A New Strategy to Improve Vineyard Resilience: Grapevine Morphological Adaptation to Short-Term Nitrogen Deficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Plan
2.2. Hydroponic Growing Conditions
2.3. Analytical Methods and Data Elaborations
3. Results
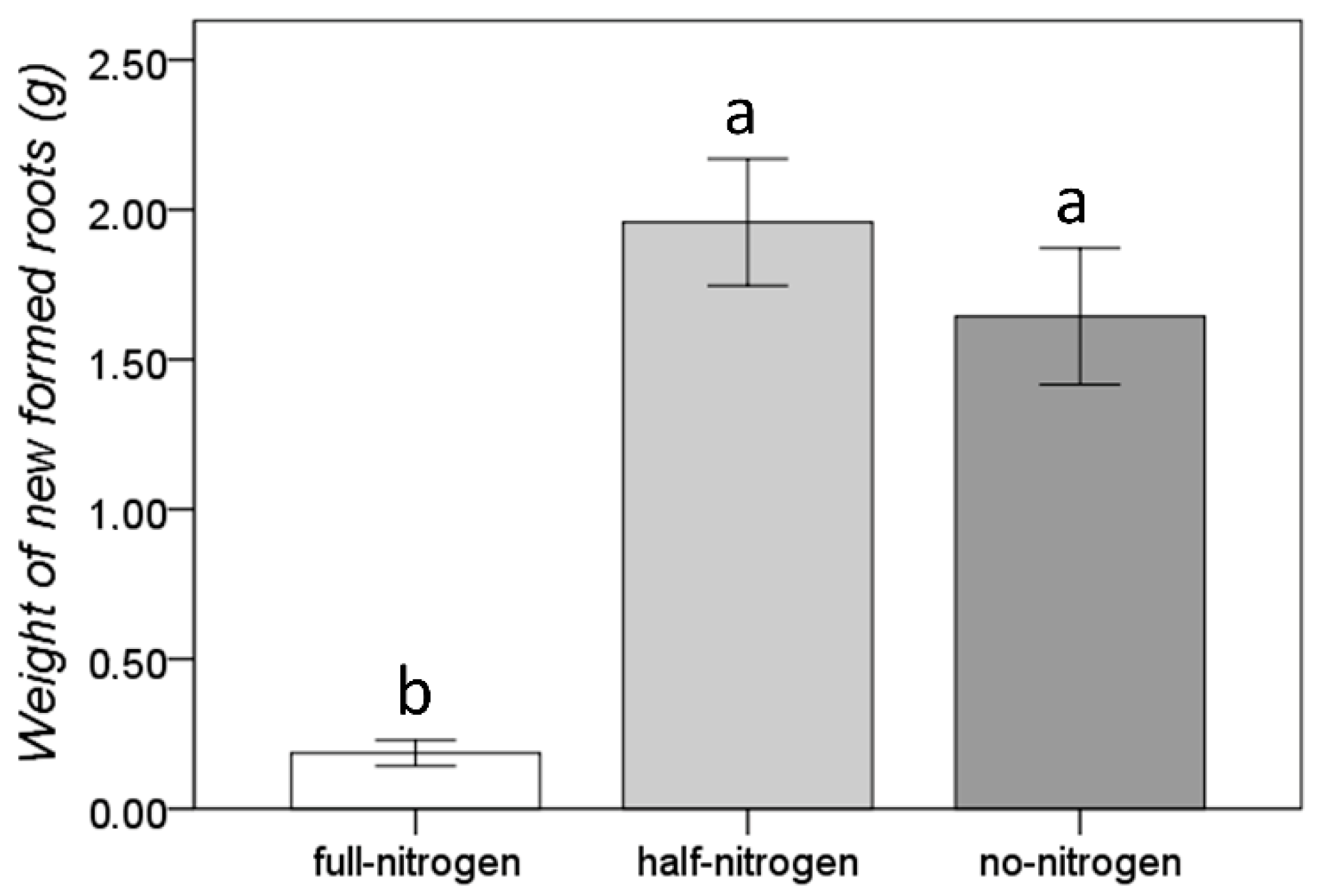
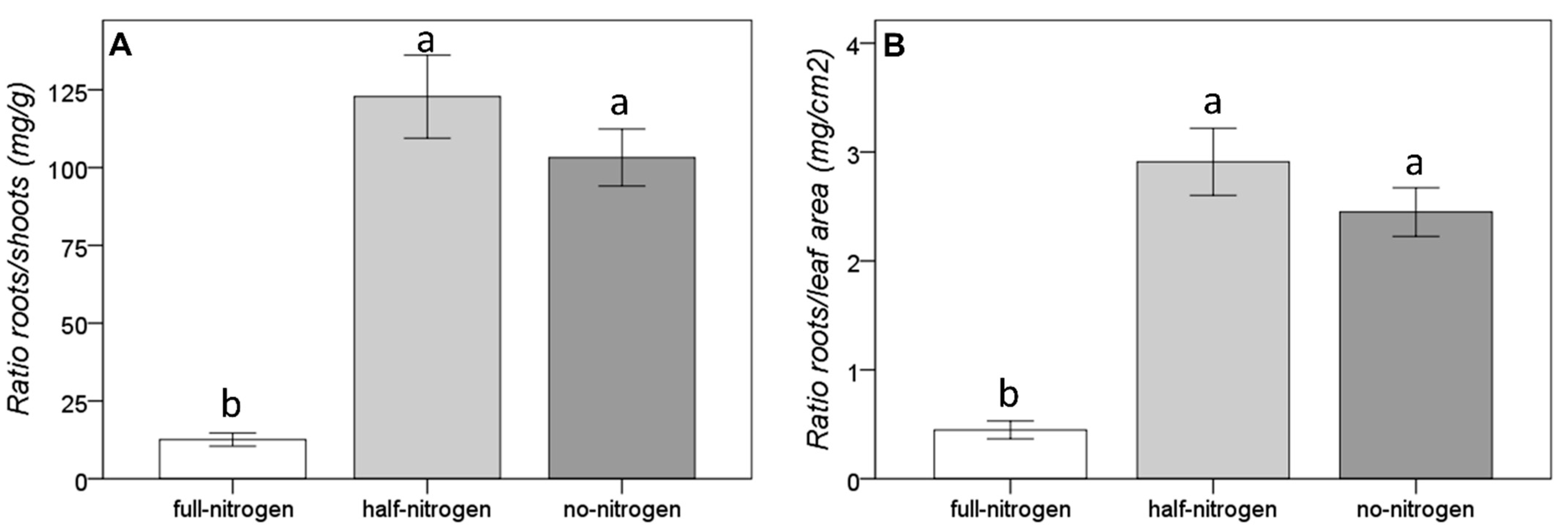
3.1. Morphological Adaptation to the Short-Term Nitrogen Deficiency
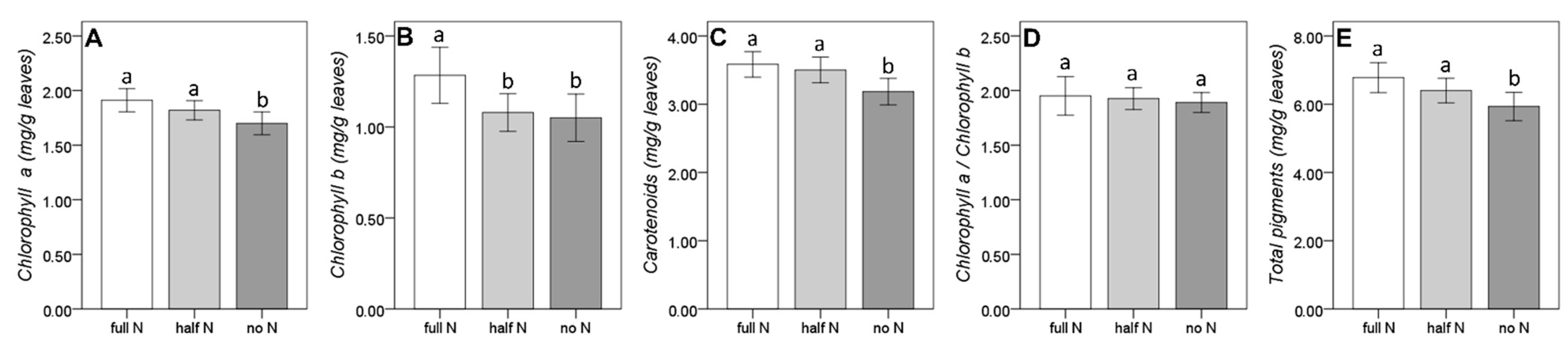
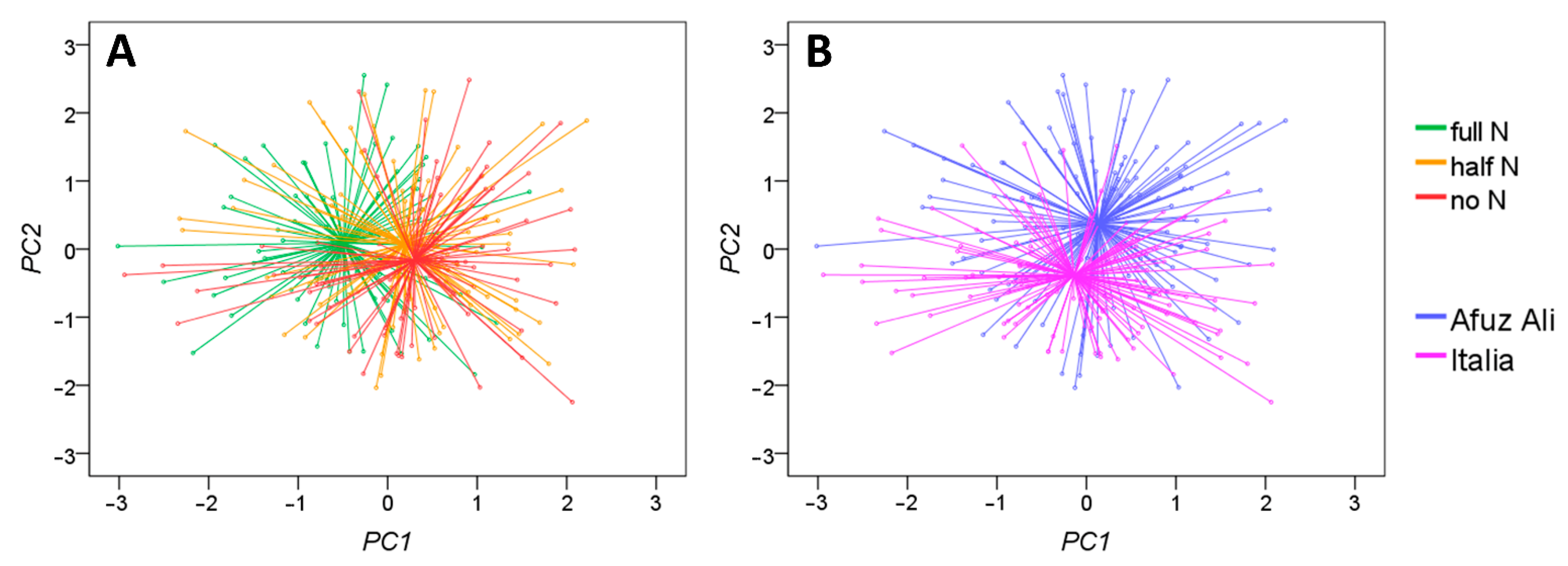
3.2. Shoot Morphology Indexes of Incipient Nitrogen Deficiency
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rustioni, L.; Cola, G.; Maghradze, D.; Abashidze, E.; Argiriou, A.; Aroutiounian, R.; Brazão, J.; Chipashvili, R.; Cocco, M.; Cornea, V.; et al. Description of the Vitis vinifera L. phenotypic variability in eno-carpological traits by a Euro-Asiatic collaborative network among ampelographic collections. Vitis 2019, 58, 37–46. [Google Scholar]
- Lovisolo, C.; Perrone, I.; Carra, A.; Ferrandino, A.; Flexas, J.; Medrano, H.; Schubert, A. Drought-induced changes in development and function of grapevine (Vitis spp.) organs and in their hydraulic and non-hydraulic interactions at the whole-plant level: A physiological and molecular update. Funct. Plant Biol. 2010, 37, 98–116. [Google Scholar] [CrossRef]
- Bisson, L.F.; Waterhouse, A.L.; Ebeler, S.E.; Walker, M.A.; Lapsley, J.T. The present and future of the international wine industry. Nature 2002, 418, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Cifre, J.; Bota, J.; Escalona, J.; Medrano, H.; Flexas, J. Physiological tools for irrigation scheduling in grapevine (Vitis vinifera L.): An open gate to improve water-use efficiency? Agric. Ecosyst. Environ. 2005, 106, 159–170. [Google Scholar] [CrossRef]
- Chalmers, Y.; Kelly, G.; Krstic, M. Partial Rootzone Drying of Vitis Vinifera cv. ‘shiraz’ Winegrapes in a Semi-Arid Climate. Acta Hortic. 2004, 664, 133–138. [Google Scholar] [CrossRef]
- Gambetta, G.; Manuck, C.M.; Drucker, S.T.; Shaghasi, T.; Fort, K.; Matthews, M.A.; Walker, M.A.; McElrone, A.J. The relationship between root hydraulics and scion vigour across Vitis rootstocks: What role do root aquaporins play? J. Exp. Bot. 2012, 63, 6445–6455. [Google Scholar] [CrossRef]
- de Orduña, R.M. Climate change associated effects on grape and wine quality and production. Food Res. Int. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Padgett-Johnson, M.; Williams, L.E.; Walker, M.A. Vine water relations, gas exchange, and vegetative growth of seventeen Vitis species grown under irrigated and nonirrigated con conditions in California. J. Am. Soc. Hortic. Sci. 2003, 128, 269–276. [Google Scholar] [CrossRef]
- Rustioni, L.; Ciacciulli, A.; Grossi, D.; Brancadoro, L.; Failla, O. Stem Xylem Characterization for Vitis Drought Tolerance. J. Agric. Food Chem. 2016, 64, 5317–5323. [Google Scholar] [CrossRef]
- Bianchi, D.; Grossi, D.; Tincani, D.T.; Di Lorenzo, G.S.; Brancadoro, L.; Rustioni, L. Multi-parameter characterization of water stress tolerance in Vitis hybrids for new rootstock selection. Plant Physiol. Biochem. 2018, 132, 333–340. [Google Scholar] [CrossRef]
- Bianchi, D.; Grossi, D.; Di Lorenzo, G.S.; Ying, Y.Z.; Rustioni, L.; Brancadoro, L. Phenotyping of the “G series” Vitis hybrids: First screening of the mineral composition. Sci. Hortic. 2020, 264, 109155. [Google Scholar] [CrossRef]
- Ortega-Farias, S.; Fereres, E.; Sadras, V.O. Special issue on water management in grapevines. Irrig. Sci. 2012, 30, 335–337. [Google Scholar] [CrossRef][Green Version]
- Gómez-Del-Campo, M.; Ruiz, C.; Baeza, P.; Lissarrague, J.R. Drought adaptation strategies of four grapevine cultivars (Vitis vinifera L.): Modification of the properties of the leaf area. OENO One 2003, 37, 131–143. [Google Scholar] [CrossRef]
- Dinu, D.G.; Bianchi, D.; Mamasakhlisashvili, L.; Quarta, C.; Brancadoro, L.; Maghradze, D.; Cardinale, M.; Rustioni, L. Effects of genotype and environmental conditions on grapevine (Vitis vinifera L.) shoot morphology. Vitis 2021, 60, 85–91. [Google Scholar] [CrossRef]
- Green, S.R.; Kirkham, M.; Clothier, B.E. Root uptake and transpiration: From measurements and models to sustainable irrigation. Agric. Water Manag. 2006, 86, 165–176. [Google Scholar] [CrossRef]
- Gambetta, G.A. Water Stress and Grape Physiology in the Context of Global Climate Change. J. Wine Econ. 2016, 11, 168–180. [Google Scholar] [CrossRef]
- Jackson, R.B.; Sperry, J.S.; Dawson, T.E. Root water uptake and transport: Using physiological processes in global predictions. Trends Plant Sci. 2000, 5, 482–488. [Google Scholar] [CrossRef]
- Canadell, J.; Jackson, R.B.; Ehleringer, J.B.; Mooney, H.A.; Sala, O.; Schulze, E.-D. Maximum rooting depth of vegetation types at the global scale. Oecologia 1996, 108, 583–595. [Google Scholar] [CrossRef]
- Archer, E.; Strauss, H. The Effect of Vine Spacing on Some Physiological Aspects of Vitis vinifera L. (cv. Pinot noir). South Afr. J. Enol. Vitic. 2017, 11, 76–87. [Google Scholar] [CrossRef][Green Version]
- Sun, X.; Chen, F.; Yuan, L.; Mi, G. The physiological mechanism underlying root elongation in response to nitrogen deficiency in crop plants. Planta 2020, 251, 84. [Google Scholar] [CrossRef]
- Mariani, L.; Ferrante, A. Agronomic Management for Enhancing Plant Tolerance to Abiotic Stresses—Drought, Salinity, Hypoxia, and Lodging. Horticulturae 2017, 3, 52. [Google Scholar] [CrossRef]
- Koevoets, I.T.; Venema, J.H.; Elzenga, J.T.M.; Testerink, C. Roots Withstanding their Environment: Exploiting Root System Architecture Responses to Abiotic Stress to Improve Crop Tolerance. Front. Plant Sci. 2016, 7, 1335. [Google Scholar] [CrossRef] [PubMed]
- Mickelbart, M.V.; Hasegawa, P.M.; Bailey-Serres, J. Genetic mechanisms of abiotic stress tolerance that translate to crop yield stability. Nat. Rev. Genet. 2015, 16, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Mi, G.; Chen, F.; Wu, Q.; Lai, N.; Yuan, L.; Zhang, F. Ideotype root architecture for efficient nitrogen acquisition by maize in intensive cropping systems. Sci. China Life Sci. 2010, 53, 1369–1373. [Google Scholar] [CrossRef]
- Köse, B.; Karabulut, B.; Ceylan, K. Effect of Rootstock on Grafted Grapevine Quality Europ. J. Hort. Sci. 2014, 79, 197. [Google Scholar]
- Corso, M.; Bonghi, C. Grapevine rootstock effects on abiotic stress tolerance. Plant Sci. Today 2014, 1, 108–113. [Google Scholar] [CrossRef]
- Bauerle, T.L.; Smart, D.R.; Bauerle, W.L.; Stockert, C.; Eissenstat, D.M. Root foraging in response to heterogeneous soil moisture in two grapevines that differ in potential growth rate. New Phytol. 2008, 179, 857–866. [Google Scholar] [CrossRef]
- Alsina, M.M.; Smart, D.R.; Bauerle, T.; De Herralde, F.; Biel, C.; Stockert, C.; Negron, C.; Save, R. Seasonal changes of whole root system conductance by a drought-tolerant grape root system. J. Exp. Bot. 2010, 62, 99–109. [Google Scholar] [CrossRef]
- Franco, J.A.; Bañón, S.; Vicente, M.J.; Miralles, J.; Martínez-Sánchez, J.J. Review Article: Root development in horticultural plants grown under abiotic stress conditions–A review. J. Hortic. Sci. Biotechnol. 2011, 86, 543–556. [Google Scholar] [CrossRef]
- Wells, C.E.; Eissenstat, D.M. Beyond the Roots of Young Seedlings: The Influence of Age and Order on Fine Root Physiology. J. Plant Growth Regul. 2002, 21, 324–334. [Google Scholar] [CrossRef]
- Maurel, C.; Simonneau, T.; Sutka, M. The significance of roots as hydraulic rheostats. J. Exp. Bot. 2010, 61, 3191–3198. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.-J.; Yang, H.-Q.; Wang, J.-X. Arginine metabolism in roots and leaves of apple (Malus domestica Borkh.): The tissue-specific formation of both nitric oxide and polyamines. Sci. Hortic. 2009, 119, 147–152. [Google Scholar] [CrossRef]
- Vilanova, M.; Fandiño, M.; Frutos-Puerto, S.; Cancela, J.J. Assessment fertigation effects on chemical composition of Vitis vinifera L. cv. Albariño. Food Chem. 2018, 278, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.P.; Merkt, N.; Zörb, C. Different nitrogen (N) forms affect responses to N form and N supply of rootstocks and grafted grapevines. Plant Sci. 2018, 277, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Álvarez, E.P.; Martínez-Vidaurre, J.M.; Martín, I.; García-Escudero, E.; Peregrina, F. Relationships among Soil Nitrate Nitrogen and Nitrogen Nutritional Status, Yield Components, and Must Quality in Semi-arid Vineyards from Rioja AOC, Spain. Commun. Soil Sci. Plant Anal. 2013, 44, 232–242. [Google Scholar] [CrossRef]
- Akin, A.; Dardeniz, A.; Ates, F.; Celik, M. Effects of various crop loads and leaf fertilizer on grapevine yield and quality. J. Plant Nutr. 2012, 35, 1949–1957. [Google Scholar] [CrossRef]
- Coipel, J.; Lovelle, B.R.; Sipp, C.; Van Leeuwen, C. “Terroir” effect, as a result of enviromental stess, depends more on soil depth than on soil type (Vitis vinifera L. cv. Grenache Noir, Côtes du Rhône, France, 2000). OENO One 2006, 40, 177–185. [Google Scholar] [CrossRef]
- Delgado, J.A.; Kowalski, K.; Tebbe, C. The first Nitrogen Index app for mobile devices: Using portable technology for smart agricultural management. Comput. Electron. Agric. 2013, 91, 121–123. [Google Scholar] [CrossRef]
- Xu, X.-J.; Li, Q.-Y.; Song, X.-H.; Qi-Rong, S.; Cai-Xia, D. Dynamic Regulation of Nitrogen and Organic Acid Metabolism of Cherry Tomato Fruit as Affected by Different Nitrogen Forms. Pedosphere 2012, 22, 67–78. [Google Scholar] [CrossRef]
- Roubelakis-Angelakis, K.A.; Kliewer, W.M. Nitrogen Metabolism in Grapevine. Hortic. Rev. 1992, 14, 407–452. [Google Scholar] [CrossRef]
- Grechi, I.; Vivin, P.; Hilbert, G.; Milin, S.; Robert, T.; Gaudillère, J.-P. Effect of light and nitrogen supply on internal C:N balance and control of root-to-shoot biomass allocation in grapevine. Environ. Exp. Bot. 2007, 59, 139–149. [Google Scholar] [CrossRef]
- Robinson, D. Compensatory Changes in the Partitioning of Dry Matter in Relation to Nitrogen Uptake and Optimal Variations in Growth. Ann. Bot. 1986, 58, 841–848. [Google Scholar] [CrossRef]
- Keller, M.; Koblet, W. Dry matter and leaf area partitioning, bud fertility and second season growth of Vitis vinifera L: Responses to Nitrogen supply and limiting irradiance. Vitis 1995, 34, 77–83. [Google Scholar]
- Rustioni, L.; Grossi, D.; Brancadoro, L.; Failla, O. Iron, magnesium, nitrogen and potassium deficiency symptom discrimination by reflectance spectroscopy in grapevine leaves. Sci. Hortic. 2018, 241, 152–159. [Google Scholar] [CrossRef]
- Metay, A.; Magnier, J.; Guilpart, N.; Christophe, A. Nitrogen supply controls vegetative growth, biomass and nitrogen allocation for grapevine (cv. Shiraz) grown in pots. Funct. Plant Biol. 2015, 42, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Somkuwar, R.G.; Satisha, J.; Ramteke, S.D. Berry weight, quality and cane biochemistry changes in relation to cane thickness of own-rooted and grafted ‘Tas-A-Ganesh’ grape. J. Hortl. Sci. 2013, 8, 30–34. [Google Scholar]
- Easlon, H.M.; Bloom, A.J. Easy Leaf Area: Automated digital image analysis for rapid and accurate measurement of leaf area. Appl. Plant Sci. 2014, 2, 1400033. [Google Scholar] [CrossRef]
- Holm, G. Chlorophyll Mutations in Barley. Acta Agric. Scand. 1954, 4, 457–471. [Google Scholar] [CrossRef]
- Zapata, C.; Deléens, E.; Chaillou, S.; Magné, C. Partitioning and mobilization of starch and N reserves in grapevine (Vitis vinifera L.). J. Plant Physiol. 2004, 161, 1031–1040. [Google Scholar] [CrossRef]
- Najar, A.; Landhäusser, S.M.; Whitehill, J.G.A.; Bonello, P.; Erbilgin, N. Reserves Accumulated in Non-Photosynthetic Organs during the Previous Growing Season Drive Plant Defenses and Growth in Aspen in the Subsequent Growing Season. J. Chem. Ecol. 2013, 40, 21–30. [Google Scholar] [CrossRef]
- Stefanello, L.O.; Schwalbert, R.; Schwalbert, R.A.; De Conti, L.; Kulmann, M.S.D.S.; Garlet, L.P.; Silveira, M.L.R.; Sautter, C.K.; de Melo, G.W.B.; Rozane, D.E.; et al. Nitrogen supply method affects growth, yield and must composition of young grape vines (Vitis vinifera L. cv Alicante Bouschet) in southern Brazil. Sci. Hortic. 2019, 261, 108910. [Google Scholar] [CrossRef]
- Lacroux, F.; Trégoat, O.; Van Leeuwen, C.; Pons, A.; Tominaga, T.; Lavigne-Cruège, V.; Dubourdieu, D. Effect of foliar nitrogen and sulphur application on aromatic expression of Vitis vinifera L. cv. Sauvignon blanc. OENO One 2008, 42, 125–132. [Google Scholar] [CrossRef]
- Romero, I.; Benito, A.; Domínguez, N.; García-Escudero, E.; Martín, I. Leaf blade and petiole nutritional diagnosis for Vitis vinifera L. cv. Tempranillo by deviation from optimum percentage method. Span. J. Agric. Res. 2014, 12, 206. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids–Measurement and Characterisation by UV-VIS. In Current Protocols in Food Analytical Chemistry (CPFA), (Supplement 1), F4.3.1–F 4.3.8; John Wiley: New York, NY, USA, 2001. [Google Scholar]
- Rustioni, L.; Rocchi, L.; Di Meo, F.; Failla, O.; Trouillas, P. Advances in grape phenotyping: Pigment characterization by reflectance and theoretical chemistry. Vitis 2015, 54, 173–174. [Google Scholar]
- Rocchi, L.; Rustioni, L.; Failla, O. Chlorophyll and carotenoid quantifications in white grape (Vitis vinifera L.) skins by reflectance spectroscopy. VITIS-J. Grapevine Res. 2016, 55, 11–16. [Google Scholar] [CrossRef]
- Steele, M.R.; Gitelson, A.A.; Rundquist, D.C. A Comparison of Two Techniques for Nondestructive Measurement of Chlorophyll Content in Grapevine Leaves. Agron. J. 2008, 100, 779–782. [Google Scholar] [CrossRef]
- Shaahan, M.M.; El-Sayed, A.; El-Nour, E.A. Predicting nitrogen, magnesium and iron nutritional status in some perennial crops using a portable chlorophyll meter. Sci. Hortic. 1999, 82, 339–348. [Google Scholar] [CrossRef]
- Setshedi, K.J.; Mutingwende, N.; Ngqwala, N.P. The Use of Artificial Neural Networks to Predict the Physicochemical Characteristics of Water Quality in Three District Municipalities, Eastern Cape Province, South Africa. Int. J. Environ. Res. Public Health 2021, 18, 5248. [Google Scholar] [CrossRef]
- Rustioni, L.; Grossi, D.; Brancadoro, L.; Failla, O. Characterization of iron deficiency symptoms in grapevine (Vitis spp.) leaves by reflectance spectroscopy. Plant Physiol. Biochem. 2017, 118, 342–347. [Google Scholar] [CrossRef]
- Carter, G.A.; Knapp, A.K. Leaf optical properties in higher plants: Linking spectral characteristics to stress and chlorophyll concentration. Am. J. Bot. 2001, 88, 677–684. [Google Scholar] [CrossRef]
- Bavaresco, L. Relationship between chlorosis occurrence and mineral composition of grapevine leaves and berries. Commun. Soil Sci. Plan 1997, 28, 13–21. [Google Scholar] [CrossRef]
- Mu, X.; Chen, Y. The physiological response of photosynthesis to nitrogen deficiency. Plant Physiol. Biochem. 2020, 158, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Prieto, J.A.; Louarn, G.; Peña, J.P.; Ojeda, H.; Simonneau, T.; Lebon, E. A leaf gas exchange model that accounts for intra-canopy variability by considering leaf nitrogen content and local acclimation to radiation in grapevine (Vitis vinifera L.). Plant Cell Environ. 2012, 35, 1313–1328. [Google Scholar] [CrossRef] [PubMed]
- Vrignon-Brenas, S.; Aurélie, M.; Romain, L.; Shiva, G.; Alana, F.; Myriam, D.; Gaëlle, R.; Anne, P. Gradual responses of grapevine yield components and carbon status to nitrogen supply. OENO One 2019, 53, 289–306. [Google Scholar] [CrossRef]

| Nutrient Solutions | Full-Nitrogen | Half-Nitrogen | No-Nitrogen |
|---|---|---|---|
| Ca(NO3)2 | 2 | 1 | 0 |
| CaSO4 | 0 | 1 | 2 |
| KNO3 | 0.75 | 0.375 | 0 |
| MgSO4 | 0.65 | 0.65 | 0.65 |
| KH2PO4 | 0.5 | 1.075 | 1.65 |
| H3BO3 | 0.005 | 0.005 | 0.005 |
| MnSO4 | 0.001 | 0.001 | 0.001 |
| CuSO4 | 0.0005 | 0.0005 | 0.0005 |
| FeIII EDTA | 0.08 | 0.08 | 0.08 |
| ZnSO4 | 0.0005 | 0.0005 | 0.0005 |
| (NH4)6Mo7O24 | 0.00005 | 0.00005 | 0.00005 |
| Regression Factor Score | |||||
|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | PC5 | |
| N availability | 0.000 | 0.100 | 0.002 | 0.000 | 0.000 |
| Variety | 0.004 | 0.000 | 0.005 | 0.000 | 0.001 |
| Rootstock | 0.121 | 0.276 | 0.643 | 0.033 | 0.022 |
| Internode | 0.000 | 0.020 | 0.000 | 0.000 | 0.000 |
| Variety × Rootstock | 0.000 | 0.128 | 0.016 | 0.024 | 0.004 |
| Variety × N availability | 0.001 | 0.866 | 0.055 | 0.514 | 0.005 |
| Variety × internode | 0.717 | 0.389 | 0.102 | 0.805 | 0.011 |
| Rootstock × N availability | 0.000 | 0.001 | 0.026 | 0.227 | 0.342 |
| Rootstock × Internode | 0.687 | 0.875 | 0.940 | 0.983 | 0.828 |
| N availability × Internode | 0.691 | 0.123 | 0.098 | 0.954 | 0.731 |
| Variety × Rootstock × N availability | 0.010 | 0.001 | 0.010 | 0.003 | 0.244 |
| Variety × Rootstock × Internode | 0.777 | 0.859 | 0.890 | 0.727 | 0.471 |
| Variety × N availability × Internode | 0.246 | 0.936 | 0.195 | 0.612 | 0.582 |
| Rootstock × N availability × Internode | 0.472 | 0.950 | 0.753 | 0.988 | 0.524 |
| Variety × Rootstock × N availability × Internode | 0.680 | 0.907 | 0.956 | 0.780 | 0.596 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinu, D.G.; Popescu, C.F.; Sumedrea, D.I.; Manolescu, A.E.; Pandelea, L.M.; Rustioni, L. A New Strategy to Improve Vineyard Resilience: Grapevine Morphological Adaptation to Short-Term Nitrogen Deficiency. Agronomy 2022, 12, 1355. https://doi.org/10.3390/agronomy12061355
Dinu DG, Popescu CF, Sumedrea DI, Manolescu AE, Pandelea LM, Rustioni L. A New Strategy to Improve Vineyard Resilience: Grapevine Morphological Adaptation to Short-Term Nitrogen Deficiency. Agronomy. 2022; 12(6):1355. https://doi.org/10.3390/agronomy12061355
Chicago/Turabian StyleDinu, Daniel Grigorie, Carmen Florentina Popescu, Dorin Ioan Sumedrea, Andreea Elena Manolescu, Letitia Mariana Pandelea, and Laura Rustioni. 2022. "A New Strategy to Improve Vineyard Resilience: Grapevine Morphological Adaptation to Short-Term Nitrogen Deficiency" Agronomy 12, no. 6: 1355. https://doi.org/10.3390/agronomy12061355
APA StyleDinu, D. G., Popescu, C. F., Sumedrea, D. I., Manolescu, A. E., Pandelea, L. M., & Rustioni, L. (2022). A New Strategy to Improve Vineyard Resilience: Grapevine Morphological Adaptation to Short-Term Nitrogen Deficiency. Agronomy, 12(6), 1355. https://doi.org/10.3390/agronomy12061355







