Development of the PARMS Markers of the Waxy Gene and Utilization in Discriminating Wild Accessions, and Cultivated Rice (Oryza sativa L.) with Different Eating and Cooking Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. DNA Extraction and Quality Analysis
2.3. Identification of the SNP Sites and Primer Design
2.4. PARMS Genotyping Analysis
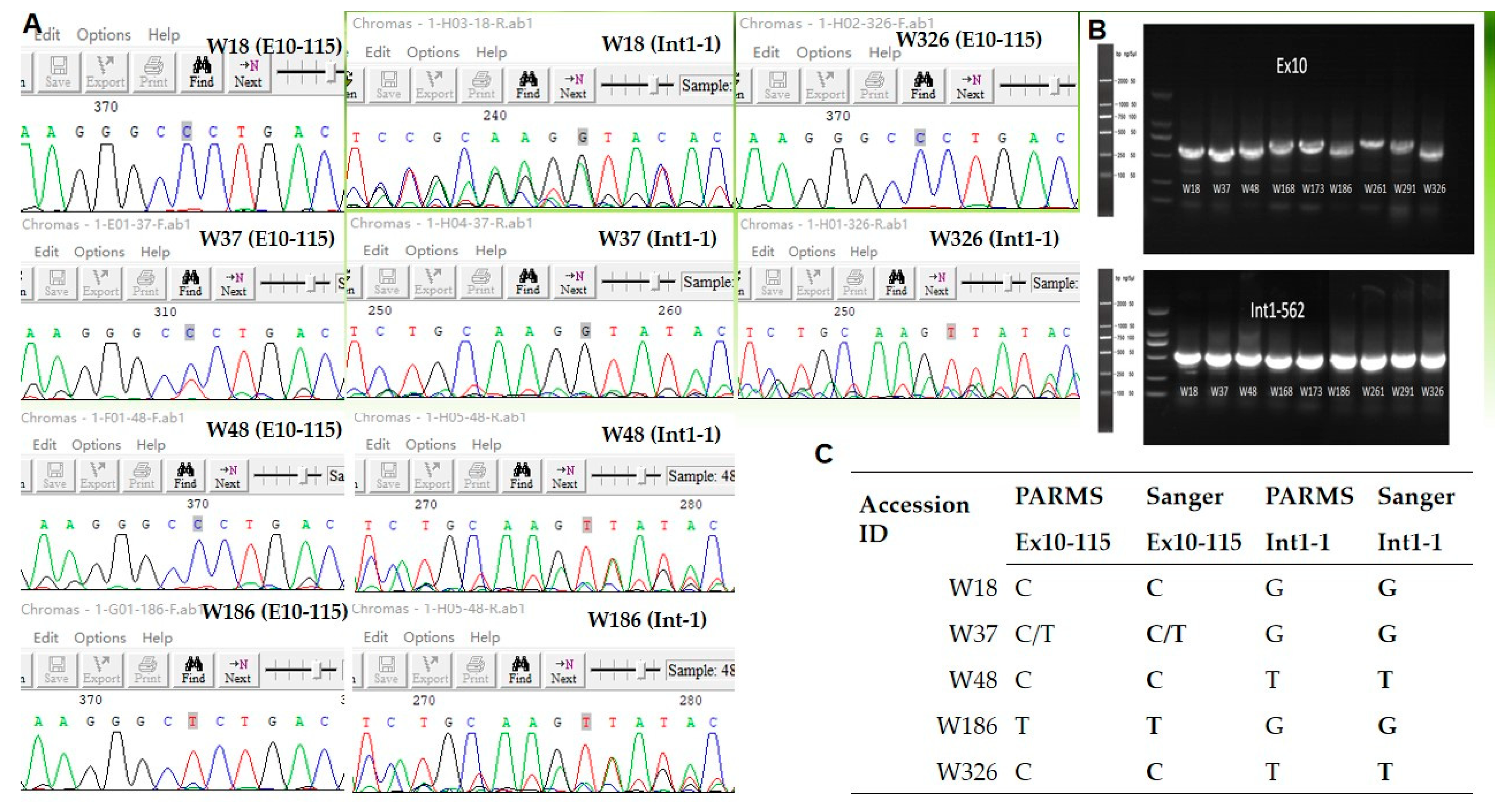
2.5. Validation of Genotyping Results through Sanger Sequencing
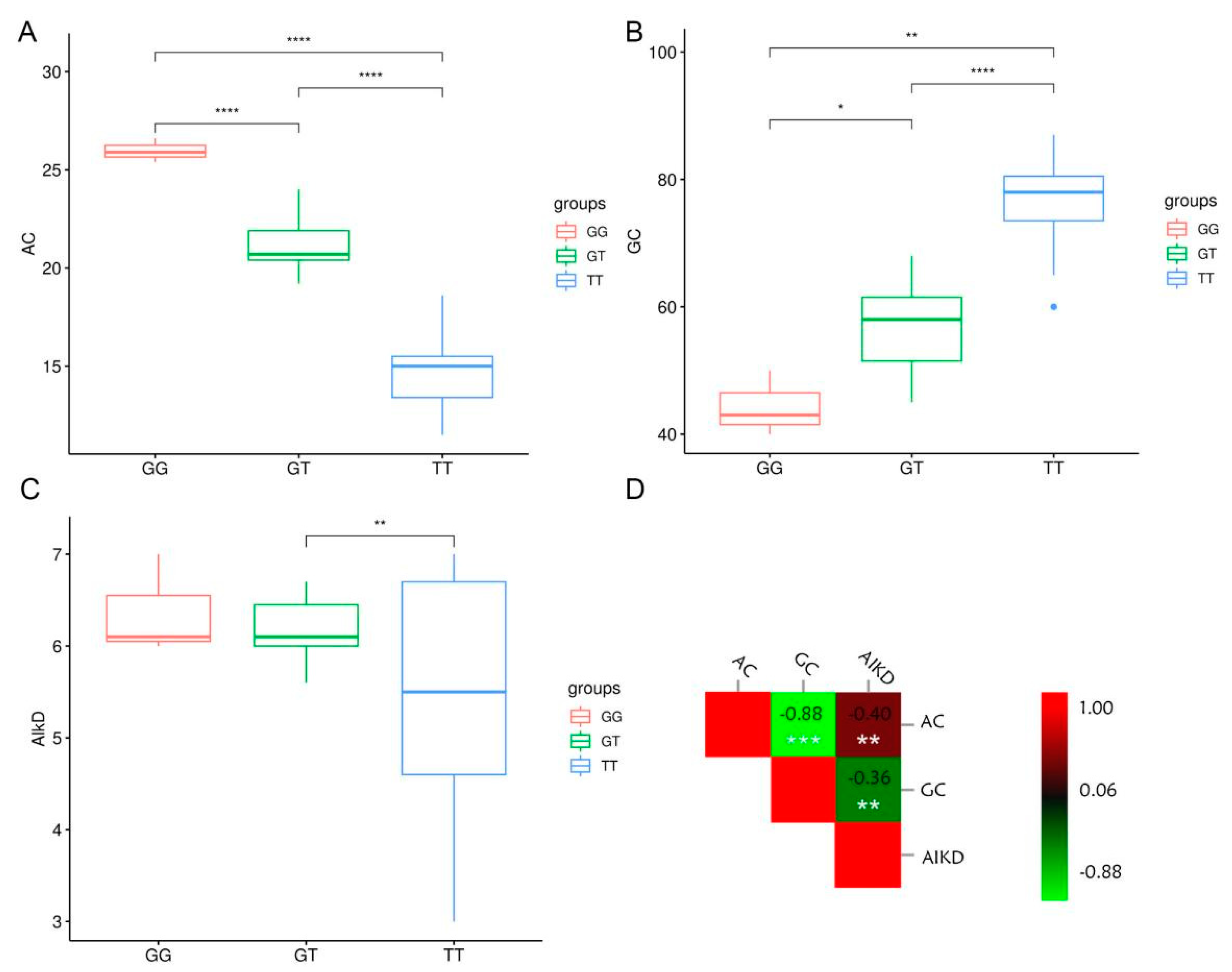
3. Results
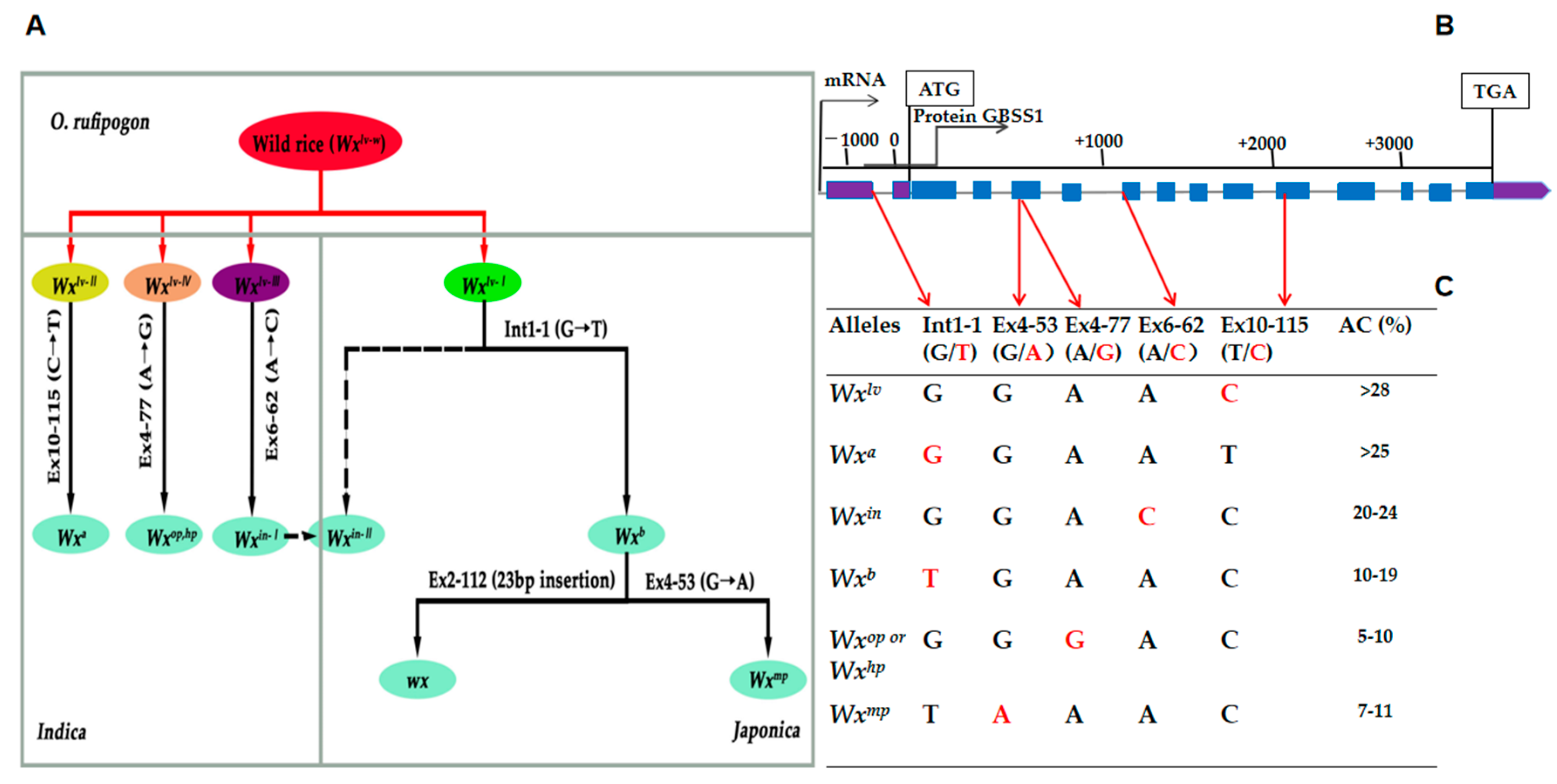
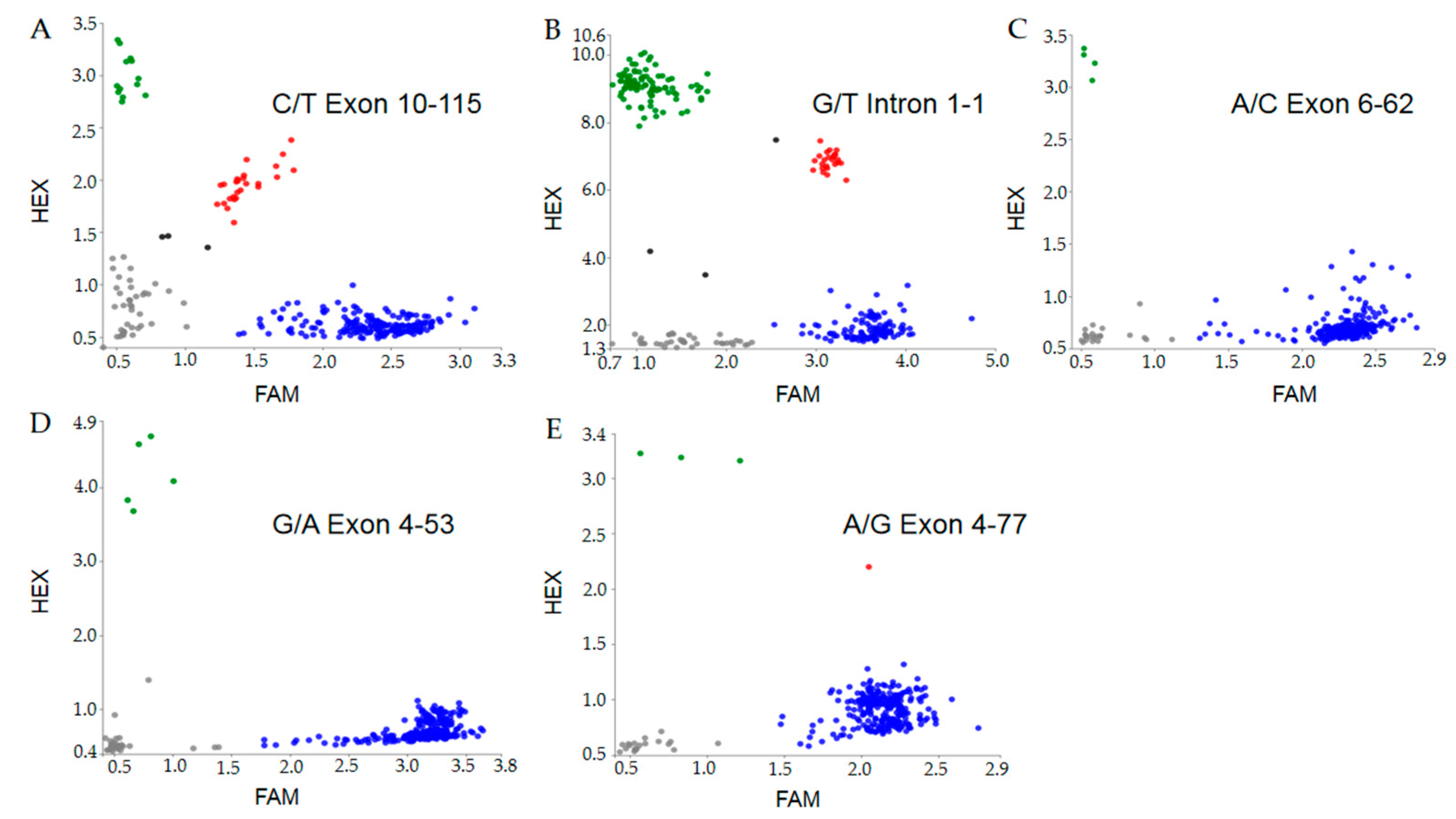
3.1. Development of PARMS Marker of the Wx Alleles
3.2. Validation of PARMS Markers through the Genotyping of Rice Cultivars and Accessions
3.3. Validation of PARMS Genotyping Results from Wild Accessions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAC | Apparent Amylose Content |
| AC | Amylose Content |
| AlkD | Alkali Digestion |
| AS-PCR | Allelic Specific PCR |
| CAPS | Cleaved amplified polymorphic sequence |
| CTAB | Cetyltrimethylammonium Bromide |
| ECQ | Eating and Cooking Quality |
| GBSS1 | Granule Bound-Starch Synthase 1 |
| GC | Gel Consistency |
| GrL | Grain Length |
| INDEL | Insertion–Deletion |
| MAS | Marker-Assisted Selection |
| MAB | Marker-Assisted Breeding |
| SNP | Single Nucleotide Polymorphism |
| SSR | Simple Sequence Repeats |
| KASP | Kompetitive Allele-specific |
| PARMS | Penta-primer amplification refractory mutation system |
| PCR | Polymerase Chain Reaction |
References
- Fahad, S.; Adnan, M.; Noor, M.; Arif, M.; Alam, M.; Khan, I.A.; Wang, D. Major constraints for global rice production. In Advances in Rice Research for Abiotic Stress Tolerance; Woodhead Publishing: Sawston, UK, 2019; pp. 1–22. [Google Scholar] [CrossRef]
- Mackon, E.; Ma, Y.; Jeazet Dongho Epse Mackon, G.C.; Usman, B.; Zhao, Y.; Li, Q.; Liu, P. Computational and Transcriptomic Analysis Unraveled OsMATE34 as a Putative Anthocyanin Transporter in Black Rice (Oryza sativa L.) Caryopsis. Genes 2021, 12, 583. [Google Scholar] [CrossRef] [PubMed]
- Muthayya, S.; Sugimoto, J.D.; Montgomery, S.; Maberly, G.F. An overview of global rice production, supply, trade, and consumption. Ann. N. Y. Acad. Sci. 2014, 1324, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Sarma, R.; Swamy, H.V.V.K.; Shashidhar, H.E. Dealing with zinc and iron deficiency in rice: Combine strategies to fight hidden hunger in developing countries. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1887–1895. [Google Scholar] [CrossRef]
- Zhou, H.; Xia, D.; He, Y. Rice grain quality-traditional traits for high quality rice and health-plus substances. Mol. Breed. 2020, 40, 1. [Google Scholar] [CrossRef]
- Juliano, B.O. Rice Chemistry and Quality; Philippine Rice Research Institute: Muñoz, Philippines, 2004; 480p.
- Tian, Z.X.; Qian, Q.; Liu, Q.Q.; Yan, M.X.; Liu, X.F.; Yan, C.J.; Liu, G.F.; Gao, Z.Y.; Tang, S.Z.; Zeng, D.L.; et al. Allelic diversities in rice starch biosynthesis lead to a diverse array of rice eating and cooking qualities. Proc. Natl. Acad. Sci. USA 2009, 106, 21760–21765. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.J.; Li, M.; Fang, Y.W.; Liu, F.C.; Lu, Y.; Meng, Q.C.; Peng, J.C.; Yi, X.H.; Gu, M.H.; Yan, C.J. Diversification of the waxy gene is closely related to variations in rice eating and cooking quality. Plant. Mol. Biol. Rep. 2012, 30, 462–469. [Google Scholar] [CrossRef]
- Zhou, H.; Xia, D.; Zhao, D.; Li, Y.; Li, P.; Wu, B.; Gao, G.; Zhang, Q.; Wang, G.; Xiao, J.; et al. The origin of Wxla provides new insights into the improvement of grain quality in rice. J. Integr. Plant Biol. 2021, 63, 878–888. [Google Scholar] [CrossRef]
- Bao, J.S.; Shen, S.Q.; Sun, M.; Corke, H. Analysis of genotypic diversity in the starch physico-chemical properties of non waxy rice: Apparent amylose content, pasting viscosity and gel texture. Starch-Stärke 2006, 58, 259–267. [Google Scholar] [CrossRef]
- Huang, M.; Li, X.; Hu, L.; Xiao, Z.; Chen, J.; Cao, F. Comparing texture and digestion properties between white and brown rice of indica cultivars preferred by Chinese consumers. Sci. Rep. 2021, 11, 19054. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, Y.; Chen, S.; Liu, X.; Zhu, J.; Zhou, L.; Lu, Y.; Li, Q.; Fan, X.; Tang, S.; et al. A rare Waxy allele coordinately improves rice eating and cooking quality and grain transparency. J. Integ. Plant Biol. 2020, 63, 889–901. [Google Scholar] [CrossRef]
- Juliano, B.O. Criteria and test for rice grain quality. In Rice Chemistry and Technology; ACC: St. Paul, MN, USA, 1985; pp. 443–524. [Google Scholar] [CrossRef]
- Roy, S.; Banerjee, A.; Basak, N.; Bagchi, T.B.; Mandal, N.P.; Patra, B.C.; Misra, A.K.; Singh, S.K.; Rathi, R.S.; Pattanayak, A. Genetic diversity analysis of specialty glutinous and low-amylose rice (Oryza sativa L.) landraces of Assam based on Wx locus and microsatellite diversity. J. Biosci. 2020, 45, 86. [Google Scholar] [CrossRef]
- He, P.; Li, S.G.; Qian, Q.; Ma, Y.Q.; Li, J.Z.; Wang, W.M.; Chen, Y.; Zhu, L.H. Genetic analysis of rice grain quality. Theor. Appl. Genet. 1999, 98, 502–508. [Google Scholar] [CrossRef]
- Vandeputte, G.E.; Delcour, J.A. From sucrose to starch granule to starch physical behaviour: A focus on rice starch. Carb. Pol. 2004, 58, 245–266. [Google Scholar] [CrossRef]
- Traore, K.; McClung, A.M.; Chen, M.H.; Fjellstrom, R. Inheritance of flour paste viscosity is associated with a rice Waxy gene exon 10 SNP marker. J. Cereal Sci. 2011, 53, 37–44. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, S.; Ren, X.; Lu, Y.; Liu, D.; Cai, X.; Li, Q.; Gao, J.; Liu, Q. Molecular structure and physicochemical properties of starches from rice with different amylose contents resulting from modification of OsGBSSI activity. J. Agric. Food Chem. 2017, 65, 2222–2232. [Google Scholar] [CrossRef]
- Kumar, I.; Khush, G.S. Gene dosage effects of amylose content in rice endosperm. Jpn. J. Genet. 1986, 61, 559–568. [Google Scholar] [CrossRef] [Green Version]
- Cheng, A.; Ismail, I.; Osman, M.; Hashim, H. Simple and Rapid Molecular Techniques for Identification of Amylose Levels in Rice Varieties. Mol. Sci. 2012, 13, 6156–6166. [Google Scholar] [CrossRef] [Green Version]
- Biselli, C.; Cavalluzzo, D.; Perrini, R.; Gianinetti, A.; Bagnaresi, P.; Urso, S.; Orasen, G.; Desiderio, F.; Lupotto, E.; Cattivelli, L.; et al. Improvement of marker-based predictability of apparent amylose content in japonica rice through GBSSI allele mining. Rice 2014, 1, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Zhu, J.; Chen, S.; Fan, X.; Li, Q.; Lu, Y.; Wang, M.; Yu, H.; Yi, C.; Tang, S. WxLv, the Ancestral Allele of Rice waxy gene. Mol. Plant. 2019, 12, 1157–1166. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.; Peng, Y.; Mao, B.; Lv, Q.; Yuan, D.; Liu, X.; Zhao, B. Allelic variations of the Wx locus in cultivated rice and their use in the development of hybrid rice in China. PLoS ONE 2020, 15, e0232279. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Zheng, F.Q.; Shen, G.Z.; Gao, J.P.; Snustad, D.P.; Li, M.G.; Zhang, J.L.; Hong, M.M. The amylose content in rice endosperm is related to the post-transcriptional regulation of the waxy gene. Plant J. 1995, 7, 613–622. [Google Scholar] [CrossRef]
- Cai, X.L.; Wang, Z.Y.; Xing, Y.Y.; Zhang, J.L.; Hong, M.M. Aberrant splicing of intron 1 leads to the heterogeneous 5‘UTR and decreased expression of waxy gene in rice cultivars of intermediate amylose content. Plant J. 1998, 14, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Crofts, N.; Itoh, A.; Abe, M.; Miura, S.; Oitome, N.F.; Bao, J.; Fujita, N. Three major nucleotide polymorphisms in the waxy gene correlated with the amounts of extra-long chains of amylopectin in rice cultivars with S or L-type Amylopectin. J. Appl. Glycosci. 2019, 66, 37–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larkin, P.D.; Park, W.D. Association of waxy gene single nucleotide polymorphisms with starch characteristics in rice (Oryza sativa L.). Mol. Breed. 2003, 12, 335–339. [Google Scholar] [CrossRef]
- Mikami, I.; Aikawa, M.; Hirano, H.Y.; Sano, Y. Altered tissue-specific expression at the wx gene of the opaque mutants in rice. Euphytica 1999, 105, 91–97. [Google Scholar] [CrossRef]
- Liu, L.L.; Ma, X.D.; Liu, S.J.; Zhu, C.L.; Jiang, L.; Wang, Y.H.; Shen, Y.; Ren, Y.L.; Dong, H.; Chen, L.M.; et al. Identification and characterization of a novel waxy allele from yunnan rice landrace. Plant Mol. Biol. 2009, 71, 609–626. [Google Scholar] [CrossRef]
- Wanchana, S.; Toojinda, T.; Tragoonrung, S.; Vanavichit, A. Duplicated coding sequence in the waxy allele of tropical glutinous rice (Oryza sativa L.). Plant Sci. 2003, 165, 1193–1199. [Google Scholar] [CrossRef]
- Isshiki, M.; Morino, K.; Nakajima, M.; Okagaki, R.J.; Wessler, S.R.; Izawa, T.; Shimamoto, K. A naturally occurring functional allele of the rice waxy locus has a GT to TT mutation at the 5′ splice site of the first intron. Plant J. 1998, 15, 133–138. [Google Scholar] [CrossRef] [Green Version]
- Usman, B.; Zhao, N.; Nawaz, G.; Qin, B.; Liu, F.; Liu, Y.; Li, R. CRISPR/Cas9 Guided Mutagenesis of Grain Size 3 Confers Increased Rice (Oryza sativa L.) Grain Length by Regulating Cysteine Proteinase Inhibitor and Ubiquitin-Related Proteins. Int. J. Mol. Sci. 2021, 22, 3225. [Google Scholar] [CrossRef]
- Cai, X.L.; Liu, Q.Q.; Tang, S.Z.; Gu, M.H.; Wang, Z.Y. Development of a molecular marker for screening the rice cultivars with intermediate amylose content in Oryza sativa subsp. indica. J. Plant Physiol. Mol. Biol. 2002, 28, 137–144. [Google Scholar]
- Liu, Q.Q.; Li, Q.F.; Cai, X.L.; Wang, H.M.; Tang, S.Z.; Yu, H.X.; Wang, Z.Y.; Gu, M.H. Molecular Marker-Assisted selection for Improved Cooking and Eating Quality of two Elite Parents of Hybrid Rice. Crop Sci. 2006, 46, 2354–2360. [Google Scholar] [CrossRef]
- Gao, L.; Zhou, M.; Chen, R.; Gao, H.; Yan, Q.; Zhou, W.; Deng, G. Developping and validating the Functional Marker of Rice Waxy Gene, M-WX. Rice Gen.Genet. 2012, 10, 61–65. [Google Scholar] [CrossRef]
- Chen, H.; Shan, J.; Yang, K.; Wang, Y.Y.; Lu, C.M. Abundant Variation of Waxy Gene in Yunnan Rice Landraces and Molecular Characterization of a Novel Wx-zm Allele. Crop Sci. 2014, 54, 2152–2159. [Google Scholar] [CrossRef]
- Chen, Z.H.; Wang, F.Q.; Xu, Y.; Wang, J.; Li, W.Q.; Fan, F.J.; Zhong, W.G.; Yang, J. The distribution of low Amylose Content Allele Wx-mp in Japonica Rice. J. Plant Genet. Res. 2019, 20, 975–981. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, S.; Yang, G.; Zha, W.; Cai, H.; Li, S.; Chen, Z.; Liu, K.; Xu, H.; You, A. A perfect functional marker for the gene of intermediate amylose content Wx-in in rice (Oryza Sativa L.). Crop Breed. Appl. Biotech. 2018, 18, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Wang, J.; Fan, F.J.; Zhu, J.Y.; Chen, T.; Wang, C.L.; Zheng, T.Q.; Zhang, J.; Zhong, W.G.; Xu, J.L. Development of AS-PCR marker based on a key mutation confirmed by resequencing of Wx-mp in Milky Princess and its application in japonica soft rice (Oryza sativa L.). Plant Breed. 2013, 132, 595–603. [Google Scholar] [CrossRef]
- Sakthivel, K.; Shobha Rani, N.; Pandey, M.K.; Sivaranjani, A.K.P.; Neeraja, C.N.; Balachandran, S.M.; Sheshu Madhav, M.; Viraktamath, B.C.; Prasad, G.S.V.; Sundaram, R.M. Development of a simple functional marker for fragrance in rice and its validation in Indian Basmati and non-Basmati fragrant rice varieties. Mol. Breed. 2009, 24, 185–190. [Google Scholar] [CrossRef]
- Poczai, P.; Varga, I.; Laos, M.; Cseh, A.; Bell, N.; Valkonen, J.P.; Hyvönen, J. Advances in plant gene-targeted and functional markers: A review. Plant Methods 2013, 9, 6. [Google Scholar] [CrossRef] [Green Version]
- Rosas, J.E.; Bonnecarrère, V.; Pérez de Vida, F. One-step, codominant detection of imidazolinone resistance mutations in weedy rice (Oryza sativa L.). Electron. J. Biotechnol. 2014, 17, 95–101. [Google Scholar] [CrossRef] [Green Version]
- Dennis Lo, Y.M. The Amplification Refractory Mutation System. In Clinical Applications of PCR; Lo, Y.M.D., Ed.; Methods in Molecular Medicine™; Humana Press: Totowa, NJ, USA, 1998; Volume 16, pp. 61–69. [Google Scholar] [CrossRef]
- Gao, J.; Liang, H.; Huang, J.; Qing, D.; Wu, H.; Zhou, W.; Chen, W.; Pan, Y.; Dai, G.; Gao, L.; et al. Development of the PARMS marker of the TAC1 gene and its utilization in rice plant architecture breeding. Euphytica 2021, 217, 49. [Google Scholar] [CrossRef]
- Lu, J.; Hou, J.; Ouyang, Y.; Luo, H.; Zhao, J.; Mao, C.; Han, M.; Wang, L.; Xiao, J.; Yang, Y.; et al. A direct PCR–based SNP marker–assisted selection system (D-MAS) for different crops. Mol. Breed. 2020, 40, 9. [Google Scholar] [CrossRef]
- Sayadi Maazou, A.-R.; Gedil, M.; Adetimirin, V.O.; Meseka, S.; Mengesha, W.; Babalola, D.; Offornedo, Q.N.; Menkir, A. Comparative assessment of effectiveness of alternative genotyping assays for characterizing carotenoids accumulation in tropical maize inbred lines. Agronomy 2021, 11, 2022. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, R.; Wang, M.; Hong, M.; Zhu, L.; Li, X.; Guo, R.; Wu, G.; Zeng, X. Development of the PARMS Marker of the Dominant Genic Male Sterility (DGMS) Line and Its Utilization in Rapeseed (Brassica napus L.) Breeding. Plants 2022, 11, 421. [Google Scholar] [CrossRef] [PubMed]
- Neelam, K.; Brown-Guedira, G.; Huang, L. Development and validation of a breeder-friendly KASPar marker for wheat leaf rust resistance locus Lr21. Mol. Breed. 2013, 31, 233–237. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, L.; Zhao, Y.; Guo, H.; Li, J.; Rashid, M.; Lu, C.; Zhou, W.; Yang, X.; Liang, Y.; et al. Natural Variation in OsMKK3 Contributes to Grain Size and Chalkiness in Rice. Front. Plant Sci. 2021, 12, 784037. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2020; 260p. [Google Scholar]
- Allen, G.C.; Flores-Vergara, M.A.; Krasynanski, S.; Kumar, S.; Thompson, W.F. A modified protocol for rapid DNA isolation from plant tissues using cetyltrimethylammonium bromide. Nat. Protoc. 2006, 1, 2320–2325. [Google Scholar] [CrossRef]
- Mikami, I.; Uwatoko, N.; Ikeda, Y.; Yamaguchi, J.; Hirano, H.Y.; Suzuki, Y.; Sano, Y. Allelic diversification at the Wx locus in landraces of Asian rice. Theor. Appl. Genet. 2008, 116, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.A.; Daygon, V.D.; Resurreccion, A.P.; Cuevas, R.P.; Corpuz, H.M.; Fitzgerald, M.A. A single nucleotide polymorphism in the Waxy gene explains a significant component of gel consistency. Theor. Appl. Genet. 2011, 123, 519–525. [Google Scholar] [CrossRef]
- Kongrese, N.; Juliano, B.O. Physicochemical properties of Rice grain and Starch from lines differing in Amylose content and Gelatinization temperature. J. Sci. Food Agric. 1972, 20, 714–718. [Google Scholar]
- Cagampang, G.B.; Perez, C.M.; Juliano, B.O. A gel consistency test for eating quality of rice. J. Sci. Food Agric. 1973, 24, 1589–1594. [Google Scholar] [CrossRef]
- Khush, G.S.; Paule, C.M.; De la Cruz, N.M. Rice grain quality evaluation and improvement at IRRI. In Chemical Aspects of Rice Grain; International Rice Research Institute: Los Banos, Philippines, 1979; pp. 21–31. [Google Scholar]
- Wang, J.; Hu, P.; Chen, Z.; Liu, Q.; Wei, C. Progress in high-amylose cereal crops through inactivation of starch branching enzymes. Front. Plant Sci. 2017, 8, 469. [Google Scholar] [CrossRef] [Green Version]
- Farias, C.J.F.; Cruz De La, N.M. Cooking and eating characteristics in upland and irrigated rice varieties. Pesqui. Agropecu. Bras. 1995, 30, 115–120. [Google Scholar]
- Hirano, H.Y.; Eiguchi, M.; Sano, Y. A single base change altered the regulation of the Waxy gene at the posttranscriptional level during the domestication of rice. Mol. Biol. Evol. 1998, 15, 978–987 doiorg/101093/oxfordjournalsmolbeva026013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Civán, P.; Craig, H.; Cox, C.; Brown, T.A. Multiple domestications of Asian rice. Nat. Plants 2016, 2, 16037. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Lu, H.; Jiang, L.; Zhang, J.; Yang, X.; Huan, X.; He, K.; Wang, C.; Wu, N. Dating rice remains through phytolith carbon-14 study reveals domestication at the beginning of the Holocene. Proc. Natl. Acad. Sci. USA 2017, 114, 6486–6491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Han, B. Rice domestication occurred through single origin and multiple introgressions. Nat. Plants 2015, 9, 15207. [Google Scholar] [CrossRef] [PubMed]
| Cultivar | Hybrid (H) or Inbred (I) | AC | Gel Consistency (GC) | Alkali Digestion (AlkD) | Grain Length (GrL) (mm) | Length–Width Ratio (L/W) | 1000-Grain Weight (TGW) (g) |
|---|---|---|---|---|---|---|---|
| Guangxin 5113 | H | 20.4 | 61.0 | 6.0 | 7.0 | 3.2 | 24.0 |
| Hualiangyou 338 | H | 24 | 78.0 | 6.6 | 9.1 | 3.4 | 24.0 |
| Zhaofengyou 9958 | H | 20.7 | 78.0 | 5.9 | 7.3 | 3.4 | 23.5 |
| Yongfengyou 9802 | H | 14.6 | 83.0 | 7.0 | 10.2 | 3.4 | 24.3 |
| Guang8youhuahzhan | H | 16.1 | 55.5 | 3.1 | 9.1 | 3.4 | 20.2 |
| Teyou 2278 | H | 26.6 | 40.0 | 7.0 | 8.5 | 2.7 | 26.1 |
| Youxianglongsimiao | H | 15.5 | 78.0 | 6.9 | 8.7 | 4.4 | 22.1 |
| Hlianyou 6839 | H | 12.3 | 86.0 | 4.5 | 10.5 | 3.2 | 30.1 |
| Hengfengyou 666 | H | 13.1 | 76.0 | 4.6 | 9.7 | 3.5 | 27.3 |
| Hengfengyou 426 | H | 11.5 | 78.0 | 3.0 | 9.5 | 3.3 | 26.26 |
| Hengfengyou 7166 | H | 19.2 | 58.0 | 5.6 | 6.6 | 3.2 | 26.7 |
| Hengfengyoujinsimiao | H | 12.5 | 76.0 | 4.3 | 6.7 | 3.4 | 23.6 |
| Hengfengyouhuazhan | H | 15.1 | 76.5 | 3.0 | 7.8 | 3.5 | 24.1 |
| Guangheyouhuazhan | H | 12.7 | 82.0 | 6.8 | 6.5 | 3.4 | 23.6 |
| Shenliangyou 8386 | H | 11.5 | 78.0 | 3.2 | 7.5 | 3.5 | 25.4 |
| Guang8youxiangsimiao | H | 15.4 | 76.0 | 6.7 | 9.1 | 4.2 | 20.8 |
| Hengfengyouyuxiang | H | 14.6 | 80.0 | 7.0 | 10.2 | 3.8 | 25.6 |
| Guang8you 165 | H | 15.4 | 65.0 | 6.8 | 9.0 | 3.4 | 21.7 |
| H liangyou 5872 | H | 16.2 | 80.0 | 3.0 | 9.2 | 3.2 | 28.9 |
| Wuyou 305 | H | 13.4 | 74.4 | 5.5 | 6.7 | 3.1 | 23.7 |
| Shenyou 9569 | H | 12.5 | 83.0 | 6.6 | 6.5 | 3.0 | 26.4 |
| Teyou 6811 | H | 25.9 | 43.0 | 6.1 | 9.4 | 2.2 | 25.3 |
| Hengfengyou 777 | H | 11.6 | 82.0 | 3.3 | 6.8 | 3.1 | 25.6 |
| Wushansimiao | I | 17.5 | 66.7 | 6.8 | 9.1 | 3.1 | 22.4 |
| Quanxiangyou 822 | H | 15.1 | 86.0 | 6.3 | 8.5 | 3.6 | 24.0 |
| Teyou 582 | H | 21.6 | 38.0 | 6.7 | 9.5 | 2.2 | 24.9 |
| Teyou 831 | H | 25.4 | 50.0 | 6.0 | 8.3 | 2.4 | 26.9 |
| Quanyousimiao | H | 16 | 72.0 | 7.0 | 6.7 | 3.2 | 26.4 |
| Quanxiangyoumeizhan | H | 13.6 | 76.0 | 4.8 | 10.2 | 3.8 | 23.9 |
| Quanyou 123 | H | 16.9 | 82.0 | 4.9 | 9.9 | 3.3 | 28.8 |
| Hexin 5 hao | I | 13.6 | 80.0 | 4.9 | 10.2 | 3.7 | 20.9 |
| Fengtianyou 553 | H | 15 | 78.0 | 5.4 | 9.2 | 3.5 | 22.4 |
| Shenliangyou 1173 | H | 15.2 | 54.0 | 6.9 | 9.3 | 3.2 | 21.7 |
| Y liangyou 1173 | H | 13.3 | 80.0 | 5.0 | 7.2 | 3.2 | 20.6 |
| Fengtianyou 089 | H | 14.9 | 70.0 | 5.6 | 8.5 | 3.6 | 22.2 |
| Zhuangxiangyoubaijin 5 | H | 15.2 | 76.0 | 6.1 | 6.6 | 4.1 | 20.8 |
| Taiyou 2068 | H | 14.4 | 76.0 | 4.5 | 8.5 | 3.8 | 24.2 |
| Qianliangyou 8 hao | H | 15.8 | 67.0 | 6.8 | 9.2 | 3.3 | 24.6 |
| Changliangyou 8 hao | H | 17.2 | 69.0 | 6.3 | 8.8 | 3.4 | 24.1 |
| Shenyou 9516 | H | 18.6 | 77.0 | 4.6 | 8.1 | 3.2 | 27.5 |
| Heliangyou 713 | H | 15 | 76.0 | 5.5 | 9.2 | 3.0 | 23.7 |
| Y liangyou 087 | H | 15.3 | 66.0 | 6.2 | 6.7 | 3.0 | 25.8 |
| Qianliangyouhuazhan | H | 15 | 80.5 | 5.4 | 9.1 | 3.2 | 22.9 |
| Changliangyou 6 hao | H | 15.7 | 78.0 | 7.0 | 9.1 | 3.6 | 25.2 |
| Heliangyou 1 hao | H | 15 | 73.5 | 5.5 | 9.0 | 3.0 | 23.6 |
| Guoliangyou 633 | H | 15.8 | 72.0 | 6.5 | 8.9 | 3.7 | 19.6 |
| Teyou 7671 | H | 23.3 | 48.0 | 6.5 | 8.5 | 2.7 | 27.6 |
| Teyou 7571 | H | 21.9 | 55.0 | 6.4 | 8.6 | 2.5 | 27.5 |
| T You 682 | H | 19.9 | 60.0 | 6.0 | 10.1 | 2.9 | 27.7 |
| Teyou 679 | H | 20.7 | 45.0 | 6.0 | 8.0 | 2.3 | 27.3 |
| Guihefeng | I | 14.2 | 78.0 | 6.1 | 6.8 | 3.5 | 20.9 |
| Y liangyou 5806 | H | 13.4 | 85.0 | 5.6 | 6.8 | 3.0 | 26.7 |
| Teyou 913 | H | 21.9 | 72.0 | 6.3 | 8.5 | 2.6 | 29.4 |
| Teyou 986 | H | 20.4 | 36.0 | 6.1 | 6.9 | 2.1 | 28.7 |
| Naide 606 | I | 13.8 | 76.0 | 4.8 | 9.3 | 3.1 | 21.5 |
| Marker Name | SNP Type | Sequences | ||
|---|---|---|---|---|
| Primer X-FAM (Forward 1, 5’-3’) | Primer Y-HEX (Forward 2, 5’-3’) | Primer C (Reverse, 5’-3’) | ||
| PM-Wxlv | (T/C) | 5′GAAGGTGACCAAGTTCATGCTCTGGAGGAACAGAAGGGCC-3′ | 5′GAAGGTCGGAGTCAACGGATTGCTGGAGGAACAGAAGGGCT-3′ | 5′GAGCTCCGGGATGGCG-3′ |
| PM-Wxa | (G/T) | 5′GAAGGTGACCAAGTTCATGCTATCAGGAAGAACATCTGCAAGG-3′ | 5′GAAGGTCGGAGTCAACGGATTCATCAGGAAGAACATCTGCAAGT-3′ | 5′GATCTGAATAAGAGGGGAAACAAA-3′ |
| PM-Wxin | (A/C) | 5′GAAGGTGACCAAGTTCATGCTACAACCCATACTTCAAAGGAACTTA-3′ | 5′GAAGGTCGGAGTCAACGGATTCAACCCATACTTCAAAGGAACTTC-3′ | 5′AATTAGTCTGATCATCATGGATTCC-3′ |
| PM-Wxb | (G/T) | 5′GAAGGTGACCAAGTTCATGCTATCAGGAAGAACATCTGCAAGG-3′ | 5′GAAGGTCGGAGTCAACGGATTCATCAGGAAGAACATCTGCAAGT-3′ | 5′GATCTGAATAAGAGGGGAAACAAA-3′ |
| PM-Wxop, PM-Wxhp | (A/G) | 5′GAAGGTGACCAAGTTCATGCTCTCCAGGAATGACGGATGGT-3′ | 5′GAAGGTCGGAGTCAACGGATTCCAGGAATGACGGATGGC-3′ | 5′AGCGTGGAGTCGACCGTG-3′ |
| PM-Wxmp | (G/A) | 5′GAAGGTGACCAAGTTCATGCTTGAACACACGGTCGACTCCAC-3′ | 5′GAAGGTCGGAGTCAACGGATTTGAACACACGGTCGACTCCAT-3′ | 5′GGTTGCAGACAGGTACGAGAGG-3′ |
| Types | Name | Hybrid (H) or Inbred (I) | Wxa and Wxb Int1-1 (G/T) | Wxmp Ex4-53 (G/A) | Wxop Ex4-77 (A/G) | Wxin Ex6-62 (A/C) | Wxlv Ex10-115 (C/T) | Wx Alleles/Genotypes |
|---|---|---|---|---|---|---|---|---|
| Cultivars | Teyou 2278 | H | G | G | A | A | T | Wxa |
| Hengfengyou 7166 | H | G/T | G | A | A | C/T | Wxa/Wxb | |
| Shenliangyou 8386 | H | T | G | A | A | C | Wxb | |
| Teyou 6811 | H | G | G | A | A | T | Wxa | |
| Yliangyou 286 | H | T | G | A | A | C | Wxb | |
| Teyou 831 | H | G | G | A | A | T | Wxa | |
| Quanyou 123 | H | T | G | A | A | C | Wxb | |
| Fengtianyou 553 | H | T | G | A | A | C | Wxb | |
| Guangxin 5113 | H | G/T | G | A | A | C/T | Wxa/Wxb | |
| Zhuangxiangyoubaijin 5 | H | T | G | A | A | C | Wxb | |
| Changliangyou 8 hao | H | T | G | A | A | C | Wxb | |
| Guoliangyou 633 | H | T | G | A | A | C | Wxb | |
| Teyou 7671 | H | G/T | G | A | A | C/T | Wxa/Wxb | |
| Teyou 913 | H | G/T | G | A | A | C/T | Wxa/Wxb | |
| Zhaofengyou 9958 | H | G/T | G | A | A | C/T | Wxa/Wxb | |
| wild accessions (*) | W72 | G | G | A | A | C/T | Wxlv/Wxa | |
| W165 | G | G | A | A | C | Wxlv | ||
| W186 | G | G | A | A | T | Wxa | ||
| W171 | G | G | A | A | C | Wxlv | ||
| W173 | T | G | A | A | C | Wxb | ||
| W189 | G | G | A | A | C/T | WxlvWxa | ||
| W284 | G | G | A | A | C | Wxlv | ||
| W290 | G | G | A | A | C | Wxlv | ||
| W321 | G | G | A | A | C | Wxlv | ||
| W326 | T | G | A | A | C | Wxb | ||
| W334 | G | G | A | A | C | Wxlv | ||
| W338 | G | G | A | A | C | Wxlv | ||
| Control samples (sterile, hybrid, and restorer lines | Nipponbare | I | T | G | A | A | C | Wxb |
| IR24 | I | T | G | A | A | C | Wxb | |
| Zhong A | H | G | G | A | A | T | Wxa | |
| Ce 64-7 | I | G | G | A | A | T | Wxa | |
| Tianyouhuazhan | H | G/T | G | A | A | C/T | Wxa/Wxb | |
| Kasalath, | I | G | G | A | A | C | Wxlv | |
| R402 | I | G | G | A | A | C | Wxlv | |
| Basmati | I | G | G | A | C | C | Wxin | |
| IR64 | I | G | G | A | C | C | Wxin | |
| Nangeng 46 | I | T | A | A | A | C | Wxmp | |
| Huzaoxiang | I | T | A | A | A | C | Wxmp | |
| ZHN63 | I | G | G | G | A | C | Wxop | |
| Haomuxi | I | G | G | G | A | C | Wxop |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeazet Dongho Epse Mackon, G.C.; Mackon, E.; Ma, Y.; Zhao, Y.; Yao, Y.; Dai, X.; Liu, P. Development of the PARMS Markers of the Waxy Gene and Utilization in Discriminating Wild Accessions, and Cultivated Rice (Oryza sativa L.) with Different Eating and Cooking Quality. Agronomy 2022, 12, 1294. https://doi.org/10.3390/agronomy12061294
Jeazet Dongho Epse Mackon GC, Mackon E, Ma Y, Zhao Y, Yao Y, Dai X, Liu P. Development of the PARMS Markers of the Waxy Gene and Utilization in Discriminating Wild Accessions, and Cultivated Rice (Oryza sativa L.) with Different Eating and Cooking Quality. Agronomy. 2022; 12(6):1294. https://doi.org/10.3390/agronomy12061294
Chicago/Turabian StyleJeazet Dongho Epse Mackon, Guibeline Charlie, Enerand Mackon, Yafei Ma, Yitong Zhao, Yuhang Yao, Xianggui Dai, and Piqing Liu. 2022. "Development of the PARMS Markers of the Waxy Gene and Utilization in Discriminating Wild Accessions, and Cultivated Rice (Oryza sativa L.) with Different Eating and Cooking Quality" Agronomy 12, no. 6: 1294. https://doi.org/10.3390/agronomy12061294
APA StyleJeazet Dongho Epse Mackon, G. C., Mackon, E., Ma, Y., Zhao, Y., Yao, Y., Dai, X., & Liu, P. (2022). Development of the PARMS Markers of the Waxy Gene and Utilization in Discriminating Wild Accessions, and Cultivated Rice (Oryza sativa L.) with Different Eating and Cooking Quality. Agronomy, 12(6), 1294. https://doi.org/10.3390/agronomy12061294





