rosell, C.M.; Barro, F.; Sousa, C.; Mena, M.C. Cereals for developing gluten-free products and analytical tools for gluten detection. J. Cereal Sci. 2014, 59, 354–364. [Google Scholar] [CrossRef] [Green Version]
- Birla, D.S.; Malik, K.; Sainger, M.; Chaudhary, D.; Jaiwal, R.; Jaiwal, P.K. Progress and challenges in improving the nutritional quality of rice (Oryza sativa L.). Crit. Rev. Food Sci. Nutr. 2017, 57, 2455–2481. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, H.; Guo, B.; Xu, K.; Dai, Q.; Wei, H.; Gao, H.; Hu, Y.; Cui, P.; Hou, Z. Effects of nitrogen level on yield and quality of Japonica soft super rice. J. Integr. Agric. 2017, 16, 1018–1027. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, M.; Sun, S.; Zou, Y.; Yin, S.; Liu, Y.; Tang, S.; Gu, M.; Yang, Z.; Yan, C. Natural variation of OsGluA2 is involved in grain protein content regulation in rice. Nat. Commun. 2019, 10, 1949. [Google Scholar] [CrossRef]
- Xuan, Y.; Yi, Y.; Liang, H.; Wei, S.; Chen, N.; Jiang, L.; Ali, I.; Ullah, S.; Wu, X.; Cao, T.; et al. Amylose content and RVA profile characteristics of noodle rice under different conditions. Agron. J. 2020, 112, 117–129. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Wang, H.; Ai, L.; Xiong, W. Insight into protein-starch ratio on the gelatinization and retrogradation characteristics of reconstituted rice flour. Int. J. Biol. Macromol. 2020, 146, 524–529. [Google Scholar] [CrossRef]
- Martin, M.; Fitzgerald, M.A. Proteins in Rice Grains Influence Cooking Properties. J. Cereal Sci. 2002, 36, 285–294. [Google Scholar] [CrossRef]
- Baxter, G.; Blanchard, C.; Zhao, J. Effects of glutelin and globulin on the physicochemical properties of rice starch and flour. J. Cereal Sci. 2014, 60, 414–420. [Google Scholar] [CrossRef]
- Jeong, J.M.; Jeung, J.U.; Lee, B.S. Physicochemical Properties of Rice Endosperm with Different Amylose Contents. Korea J. Crop. Sci. 2013, 3, 274–283. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Hamaker, B.R. Amylopectin Fine Structure and Rice Starch Paste Breakdown. J. Cereal Sci. 2001, 34, 279–284. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch Retrogradation: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- Yu, S.; Ma, Y.; Sun, D. Impact of on starch retrogradation and texture of cooked milled rice during storage. J. Cereal Sci. 2009, 50, 139–144. [Google Scholar] [CrossRef]
- AACC. Approved Methods of the American Association of Cereal Chemists 61–02; AACC: Tusmore, Australia, 1995. [Google Scholar]
- Chen, L.; Tian, Y.; Tong, Q.; Zhang, Z.; Jin, Z. Effect of pullulan on the water distribution, microstructure and textural properties of rice starch gels during cold storage. Food Chem. 2017, 214, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Chen, J.; Zhao, J.; Chen, L.; Wang, Y. Effect of the addition of modified starch on gelatinization and gelation properties of rice flour. Int. J. Biol. Macromol. 2020, 153, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Robards, K.; Helliwell, S.; Blanchard, C. Composition and functional properties of rice. Int. J. Food Sci. Technol. 2002, 37, 849–868. [Google Scholar] [CrossRef]
- Han, X.; Hamaker, B.R. Functional and Microstructural Aspects of Soluble Corn Starch in Pastes and Gels. Starch Stärke 2000, 52, 76–80. [Google Scholar] [CrossRef]
- Cheetham, N.W.H.; Tao, L. The effects of amylose content on the molecular size of amylose, and on the distribution of amylopectin chain length in maize starches. Carbohydr. Polym. 1997, 33, 251–261. [Google Scholar] [CrossRef]
- Lu, S.; Chen, L.N.; Lii, C.Y. Correlations between the fine structure, physicochemical properties, and retrogradation of amylopectins from Taiwan rice varieties. Cereal Chem. 1997, 74, 34–39. [Google Scholar] [CrossRef]
- Charles, L.A.; Chang, H.Y.; Ko, C.W. Influence of Amylopectin Structure and Amylose Content on the Gelling Properties of Five Cultivars of Cassava Starches. J. Agr. Food Chem. 2005, 7, 2717–2725. [Google Scholar] [CrossRef]
- Vamadevan, V.; Bertoft, E. Observations on the impact of amylopectin and amylose structure on the swelling of starch granules. Food Hydrocoll. 2020, 103, 105663. [Google Scholar] [CrossRef]
- Kumar, L.; Brennan, M.A.; Mason, S.L.; Zheng, H.; Brennan, C.S. Rheological, pasting and microstructural studies of dairy protein-starch interactions and their application in extrusion-based products: A review. Starch Stärke 2017, 69, 1600273. [Google Scholar] [CrossRef]
- Jeong, H.; Lim, S. Crystallinity and Pasting Properties of Freeze-Thawed High Amylose Maize Starch. Starch Stärke 2003, 55, 511–517. [Google Scholar] [CrossRef]
- Hamaker, B.; Griffin, V. Effect of disulfide bond-containing protein on rice starch gelatinization and pasting. Cereal Chem. 1993, 70, 377–380. [Google Scholar]
- Zhang, Y.; Chen, C.; Chen, Y.; Chen, Y. Effect of rice protein on the water mobility, water migration and microstructure of rice starch during retrogradation. Food Hydrocoll. 2019, 91, 136–142. [Google Scholar] [CrossRef]
- Saleh, M.I. Protein-starch matrix microstructure during rice flour pastes formation. J. Cereal Sci. 2017, 74, 183–186. [Google Scholar] [CrossRef]
- Singh, N.; Kaur, L.; Sandhu, K.S.; Kaur, J.; Nishinari, K. Relationships between physicochemical, morphological, thermal, rheological properties of rice starches. Food Hydrocoll. 2006, 20, 532–542. [Google Scholar] [CrossRef]
- Noda, T.; Nishiba, Y.; Sato, T.; Suda, I. Properties of Starches from Several Low-Amylose Rice Cultivars. Cereal Chem. J. 2003, 80, 193–197. [Google Scholar] [CrossRef]
- Xiang, X.; Kang, C.; Xu, S.; Yang, B. Combined effects of Wx and SSIIa haplotypes on rice starch physicochemical properties. J. Sci. Food Agric. 2017, 97, 1229–1234. [Google Scholar] [CrossRef]
- Xijun, L.; Junjie, G.; Danli, W.; Lin, L.; Jiaran, Z. Effects of Protein in Wheat Flour on Retrogradation of Wheat Starch. J. Food Sci. 2014, 79, 1505–1511. [Google Scholar] [CrossRef]
- Niu, L.; Wu, L.; Xiao, J. Inhibition of gelatinized rice starch retrogradation by rice bran protein hydrolysates. Carbohydr. Polym. 2017, 175, 311–319. [Google Scholar] [CrossRef]
- Varavinit, S.; Shobsngob, S.; Varanyanond, W.; Chinachoti, P.; Naivikul, O. Effect of Amylose Content on Gelatinization, Retrogradation and Pasting Properties of Flours from Different Cultivars of Thai Rice. Starch Stärke 2003, 55, 410–415. [Google Scholar] [CrossRef]
- Min, Z.; Chao, S.; Xiaorui, W.; Naifu, W.; Yibin, Z. Effect of rice protein hydrolysates on the short-term and long-term retrogradation of wheat starch. Int. J. Biol. Macromol. 2019, 155, 1169–1175. [Google Scholar]
- Cameron, D.K.; Wang, Y. A Better Understanding of Factors That Affect the Hardness and Stickiness of Long-Grain Rice. Cereal Chem. J. 2005, 82, 113–119. [Google Scholar] [CrossRef]
- Chen, H.; Chen, D.; He, L.; Wang, T.; Lu, H.; Yang, F.; Deng, F.; Chen, Y.; Tao, Y.; Li, M.; et al. Correlation of taste values with chemical compositions and Rapid Visco Analyser profiles of 36 indica rice (Oryza sativa L.) varieties. Food Chem. 2021, 349, 129–176. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Mao, B.; Zhang, C.; Shao, Y.; Wu, T.; Hu, L.; Hu, Y.; Tang, L.; Li, Y.; Tang, W.; et al. Influence of physicochemical properties and starch fine structure on the eating quality of hybrid rice with similar apparent amylose content. Food Chem. 2021, 353, 129461. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xue, J.; Pan, G.; Wang, Q. Protein and its composition content of grains in different position and its correlation with quality traits of Japonica rice. J. Chinese Cereal Oil Ass. 2015, 30, 1–6. [Google Scholar]
- Prakash, M.; Ravi, R.; Sathish, H.S.; Shyamala, J.C.; Shwetha, M.A.; Rangrao, G.C.P. Sensory and instrumental texture measurement of thermally processed rice. J. Sens. Stud. 2005, 20, 410–420. [Google Scholar] [CrossRef]
| RRF | Protein Fraction | PV (cp) | BD (cp) | FV (cp) | SB (cp) | PeakTime (min) | PaT (°C) |
|---|---|---|---|---|---|---|---|
| High amylose | 0% | 1945 ± 46.4 Aa | 543.0 ± 19.7 Aa | 3266.5 ± 67.9 Aa | 1864.2 ± 13.8 Aa | 6.00 ± 0.04 Aa | 78.3 ± 0.4 Aa |
| High amylose | 6% | 1668 ± 115.8 Ab | 344.9 ± 46.4 Ab | 2930.3 ± 247.4 Aa | 1607.1 ± 180.4 Aa | 6.05 ± 0.22 Aa | 79.4 ± 1.3 Aa |
| High amylose | 12% | 1247 ± 126.6 Ac | 315.3 ± 64.5 Ab | 1946.1 ± 187.6 Ab | 1014.5 ± 129.0 Ab | 6.27 ± 0.13 Aa | 81.8 ± 2.1 Aa |
| Low amylose | 0% | 1581 ± 90.1 Ba | 650.6 ± 50.9 Aa | 1823.7 ± 52.5 Ba | 893.4 ± 82.1 Ba | 5.38 ± 0.00 Bb | 80.0 ± 0.5 Aa |
| Low amylose | 6% | 1281 ± 142.8 Bb | 402.2 ± 87.8 Ab | 1514 ± 203.5 Bb | 635.6 ± 149 Bb | 5.58 ± 0.09 Aa | 79.9 ± 0.5 Aa |
| Low amylose | 12% | 1135 ± 78.8 Ac | 334.6 ± 69.1 Ab | 1397.5 ± 99.6 Ab | 596.9 ± 86.2 Bb | 5.63 ± 0.10 Ba | 79.5 ± 0.5 Aa |
| Significance (F-value) | |||||||
| RRF | * | ns | * | * | * | ns | |
| Protein fraction | * | * | * | * | * | ns | |
| RRF × Protein fraction | ns | ns | * | * | ns | * | |
| RRF | Protein Fraction | To (Onset, °C) | Tp (Peak, °C) | Tc (Conclusion, °C) | ΔH(J/g) |
|---|---|---|---|---|---|
| High amylose | 0% | 66.50 ± 1.14 Ab | 73.43 ± 0.71 Ab | 80.01 ± 0.59 Ab | 10.66 ± 0.68 Aa |
| High amylose | 6% | 67.86 ± 0.81 Ab | 74.37 ± 1.04 Ab | 80.96 ± 0.63 Ab | 8.82 ± 1.17 Ab |
| High amylose | 12% | 69.21 ± 0.96 Aa | 76.43 ± 1.59 Aa | 82.56 ± 0.41 Aa | 6.80 ± 1.55 Ac |
| Low amylose | 0% | 65.15 ± 0.95 Bc | 71.15 ± 0.85 Bc | 79.17 ± 0.75 Ab | 8.87 ± 1.09 Ba |
| Low amylose | 6% | 66.71 ± 1.08 Bb | 73.28 ± 0.78 Bb | 80.64 ± 0.91 Ab | 7.21 ± 1.09 Bb |
| Low amylose | 12% | 68.04 ± 0.71 Ba | 74.06 ± 1.06 Ba | 82.00 ± 0.66 Aa | 5.97 ± 1.38 Bc |
| Significance (F-value) | |||||
| RRF | * | * | ns | * | |
| Protein fraction | * | * | * | * | |
| RRF × Protein fraction | ns | ns | ns | ns | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Dai, X.; Mackon, E.; Ma, Y.; Liu, P. Impacts of Protein from High-Protein Rice on Gelatinization and Retrogradation Properties in High- and Low-Amylose Reconstituted Rice Flour. Agronomy 2022, 12, 1431. https://doi.org/10.3390/agronomy12061431
Zhao Y, Dai X, Mackon E, Ma Y, Liu P. Impacts of Protein from High-Protein Rice on Gelatinization and Retrogradation Properties in High- and Low-Amylose Reconstituted Rice Flour. Agronomy. 2022; 12(6):1431. https://doi.org/10.3390/agronomy12061431
Chicago/Turabian StyleZhao, Yitong, Xianggui Dai, Enerand Mackon, Yafei Ma, and Piqing Liu. 2022. "Impacts of Protein from High-Protein Rice on Gelatinization and Retrogradation Properties in High- and Low-Amylose Reconstituted Rice Flour" Agronomy 12, no. 6: 1431. https://doi.org/10.3390/agronomy12061431
APA StyleZhao, Y., Dai, X., Mackon, E., Ma, Y., & Liu, P. (2022). Impacts of Protein from High-Protein Rice on Gelatinization and Retrogradation Properties in High- and Low-Amylose Reconstituted Rice Flour. Agronomy, 12(6), 1431. https://doi.org/10.3390/agronomy12061431






