The Concepts of Seed Germination Rate and Germinability: A Re-Evaluation for Cool-Season Grasses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed Material
2.2. Germination Experiment
2.3. Analysis of Germination Data
2.4. Typologies of Germination Responses to Temperature
3. Results
3.1. Effects of Temperature on Germination Time and Germination Rate
3.2. Effects of Temperature on Final Germination Percentage
3.3. Typologies of Germination Responses to Temperature
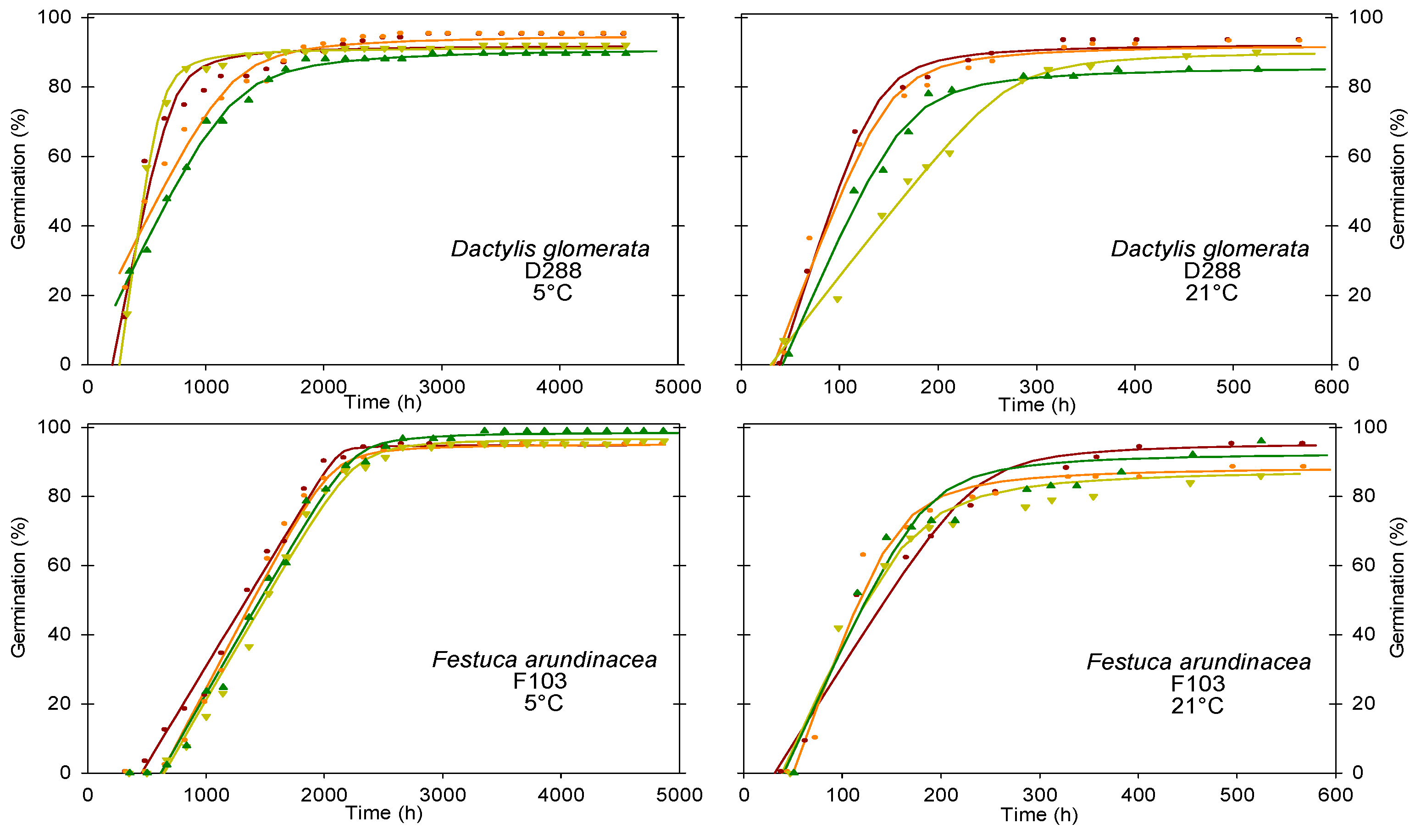
3.3.1. Type 1 Germination Dynamics–High Germinability, Fast Germination, Germinability Stable across Temperatures
3.3.2. Type 2 Germination Dynamics–High Germinability, Fast Germination (Slower than Type 1), Germinability Stable across Temperatures
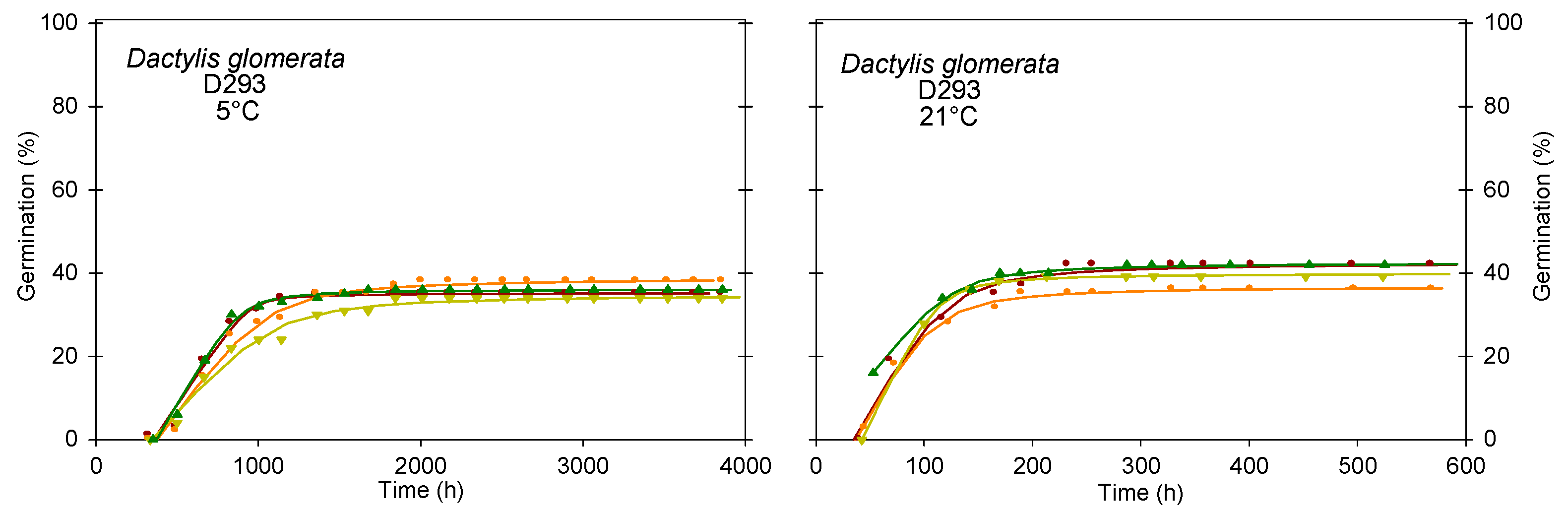
3.3.3. Type 3 Germination Dynamics–Low Germinability, Fast Germination and Germinability Stable across Temperatures
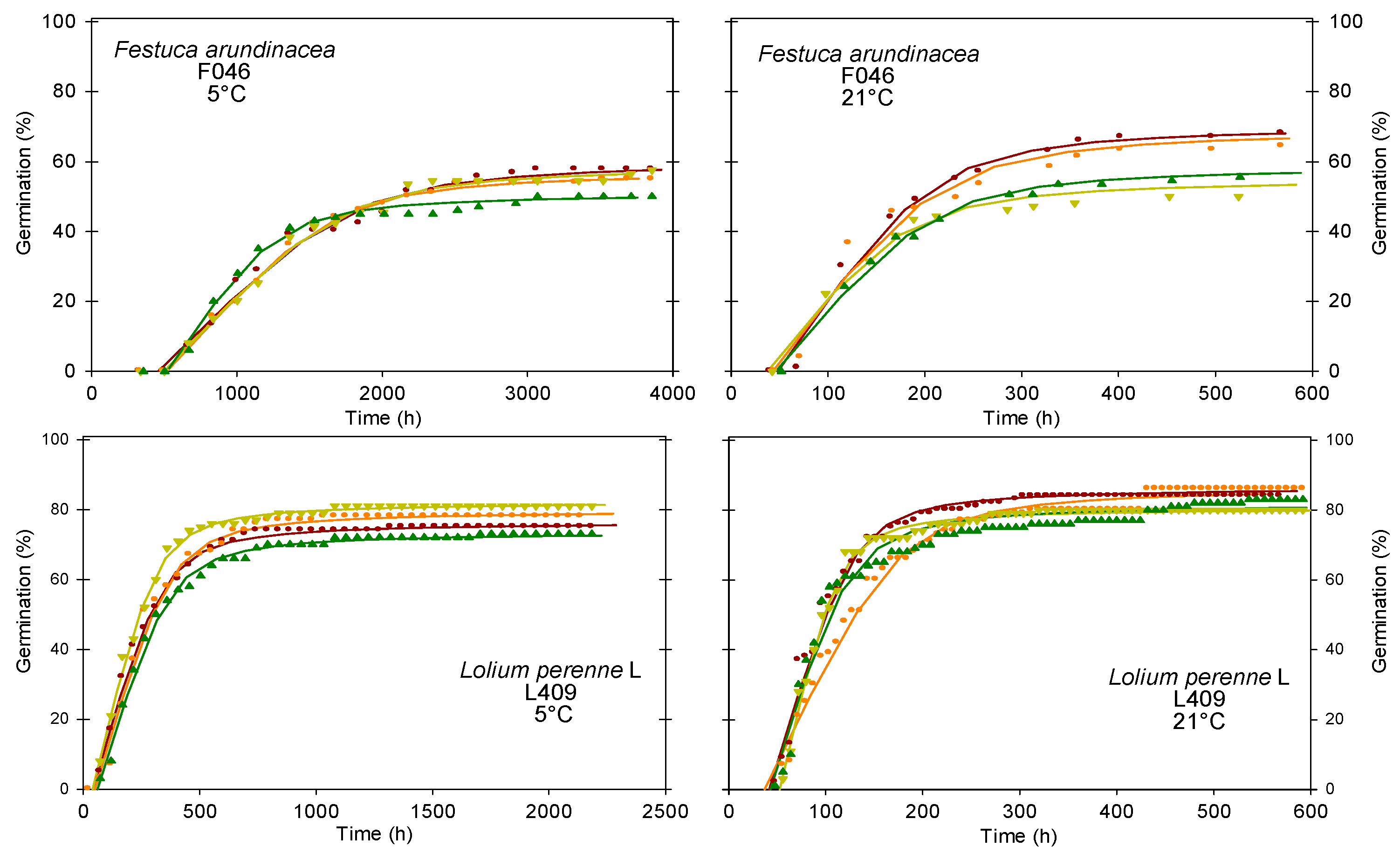
3.3.4. Type 4 Germination Dynamics–Low Germinability, Fast Germination and Germinability Stable across Temperatures
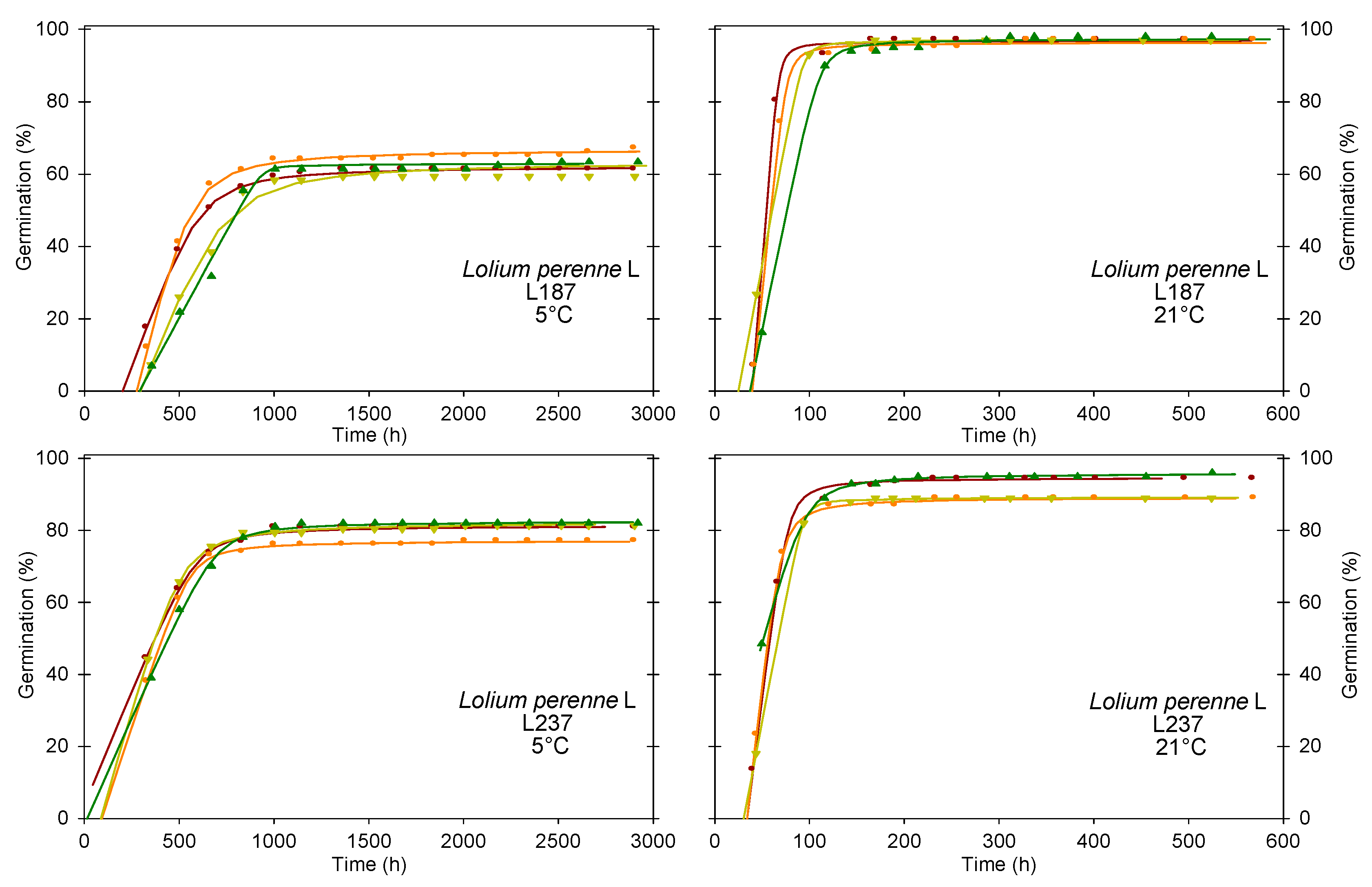
3.3.5. Type 5 Germination Dynamics–High Germinability, Fast Germination, Germinability Variable across Temperatures
3.3.6. Type 6 Germination Dynamics–High Germinability, Slow Germination, Germinability Variable across Temperatures
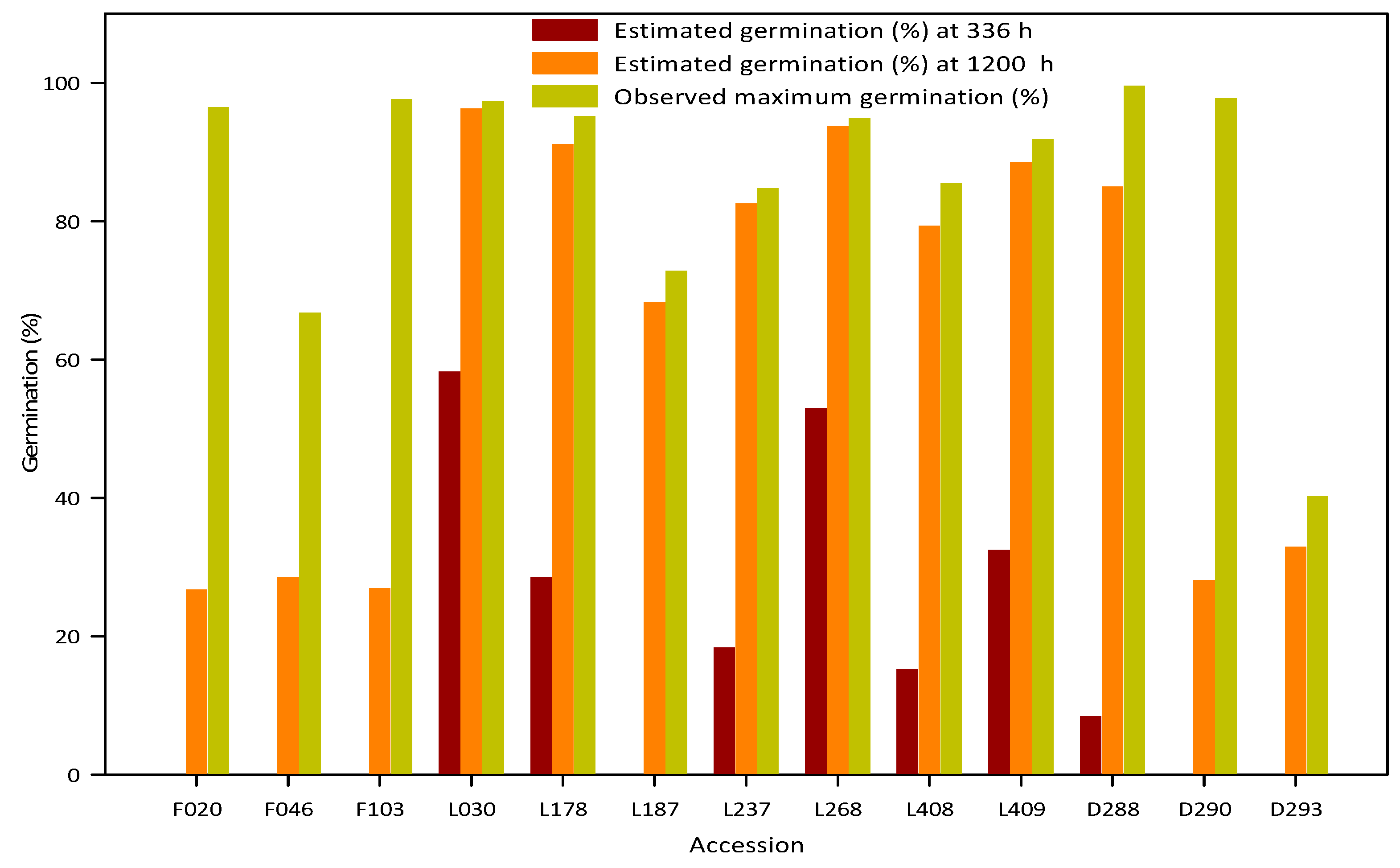
3.4. Potential Artefacts in Measuring Germinability
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bewley, J.D.; Black, M. Seeds: Physiology of Development and Germination; Springer: Boston, MA, USA, 1994; ISBN 978-0-306-44748-8. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination, 2nd ed.; Elsevier/AP: San Diego, CA, USA, 2014; ISBN 978-0-12-416677-6. [Google Scholar]
- ISTA Rules for Seed Testing; The International Seed Testing Association (ISTA) Zürichstr: Bassersdorf, Switzerland, 2015.
- Brändel, M. The Role of Temperature in the Regulation of Dormancy and Germination of Two Related Summer-Annual Mudflat Species. Aquat. Bot. 2004, 79, 15–32. [Google Scholar] [CrossRef]
- Ghaleb, W.; Ahmed, L.Q.; Escobar-Gutiérrez, A.J.; Julier, B. The History of Domestication and Selection of Lucerne: A New Perspective from the Genetic Diversity for Seed Germination in Response to Temperature and Scarification. Front. Plant Sci. 2021, 11, 578121. [Google Scholar] [CrossRef] [PubMed]
- McGinnies, W.J. Effects of Moisture Stress and Temperature on Germination of Six Range Grasses. Agron. J. 1960, 52, 159–162. [Google Scholar] [CrossRef]
- Ahmed, L.Q.; Escobar-Gutiérrez, A.J. Tall Fescue (Festuca arundinacea Schreb.) Shows Intraspecific Variability in Response to Temperature during Germination. Agronomy 2022, 12, 1245. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy; Springer: New York, NY, USA, 2013; ISBN 978-1-4614-4692-7. [Google Scholar]
- Ahmed, L.Q.; Escobar-Gutiérrez, A.J. Unexpected Intraspecific Variability of Perennial Ryegrass (Lolium perenne, L.) in Response to Constant Temperature during Germination and Initial Heterotrophic Growth. Front. Plant Sci. Plant Breed. 2022, 13, 856099. [Google Scholar] [CrossRef] [PubMed]
- Copeland, L.O.; McDonald, M.B. Principles of Seed Science and Technology, 4th ed.; Kluwer Academic Publishers: Boston, MA, USA, 2001; ISBN 978-0-7923-7322-3. [Google Scholar]
- Sachs, J. Die Abhängigkeit de Keimgung von Der Temperatur. Jahrb. Für Wiss. Bot. 1860, 2, 338–377. [Google Scholar]
- Yan, W.; Hunt, L.A. An Equation for Modelling the Temperature Response of Plants Using Only the Cardinal Temperatures. Ann. Bot. 1999, 84, 607–614. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.B.; Xu, L.Y.; Jin, X.Q.; Chen, J.H.; Lu, H.F. Effect of Temperature Regime on Germination of Seed of Perennial Ryegrass (Lolium Perenne). Grass Forage Sci. 2008, 63, 249–256. [Google Scholar] [CrossRef]
- Lu, H.; Shen, J.; Jin, X.; Hannaway, D.B.; Daly, C.; Halbleib, M.D. Determining Optimal Seeding Times for Tall Fescue Using Germination Studies and Spatial Climate Analysis. Agric. For. Meteorol. 2008, 148, 931–941. [Google Scholar] [CrossRef]
- Monks, D.P.; SadatAsilan, K.; Moot, D.J. Cardinal Temperatures and Thermal Time Requirements for Germination of Annual and Perennial Temperate Pasture Species. Agron. N. Z. 2009, 39, 95–109. [Google Scholar]
- Moot, D.; Scott, W.; Roy, A.; Nicholls, A. Base Temperature and Thermal Time Requirements for Germination and Emergence of Temperate Pasture Species. N. Z. J. Agric. Res. 2000, 43, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Larsen, S.U.; Bibby, B.M. Differences in Thermal Time Requirement for Germination of Three Turfgrass Species. Crop Sci. 2005, 45, 2030–2037. [Google Scholar] [CrossRef]
- Sharifiamina, S.; Moot, D.J.; Bloomberg, M. Calculating Hydrothermal Time to Quantify Seed Germination of Tall Fescue. J. N. Z. Grassl. 2016, 78, 163–168. [Google Scholar] [CrossRef]
- Nori, H.; Moot, D.; Black, A. Thermal time requirements for germination of four annual clover species. N. Z. J. Agric. Res. 2014, 57, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Palazzo, A.J.; Brar, G.S. The Effects of Temperature on Germination of Eleven Festuca Cultivars; DTIC: Washington, DC, USA, 1997; pp. 1–11. [Google Scholar]
- Lonati, M.; Moot, D.J.; Aceto, P.; Cavallero, A.; Lucas, R.J. Thermal Time Requirements for Germination, Emergence and Seedling Development of Adventive Legume and Grass Species. N. Z. J. Agric. Res. 2009, 52, 17–29. [Google Scholar] [CrossRef]
- Butler, T.J.; Celen, A.E.; Webb, S.L.; Krstic, D.; Interrante, S.M. Temperature Affects the Germination of Forage Legume Seeds. Crop Sci. 2014, 54, 2846. [Google Scholar] [CrossRef] [Green Version]
- Butler, T.J.; Celen, A.E.; Webb, S.L.; Krstic, D.B.; Interrante, S.M. Germination in Cool-Season Forage Grasses under a Range of Temperatures. Crop Sci. 2017, 57, 1725–1731. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; McGill, C.R.; Irving, L.J.; Kemp, P.D.; Zhou, D. A Modified Thermal Time Model to Predict Germination Rate of Ryegrass and Tall Fescue at Constant Temperatures. Crop Sci. 2013, 53, 240–249. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Ahmed, L.Q.; Durand, J.-L.; Escobar-Gutiérrez, A.J. Genetic Diversity of Alfalfa (Medicago Sativa) in Response to Temperature during Germination. Seed Sci. Technol. 2019, 47, 351–356. [Google Scholar] [CrossRef]
- Andreucci, M.P.; Moot, D.J.; Black, A.D.; Sedcole, R. A Comparison of Cardinal Temperatures Estimated by Linear and Nonlinear Models for Germination and Bulb Growth of Forage Brassicas. Eur. J. Agron. 2016, 81, 52–63. [Google Scholar] [CrossRef]
- Acosta, Y.; Santiago, F.; Escalante, D.; Mazorra, C.; Cejas, I.; Martínez-Montero, M.E.; Escobar, A.; Sershen; Hajari, E.; Lorenzo, J.C.; et al. Cryo-Exposure of Neonotonia Wightii Wigth and Am Seeds Enhances Field Performance of Plants. Acta Physiol. Plant. 2020, 42, 1–6. [Google Scholar] [CrossRef]
- Inda, L.A.; Sanmartín, I.; Buerki, S.; Catalán, P. Mediterranean Origin and Miocene–Holocene Old World Diversification of Meadow Fescues and Ryegrasses (Festuca Subgenus Schedonorus and Lolium). J. Biogeogr. 2014, 41, 600–614. [Google Scholar] [CrossRef]
- Blanco-Pastor, J.L.; Manel, S.; Barre, P.; Roschanski, A.M.; Willner, E.; Dehmer, K.J.; Hegarty, M.; Muylle, H.; Ruttink, T.; Roldán-Ruiz, I.; et al. Pleistocene Climate Changes, and Not Agricultural Spread, Accounts for Range Expansion and Admixture in the Dominant Grassland Species Lolium Perenne L. J. Biogeogr. 2019, 46, 1451–1465. [Google Scholar] [CrossRef]
- Thomson, P.A. Characterization of the Germination Response to Temperature of Species and Ecotypes. Nature 1970, 225, 827–831. [Google Scholar] [CrossRef]

| Reference | Species | Temperature Conditions | Temperature Range (°C) | Reported Germinability (%) | Reported Germination Rate | Following-Up Period (day) |
|---|---|---|---|---|---|---|
| Shen et al., 2008 [13] | Perennial ryegrass | Constant and alternating | 5–40 | Yes | No | 7–14 |
| Lu et al., 2008 [14] | Tall fescue | Constant and alternating | 5–40 | Yes | No | 7–14 |
| Monks et al., 2009 [15] | Annual and perennial temperate pasture species | Constant | 5–35 | Yes | Yes | 19 |
| Moot et al., 2000 [16] | White clover, Perennial ryegrass | Constant | 5–30 | Yes | Yes | 36 |
| Larsen and Bibby, 2005 [17] | Perennial ryegrass, Red fescue, Kentucky bluegrass | Constant | 8–24 | Yes | Yes | No germination for 3 days |
| Sharifiamina et al., 2016 [18] | Tall fescue | Constant | 5–32.5 | Yes | Yes | 50 |
| Nori et al., 2014 [19] | Annual clover species | Constant | 5–30 | Yes | Yes | 35 * |
| Palazzo and Brar, 1997 [20] | Festuca spp. | Constant | 10–30 | Yes | Yes | 28 |
| Lonati et al., 2009 [21] | Legume and grass species | Constant | 5–35 | Yes | Yes | 19 |
| Butler et al., 2014 [22] | Forage Legumes | Constant | 5–35 | Yes | Yes | 14 |
| Butler et al., 2017 [23] | Cool-Season forage grasses | Constant | 5–35 | Yes | Yes | 14 |
| Zhang et al., 2013 [24] | Ryegrass, Tall fescue | Constant | 6–43 | Yes | Yes | 44 |
| Species | Accession | ID Sample | Type | Harvest Year | Geographic Coordinates | Country | |
|---|---|---|---|---|---|---|---|
| Latitude | Longitude | ||||||
| Festuca arundinacea | 20 | DSVFa5 | Variety | Unknown | France | ||
| 46 | 500588B1 | Wild | 1988 | 35.9 | 8.9 | Tunisia | |
| 103 | 492991B2 | Wild | 1991 | 43.58333333 | 5 | France | |
| Lolium perenne | 30 | LP TGF 5 | Variety | 2015 | France | ||
| 178 | GR 3477 | Wild | 2004 | 54.32111 | 13.53194 | Germany | |
| 187 | GR 5030 | Wild | 1995 | 50.86944 | 13.73028 | Germany | |
| 237 | GR 6346 | Wild | 2002 | 45.05 | 15.1 | Croatia | |
| 268 | GR 12054 | Wild | 2005 | 51.57611 | −9.23444 | Ireland | |
| 408 | 2690??B1 | Wild | Unknown | 43.57152 | 1.787844 | France | |
| 409 | 2690??B2 | Wild | Unknown | 43.57152 | 1.787844 | France | |
| Dactylis glomerata | 288 | NGB 2735,1 | Wild | Unknown | 59.839704 | 11.474738 | Norway |
| 290 | NGB 15465,2 | Wild | Unknown | 56.511235 | 8.687534 | Denmark | |
| 293 | PORTO | variety | Unknown | 41.16 | 8.63 | Portugal | |
| Triticum aestivum | T312 | PB109C001-BC-DH33 | Variety | Unknown | Australia | ||
| T313 | PB109C001-BC-DH1 | Variety | Unknown | Australia | |||
| Species | Accession | Treatment | Final Germination Percentage (%) | Germination Start Time, tc (h) | Maximum Germination Rate, α (Proportion of Seed.h−1) | Time to 50% of Final Germination (h) | Time to 95% of Final Germination (h) | Time Last Seed Germinated (h) | Time of Last Count (h) |
|---|---|---|---|---|---|---|---|---|---|
| Festuca arundinacea | F020 | 5 °C | 97 a | 509 a | 0.05 a | 1471 a | 2344 a | 6545 | 7365 |
| 21 °C | 97 a | 40 b | 1.99 b | 68 b | 110 b | 287 | 932 | ||
| F046 | 5 °C | 56 a | 498 a | 0.06 a | 1147 a | 3281 a | 5849 | 7365 | |
| 21 °C | 64 a | 44 b | 0.44 b | 138 b | 483 b | 930 | 1462 | ||
| F103 | 5 °C | 97 a | 587 a | 0.06 a | 1393 a | 2276 a | 5517 | 7365 | |
| 21 °C | 94 a | 40 b | 0.69 b | 120 b | 295 b | 1097 | 1462 | ||
| Lolium perenne | L030 | 5 °C | 94 a | N A | 0.18 a | 137 a | 489 a | 1001 | 4554 |
| 21 °C | 96 a | N A | 1.49 b | 35 b | 73 b | 190 | 932 | ||
| L178 | 5 °C | 85 a | 58 a | 0.22 a | 299 a | 932 a | 3687 | 5517 | |
| 21 °C | 91 a | 23 a | 1.88 b | 53 b | 117 b | 328 | 932 | ||
| L187 | 5 °C | 64 b | 265 a | 0.22 a | 514 a | 1166 a | 5346 | 7365 | |
| 21 °C | 97 a | 35 b | 1.88 b | 62 b | 96 b | 244 | 910 | ||
| L237 | 5 °C | 81 b | 42 a | 0.16 a | 329 a | 794 a | 837 | 5850 | |
| 21 °C | 92 a | 25 a | 1.83 b | 56 b | 106 b | 270 | 1179 | ||
| L268 | 5 °C | 92 a | N A | N A | 135 a | 524 a | 793 | 4035 | |
| 21 °C | 98 a | N A | N A | 43 b | 80 b | 252 | 917 | ||
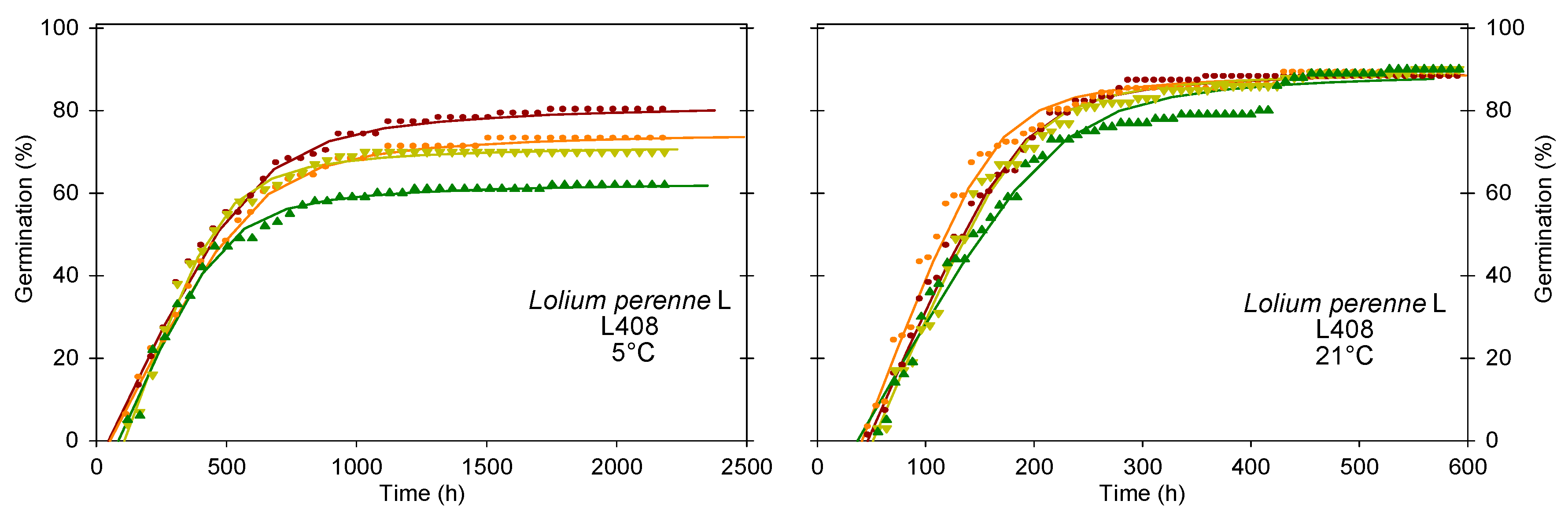
| L408 | 5 °C | 71 b | 74 a | 0.17 a | 350 a | 1261 a | 1844 | 4559 | |
| 21 °C | 90 a | 44 a | 0.66 b | 126 b | 333 b | 1920 | 2352 | ||
| L409 | 5 °C | 77 a | 48 a | 0.28 a | 225 a | 823 a | 1704 | 2184 | |
| 21 °C | 84 a | 44 a | 1.09 b | 97 b | 273 b | 664 | 1436 | ||
| Dactylis glomerata | D288 | 5 °C | 93 a | 89 a | 0.15 a | 549 a | 1461 a | 357 | 4559 |
| 21 °C | 90 a | 36 a | 0.71 b | 114 b | 263 b | 524 | 1170 | ||
| D290 | 5 °C | 99 a | 479 a | 0.05 a | 1445 a | 2319 a | 357 | 4559 | |
| 21 °C | 96 a | 37 b | 1.07 b | 85 b | 156 b | 888 | 932 | ||
| D293 | 5 °C | 36 a | 364 a | 0.06 a | 717 a | 1625 a | 448 | 4559 | |
| 21 °C | 40 a | 30 b | 0.51 b | 79 b | 222 b | 608 | 1462 | ||
| Triticum aestivum | T312 | 5 °C | 99 a | N A | N A | 211 a | 282 a | 409 | 2184 |
| 21 °C | 97 a | N A | N A | 32 b | 62 b | 236 | 952 | ||
| T313 | 5 °C | 98 a | N A | N A | 209 b | 254 a | 409 | 2184 | |
| 21 °C | 95 a | N A | N A | 24 b | 63 b | 236 | 952 |
| Species | Accession | Ratio of | Ratio of | Ratio of | Ratio of | Ratio of |
|---|---|---|---|---|---|---|
| Festuca arundinacea | F020 | 39.8 | 12.73 | 21.63 | 21.31 | 27.68 |
| F046 | 7.3 a | 11.32 a | 8.31 a | 6.79 a | 6.90 a | |
| F103 | 11.5 | 14.7 | 11.61 | 7.72 | 10.08 | |
| Lolium perenne | L030 | 8.3 | NA | 3.29 | 6.70 | NA |
| L178 | 8.6 | 2.52 | 5.64 | 7.97 | 8.03 | |
| L187 | 8.6 | 7.57 | 8.29 | 12.15 | 9.22 | |
| L237 | 11.4 a | 1.68 b | 5.88 b | 7.49 a | 9.26 a | |
| L268 | NA | NA | 3.14 | 6.55 | NA | |
| L408 | 3.9 | 1.68 | 2.78 | 3.79 | 3.37 | |
| L409 | 3.9 | 1.1 | 2.32 | 3.01 | 4.32 | |
| Dactylis glomerata | D288 | 4.7 | 2.47 | 4.82 | 5.56 | 5.90 |
| D290 | 21.4 a | 12.95 ab | 12.0 ab | 14.87 a | 20.13 a | |
| D293 | 8.5 | 12.13 | 9.08 | 7.32 | 7.20 | |
| Triticum aestivum | T312 | NA | NA | 6.59 ab | 4.55 a | NA |
| T313 | NA | NA | 8.71 | 4.03 | NA |
| Germinability (High or Low) | Germination Rate (Fast or Slow) | Temperature-Insensitive Germinability | Germinability Varying between 5 °C and 21 °C |
|---|---|---|---|
| High | Fast | Type 1 F020 | Type 5 L187 |
| L030 | L237 | ||
| L178 | |||
| L268 | |||
| D290 | |||
| T312 | |||
| T313 | |||
| Slow | Type 2 F103 | Type 6 L408 | |
| D288 | |||
| Low | Fast | Type 3 D293 | Type 7 |
| Slow | Type 4 F046 | Type 8 | |
| L409 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghaleb, W.; Ahmed, L.Q.; Wagner, M.-H.; Eprinchard-Ciesla, A.; Olivares-Rodríguez, W.E.; Perrot, C.; Chenu, K.; Norton, M.; Escobar-Gutiérrez, A.J. The Concepts of Seed Germination Rate and Germinability: A Re-Evaluation for Cool-Season Grasses. Agronomy 2022, 12, 1291. https://doi.org/10.3390/agronomy12061291
Ghaleb W, Ahmed LQ, Wagner M-H, Eprinchard-Ciesla A, Olivares-Rodríguez WE, Perrot C, Chenu K, Norton M, Escobar-Gutiérrez AJ. The Concepts of Seed Germination Rate and Germinability: A Re-Evaluation for Cool-Season Grasses. Agronomy. 2022; 12(6):1291. https://doi.org/10.3390/agronomy12061291
Chicago/Turabian StyleGhaleb, Wagdi, Lina Q. Ahmed, Marie-Hélène Wagner, Annie Eprinchard-Ciesla, Wendy E. Olivares-Rodríguez, Cédric Perrot, Karine Chenu, Mark Norton, and Abraham J. Escobar-Gutiérrez. 2022. "The Concepts of Seed Germination Rate and Germinability: A Re-Evaluation for Cool-Season Grasses" Agronomy 12, no. 6: 1291. https://doi.org/10.3390/agronomy12061291
APA StyleGhaleb, W., Ahmed, L. Q., Wagner, M.-H., Eprinchard-Ciesla, A., Olivares-Rodríguez, W. E., Perrot, C., Chenu, K., Norton, M., & Escobar-Gutiérrez, A. J. (2022). The Concepts of Seed Germination Rate and Germinability: A Re-Evaluation for Cool-Season Grasses. Agronomy, 12(6), 1291. https://doi.org/10.3390/agronomy12061291







