Cucurbitaceous Vegetables’ Gummy Stem Blight Research
Abstract
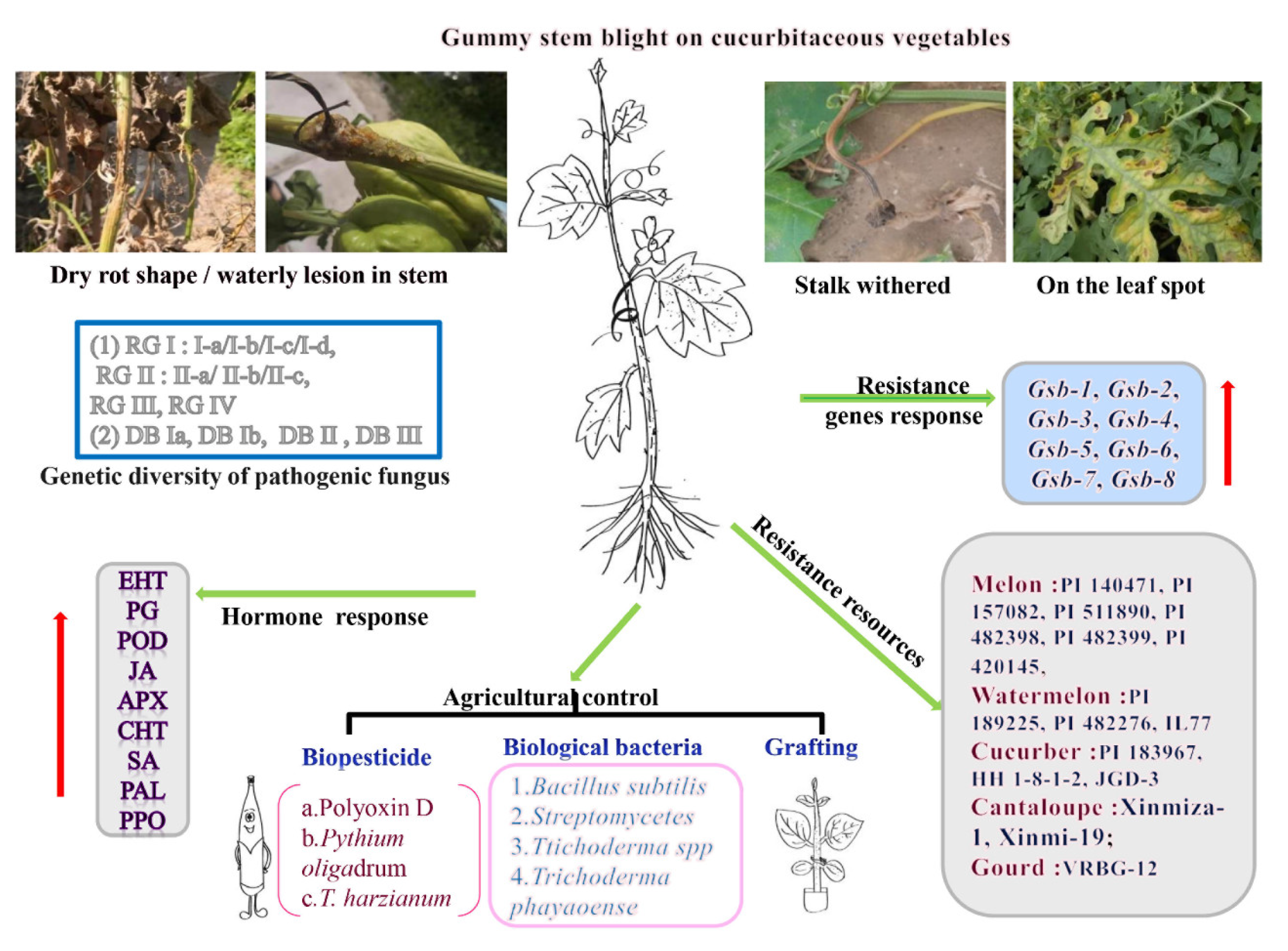
1. Introduction
2. Pathogenic Fungus Profile
2.1. Acute Symptoms
| Variety | Signs of Plants | Symptom |
|---|---|---|
| Cucumber (C. sativus) | Irregular dark brown lesions at the leaf margin [11] | Aerial mycelium (white becoming gray with age), submerged mycelium (dark olive to black) [34]; Pycnidia (sporulation aparse), perithecia (black bodies) [11]. |
| Muskmelon (C. melo) | Dry rot in stems (white) [34], leaf spots and dry rot in petiols (dark brown) [35]. | Produced a conidial mass with a white aerial mycelium at The center of colony, few pycnidia were observed [34,35]. |
| Watermelon (C. lanatus) | Angular water-soaked lesions, defoliation, dry, stem necrosis, gummy exudates, wilt and eventual death [11]; Leaf spots (dark brown) [35]. | White aerial and olivaceous mycelium, and olive to dark green or black substrate mycelium [11]; The colony surface was rough and undulated, the conidia were round-ended, cylindrical, monoseptate, and hyaline [35]. |
| Pumpkin (Cucurbita maxima) | - | Aerial mycelium (usually absent, very scanty), submerged mycelium (hyaline to brown), pycnidia (sporulation heavy), perithecia (no sporulation) [33]; Type I to type VII, type IV was the most predominant type (mostly white, a few for brown or yellow) [28]. |
| Gherkin cucumber (C. sativus) | Leaf spots (tan to blackish-brown), dry rot in stems (brown gummy exudate), brown lesions in fruits (black rot) [36]. | Conidia were hyaline, cylindrical with rounded ends, and non- or mono-septate; Ascospores were hyaline and monoseptate with two cells of differing sizes [36]. |
| Squash (Cucurbita digitata) | Irregular dark brown lesions at the leaf margin [11]. | - |
| Mellon (C. melo) | Leaf spots (brown), cankers (light brown to off-white) [11]. | Angular water-soaked lesions; the fungal showed white aerial and olivaceous mycelium, and olive to dark green or black substrate mycelium; the conidia were round-ended, cylindrical, monoseptate, and hyaline [11]. |
| Cantaloupe (C. melo var cantaloupe) | Black rot (black) and fruit decay [37]. | A brown discoloration of the net; Pycnidia and pseudothecia were not produced in black rot lesions on cantaloupe fruit [37]. |
2.2. Biological Characteristics of Pathogenic Fungus
2.3. Genetic Diversity of Pathogenic Fungus
2.4. Differentiation of Physiological Race
2.5. Genomic Characteristics
| Variety | Genetic Characteristics | References |
|---|---|---|
| Cucubits | Two to four amplified fragments were unique to all 27 isolated bacterium, 13 additional fragments were present in all D. bryoniae; | [31] |
| RG I group with a single band of 650 bp fragment while RG II group with about a 1.4 kb fragment. | [51] | |
| Muskmelon (C. melo) | The similarity in sequence identity between the rDNA ITS region was 100% and 95.0%; Nucleotide sequences of the rDNA ITS reform BLAST search from pure culture ranged from 98.2% to 99.8%; | [34] |
| Two isolates possessed a single nucleotide substitution of A to G at position 131 of the ITS 1 region. | [35] | |
| Watermelon (C. lanatus) | The isolates produced fragment sizes of approximately 120, 780, and 560 bp; | [11] |
| Two isolates possessed a single nucleotide substitution of A to G at position 131 of the ITS 1 region. | [35,57] | |
| Pumpkin (C. maxima) | Two motifs contained sequence variations unique to two groups: Type A (exhibited high similarity with one another, a typical dominant physiological Cucurbita GSB fungal group) and Type B (variant genotypic offshoots with the farthest genetic distance). | [28] |
| Melon (C. melo) | Ace 2 of Sphaerorheeafuliginea in C. melo PI 12411l conferred by an incompletely dominant gene. | [65] |
3. Resistance Breeding and Resistance Mechanism
3.1. Identification of Germplasm Resistant to Disease
| Cucurbits Species | Resistant Strains | References |
|---|---|---|
| Melon (C. melo) | PI 140471, PI 266935, PI 296345, PI 436533; | [82] |
| Pl 124111; | [65] | |
| PI 266935, PI 266934; | [83] | |
| PI 157084, PI 482399, PI 157082, PI 157080, PI 482393, PI 482402, PI 157076, PI 482408, PI 482403, PI 255478, PI 511890; | [84] | |
| MEX-2, KOR-33, JMu-15. | [85] | |
| PI 157082, PI 482398, PI 511890, PI 482399, PI 140471; | [76] | |
| PI 157076, PI 420145, PI 323498, PI 255478, PI 420147, PI 618834, PI 200819, PI 482409, PI 482402, PI 164797, PI 436534, PI 200819, PI 321004, PI 470253, PI 185111, PI 511890, PI 435345, Ames 26707; | [77] | |
| Perlita Busle S1, Valenciano Elíptico, Glaver, MR1, 2526; | [86] | |
| Charentais From 1, AC-29, C160, PI420145, PI482398, PI532830; | [78] | |
| Baipicui. | [87] | |
| Watermelon (C. lanatus) | PI 271778; | [75] |
| All-golden producer, All-sweet scarlet, A56, H25; | [88] | |
| PI 279461, PI 482379, PI 254744, PI 526233, PI 482276, PI 271771, PI 164248, PI 244019, PI 296322, PI 490383; | [89] | |
| PI 279461, PI 254744, PI 482379, PI 244019, PI 526233, PI 482276, PI 164248, PI 482284, PI 296332, PI 490383, PI 271771, PI 379243; | [90] | |
| Au-producer, All- sweet scarlet, Au-Jubilant, Sugarlee, SSDL; | [91] | |
| Smokylee, Summit, Sugarlee, Calhoun Gray, Dixie lee, Texa W5, Conqueror; | [92] | |
| M2K, L8K, M3SK, L3K; | [77] | |
| Swmx-001E-PL#13-01, WMX-001E-PL#04-01, WMX-001E-PL#02-02. | [93] | |
| Cucumber (C. sativus) | Leningradsky, Wjarnikovsky, Rheinische Vorgebirge, PI 200818, PI 339241; | [79] |
| Homegreen #2, PI 200818; | [80] | |
| PI 164433, PI 390264, Slice, M12, M17; | [67] | |
| Transamerica, Homegreen#2, Poinsett76, AR79-75, PI 390243, PI200815, PI 432855, PI 279469, LJ 90430; | [81] | |
| Sour cucumber, Chaoyou-3, HH1-8-57, HH1-8-2; | [94] | |
| HH1-8-1-2, HH1-8-5, HH1-8-1-16. | [95] | |
| Cantaloupe (C. melo var. saccharinus) | Xinmiza-1, Xinmi-19. | [96] |
| Gourd (Lagenaria siceraria (Mol.) Standl.) | BARI Lau-1, BARI Lau-2; | [18] |
| VRBG-12. | [97] |
3.2. Molecular Technique on Resistance of Breeding
3.3. The Inheritance of Resistance
3.4. Physiology and Biochemistry of GSB Resistance
4. Gene(s)/Genome Information of Resistance Mechanism
4.1. Resistance Genes
| Resistant Variety | Gene(s)/QTLs/Cucurbits Species | Genetic Diversity |
|---|---|---|
| Melon (C. melo) | Gsb-1 (from PI 140471) [76,87,109]; | A linkage distance of 5.2 cM to Gsb-1 [109]; |
| Gsb-2 (from PI 157082) [76,110]; | the linkage distance was 11.3 cM [110]; | |
| Gsb-3 (from PI 511890) [76,111]; | The gene distance between ISSR-100 and Gsb-3 is 8.3 cM [111]; | |
| Gsb-4 (from PI 482398) [76,85,112,113]; | the genetic distance between CMTA170a and Gsb-4 was 5.14 cM [112]; | |
| gsb-5 (from PI 482399) [76]; | - | |
| Gsb-6 (from PI 420145) [87,113,114]; | GSB resistance gene at a distance of 2.0cM [114]; | |
| NBS-LRR (from PI 482399) [115]; | ch 9, the first intron of MELO3C022157 linked to GSB resistance [115]; | |
| Sb-x (from 4G21) [116]; | linkage group LG1, the genetic distances were 2 cM and 3 cM respectively [116]; | |
| edr2 [117]; | T-DNA inserted in its genome [117]; | |
| PAL (from PI 420145× PI 140471) [106,118]; | - | |
| CHT [106]; | - | |
| Mc (from PI 140471) [119]; | - | |
| APX [105,106]. | - | |
| Cucurber (C. sativus) | HH1-8-1-2 (susceptible 8419) [95]; | 11 cM covering 1.299 Mbp; 12 cM spanning 3.569 Mbp with phenotypic variations of 8.7 [95]; |
| gsb-s1.1, gsbs2.1, gsb-s6.1, gsb-s6.2, gsb-s6.3 [101]; | gsb-s6.2 accounted for the highest phenotypic variation [101]; | |
| gsb3.1, gsb3.2, gsb3.3, gsb4.1, gsb5.1, gsb6.1 (From PI 183967) [120]; | Chr3, Chr4, Chr5, Chr6; Locus gsb5.1 accounted for the highest phenotypic variation (17.9%) [120]; | |
| GSB4-1, GSB4-2, GSB4-3 (PH1-8-1-2) [121]; | Chr4; The physical distance between the two markers for 375.89 kb [121]; | |
| gsb1.1, gsb2.1, gsb3.1, gsb5.1, gsb6.1 (JGD-3) [122]; | Chr1, Chr2, Chr3, Chr5, Chr6; characteristic of quantitative character inheritance, controlling by the poly-genes [122]; | |
| Csa1G65487 (‘IL77’) [123]. | Located in the 24.6-27.1 Mb [123]. | |
| Watermelon (C. slanatus) | Qgsb8.1 locus (from PI 189225) [124]; | One associated region spanning 5.7 Mb (Chr8: 10,358,659–16,101,517) [124]; |
| ClGSB3.1, ClGSB5.1, ClGSB7.1 (PI 482276) [125]; | explaining between 6.4 and 21.1% of the phenotypic variation, ClGSB5.1 includes an NBS-LRR gene, Locus ClGSB7.1 accounted for the highest phenotypic variation [125]; | |
| qLL8.1, qSB8.1, qSB6.1 [126]; | Chr8 and Chr6, explaining10.5, 10.0% and 9.7% of the phenotypic variations [126]; | |
| NBS-encoding R [Cla001821(Chr1), Cla019863 (Chr2), Cla020705 (Chr5), Cla012430, Cla012433 and Cla012439 (Chr8), Cla001017 and Cla001019 (Chr8)] [127]. | - |
4.2. Genome
4.3. circRNA
5. Prevention Methods
5.1. Grafting
5.2. Biocontrol Bacterium
6. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keinath, A.P.; Duthie, J.A. Yield and quality reductions in watermelon due to anthracnose, gummy stem blight, and black rot. Behav. Brain Sci. 1998, 36, 219–220. [Google Scholar]
- Sudisha, J.; Kumar, T.V.; Niranjana, S.R.; Shetty, H.S. First report of gummy stem blight caused by didymella bryoniae on muskmelon (cucumis melo) in India. Plant Pathol. 2004, 53, 533. [Google Scholar] [CrossRef]
- Katoch, A.; Kapoor, P.; Singh, A. The emergence of gummy stem blight: A threat to Cucurbits in India. Agrica 2017, 6, 1–6. [Google Scholar] [CrossRef]
- Nuangmek, W.; Aiduang, W.; Suwannarach, N.; Kumla, J.; Lumyong, S. First report of gummy stem blight caused by Stagonosporopsiscucurbitacearum on cantaloupe in Thailand. Can. J. Plant Pathol. 2018, 40, 306–311. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Chen, J.F. First report of Didymella bryoniae causing gummy stem blight of chayote in Taiwan. Plant Dis. 2012, 96, 1578. [Google Scholar] [CrossRef]
- Koike, S.T. First report of gummy stem blight, caused by Didymella bryoniae, on watermelon transplants in California. Plant Dis. 1997, 81, 1331. [Google Scholar] [CrossRef]
- Bala, G.; Hosein, F. Studies on gummy stem blight disease of Cucurbits in Trinidad. Trop. Agric. 1986, 63, 195–197. [Google Scholar]
- Santos, G.R.; Café-Filho, A.C. Occurrence of gummy stem blight in watermelon in the state of Tocantins caused by Didymella bryoniae. Fitopatol. Bras. 2014, 31, 208. [Google Scholar] [CrossRef][Green Version]
- El-Wakil, A.F.; Khalil, A. First report of pycnidial stage of Didymellabryoniae, the causal fungus of gummy stem blight on cantaloupe (Cucumis melo var cantalupensis) in Egypt. Egypt. J. Phytopathol. 2016, 44, 231–232. [Google Scholar] [CrossRef]
- Jensen, B.D.; Massawe, A.; Swai, I.S. First Report of Gummy Stem Blight Caused by Didymellabryoniae on Watermelon and Confirmation of the Disease on Pumpkin in Tanzania. Plant Dis. 2011, 95, 768. [Google Scholar] [CrossRef] [PubMed]
- Basim, E.; Basim, H.; Abdulai, M.; Baki, D.; Ozturk, N. Identification and characterization of Didymellabryoniae causing gummy stem blight disease of watermelon (Citrullus lanatus) in Turkey. Crop Prot. 2016, 90, 150–156. [Google Scholar] [CrossRef]
- Boughalleb, N.; Mahjoub, M.E.; Abad-Campos, P.; Pérez-Sierra, A.; Armengol, J. First report of gummy stem blight caused by Didymellabryoniae on grafted watermelon in Tunisia. Plant Dis. 2007, 91, 468. [Google Scholar] [CrossRef]
- Al-Jubouri, F.; Hussain, H.Z. The first recording of gummy stem blight disease caused by Didymellabryoniae (Stagonosporopsiscucurbitacearum) on watermelon crop in Iraq. Ann. Trop. Med. Public Health 2020, 23, e231619. [Google Scholar] [CrossRef]
- Keinath, A.P. From native plants in central Europe to cultivated crops worldwide: The emergence of Didymellabryoniae as a cucurbit pathogen. HortScience 2011, 46, 532–535. [Google Scholar] [CrossRef]
- Lee, D.H.; Mathur, S.B.; Neergaard, P. Detection and location of seed-borne inoculum of Didymellabryoniae and its transmission in seedlings of cucumber and pumpkin. J. Phytopathol. 1984, 109, 301–308. [Google Scholar] [CrossRef]
- Grube, M.; Fürnkranz, M.; Zitzenbacher, S.; Huss, H.; Berg, G. Emerging multi-pathogen disease caused by Didymella bryoniae and pathogenic bacteria on Styrian oil pumpkin. Eur. J. Plant Pathol. 2011, 131, 539–548. [Google Scholar] [CrossRef]
- Cho, M.; Cheoul, P.; Hyo, G.; Kim, H.; Tae, K. Spore production and inoculation methods for resistance screening to gummy stem blight (Mycosphaerellamelonis) in muskmelon seedlings. Hortic. Environ. Biotechnol. 1997, 38, 364–367. [Google Scholar]
- Afroz, M.; Rahman, M.A.; Nahar, M.S.; Yasmin, L.; Alam, S.M.K. Selection of resistant variety, effective organic amendment and fungicide against gummy stem blight (Didymella bryoniae) of bottle gourd. Diss. Saarc J. Agric. 2012, 10, 1–8. [Google Scholar]
- Arny, C.J.; Rowe, R.C. Effects of temperature and duration of surface wetness on spore production and infection of cucumbers by Didymella bryoniae. Phytopathology 1991, 81, 206–209. [Google Scholar] [CrossRef]
- Keinath A, P. Survival of Didymella bryoniae in buried watermelon vines in South Carolina. Plant Dis. 2002, 86, 32–38. [Google Scholar] [CrossRef][Green Version]
- Keinath A, P. Diagnostic guide for gummy stem blight and black rot on Cucurbits. Plant Health Prog. 2013, 14, 35–44. [Google Scholar] [CrossRef]
- Thinggaard, K. Attack of Didymellabryoniae on roots of cucumber. J. Phytopathol. 1987, 120, 372–375. [Google Scholar] [CrossRef]
- Steekelenburg, N. Epidemiological aspects of Didymellabryoniae, the cause of stem and fruit rot of cucumber. Neth. J. Plant Pathol. 1983, 89, 75–86. [Google Scholar] [CrossRef]
- Café-Filho, A.C.; Santos, G.R.; Laranjeira, F.F. Temporal and spatial dynamics of watermelon gummstem blight epidemics. Eur. J. Plant Pathol. 2010, 128, 473–482. [Google Scholar] [CrossRef]
- Brewer, M.T.; Rath, M.; Li, H.X. Genetic diversity and population structure of cucurbit gummy stem blight fungi based on microsatellite markers. Phytopathology 2015, 105, 815–824. [Google Scholar] [CrossRef]
- Li, H.-X.; Brewer, M.T. Spatial genetic structure and population dynamics of gummy stem blight fungi within and among watermelon fields in the southeastern United States. Phytopathology 2016, 106, 900–908. [Google Scholar] [CrossRef]
- Sun, X.-X.; You, C.; Gu, W.-Z.; Wei, Y.-H. Effect of different cultivation methods on gummy stem blight, anthracnose and yield of watermelon. China Veg. 2016, 29, 24–26, 30, (In Chinese with English Title and Abstract). [Google Scholar]
- Zhao, Q.; Wu, J.-Z.; Zhang, L.-Y.; Xu, L.-Z.; Yan, C.; Gong, Z.-P. Identification and characterization of cucurbita gummy stem blight fungi in Northeast China. J. Phytopathol. 2017, 166, 305–313. [Google Scholar] [CrossRef]
- Ren, Q.-Q.; Lu, X.-M.; Ke, S.-J.; Qian, C.-T. In vitro leaf inoculation combing with molecular marker selection to identify extreme resistance and susceptibility of melon to gummy stem blight. China Veg. 2019, 32, 13–16, (In Chinese with English Title and Abstract). [Google Scholar]
- Liu, S.-L.; Gu, X.-F.; Shi, Y.-X.; Li, B.-J.; Miao, H.; Wang, M.; Wang, Y.; Zhang, S.-P. General research situation on gummy stem blight of cucurbitacae vegetables. China Veg. 2013, 18, 1–10, (In Chinese with English Title and Abstract). [Google Scholar]
- Keinath, A.P.; Farnham, M.W.; Zitter, T.A. Morphological, pathological, and genetic differentiation of Didymella bryoniae and Phoma spp. isolated from Cucurbits. Phytopathology 1995, 85, 364–369. [Google Scholar] [CrossRef]
- Gusmini, G.; Ellington, T.L.; Wehner, T.C. Mass production of gummy stem blight spores for resistance screening. Cucurbit Genet. Coop. Rep. 2003, 26, 26–30. [Google Scholar]
- Lee, D.H. Morphological and cultural characters of Didymellabryoniae on seeds and culture media. Korean J. Mycol. 1982, 10, 7–15. [Google Scholar]
- Choi, I.Y.; Choi, J.N.; Choi, D.C.; Sharma, P.K.; Lee, W.H. Identification and characterization of the causal organism of gummy stem blight in the muskmelon (Cucumis melo L.). Mycobiology 2010, 38, 166–170. [Google Scholar] [CrossRef][Green Version]
- Li, P.-F.; Ren, R.-S.; Yao, X.-F.; Xu, J.-H.; Babu, B.; Paret, M.L.; Yang, X.-P. Identification and characterization of the causal agent of gummy stem blight from muskmelon and watermelon in East China. J. Phytopathol. 2014, 163, 314–319. [Google Scholar] [CrossRef]
- Garampalli, R.H.; Gapalkrishna, M.K.; Li, H.X.; Brewer, M.T. Two Stagonosporopsis species identified as causal agents of gummy stem blight epidemics of gherkin cucumber (Cucumis sativus) in Karnataka, India. Eur. J. Plant Pathol. 2016, 145, 507–512. [Google Scholar] [CrossRef]
- Zhang, J.-X.; Bruton, B.D.; Miller, M.E.; Isakeit, T. Relationship of developmental stage of cantaloupe fruit to black rot susceptibility and enzyme production by Didymellabryoniae. Plant Dis. 1999, 83, 1025–1032. [Google Scholar] [CrossRef]
- Furukawa, T.; Ono, Y.; Kishi, K. Gummy stem blight of balsam pear caused by Didymella bryoniae and its anamorph Phomacucurbitacearum. J. Gen. Plant Pathol. 2007, 73, 125–128. [Google Scholar] [CrossRef]
- Neergaard, E.D. Studies of Didymella bryoniae (Auersw.) Rehm: Development in the host. J. Phytopathol. 1989, 127, 107–115. [Google Scholar] [CrossRef]
- Keinath, A.P. Reproduction of Didymella bryoniae on nine species of Cucurbits under field conditions. Plant Dis. 2014, 98, 1379–1386. [Google Scholar] [CrossRef][Green Version]
- Mahapatra, S.; Rao, E.S.; Sandeepkumar, G.M.; Sriram, S. Stagonosporopsis cucurbitacearum the causal agent of gummy stem blight of watermelon in India. Australas. Plant Dis. Notes 2020, 15, 1–3. [Google Scholar] [CrossRef]
- Huang, C.-J.; Lai, Y.-R. First report of Stagonosporopsiscitrulli causing gummy stem blight of watermelon in Taiwan. J. Plant Pathol. 2018, 101, 417. [Google Scholar] [CrossRef]
- Stewart, J.E.; Turner, A.N.; Brewer, M.T. Evolutionary history and variation in host range of three Stagonosporopsis species causing gummy stem blight of Cucurbits. Fungal Biol. 2015, 119, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, A.-X.; Jiang, J.; Yang, X.-P.; Chen, H.-G. Identifiation of muskmelon gummy stem blight pathogen and its biological characters. Jiangsu J. Agric. Sci. 2008, 24, 148–152, (In Chinese with English Title and Abstract). [Google Scholar]
- Jia, J.-S.; Ma, D.-Y.; Zhang, L.; Fang, L. The new findings of the gourd crop gummy stem blight pathogene’s perfect stage in Xinjiang. Xinjiang Agric. Sci. 2003, 40, 303–304, (In Chinese with English Title and Abstract). [Google Scholar]
- Zhang, X.-J.; Wang, D.-M.; Yi, H.-P. Perfect stage identification of melon gummy stem blight pathogen in Hainan. China Veg. 2013, 6, 81–83, (In Chinese with English Title and Abstract). [Google Scholar]
- Li, H.-X.; Gottilla, T.M.; Brewer, M.T. Organization and evolution of mating-type genes in three Stagonosporopsis species causing gummy stem blight of Cucurbits and leaf spot and dry rot of papaya. Fungal Biol. 2017, 121, 849–857. [Google Scholar] [CrossRef]
- Corlett, M. A taxonomic survey of some species of Didymella and Didymella-like species. Can. J. Bot. 1981, 59, 2016–2042. [Google Scholar] [CrossRef]
- Somai, B.M.; Keinath, A.P.; Dean, R.A. Development of PCR-ELISA for detection and differentiation of Didymella bryoniae from related phoma species. Plant Dis. 2002, 86, 710–716. [Google Scholar] [CrossRef]
- Kothera, R.T.; Keinath, A.P.; Dean, R.A.; Farnham, M.W. AFLP analysis of a worldwide collection of Didymellabryoniae. Mycol. Res. 2003, 107, 297–304. [Google Scholar] [CrossRef][Green Version]
- Shim, C.K.; Seo, I.K.; Jee, H.J.; Kim, H.K. Genetic diversity of Didymellabryoniae for RAPD profiles substantiated by SCAR marker in Korea. Plant Pathol. J. 2006, 22, 36–45. [Google Scholar] [CrossRef][Green Version]
- Ling, K.-S.; Wechter, W.P.; Somai, B.M.; Walcot, R.R.; Keinath, A.P. An improved real-time PCR system for broad-spectrum detection of Didymellabryoniae, the causal agent of gummy stem blight of Cucurbits. Seed Sci. Technol. 2010, 38, 692–703. [Google Scholar] [CrossRef]
- Yao, X.-F.; Li, P.-F.; Xu, J.-H.; Zhang, M.; Ren, R.-S.; Liu, G.; Yang, X.-P. Rapid and sensitive detection of Didymella bryoniae by visual loop-mediated isothermal amplification assay. Front. Microbiol. 2016, 7, e1372. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aveskamp, M.M.; Gruyter, J.D.; Woudenberg, J.H.C.; Verkley1, G.J.M.; Crous, P.W. Highlights of the Didymellaceae: A polyphasic approach to characterize Phoma and related pleosporalean genera. Stud. Mycol. 2010, 65, 1–60. [Google Scholar] [CrossRef]
- Somai, B.M.; Dean, R.A.; Farnham, M.W.; Zitter, T.A.; Keinath, A.P. Internal transcribed spacer regions 1 and 2 and random amplified polymorphic DNA analysis of Didymellabryoniae and related Phoma species isolated from Cucurbits. Phytopathology 2002, 92, 997–1004. [Google Scholar] [CrossRef][Green Version]
- do Santos, G.R.; Ferreira, M.Á.S.V.; Pessoa-Filho, M.A.C.P.; Ferreiar, M.E.; Café-Filho, A.C. Host specificity and genetic diversity of Didymellabryoniae from Cucurbitaceae in Brazil. J. Phytopathol. 2009, 157, 265–273. [Google Scholar] [CrossRef]
- Babu, B.; Kefialew, Y.W.; Li, P.F.; Yang, X.P.; George, S.; Newberry, E.; Dufault, N.; Abate, D.; Ayalew, A.; Marois, J.; et al. Genetic characterization of Didymellabryoniae infecting watermelon and other Cucurbits in Florida and Georgia. Plant Dis. 2015, 99, 1488–1499. [Google Scholar] [CrossRef]
- Workneh, Y.K. Biorational Management of Postharvest Anthracnose on Tropical Fruits and Gummy Stem Blight on Cucurbits. Ph.D. Thesis, Addis Ababa University, Addis Ababa, Ethiopia, 2014. [Google Scholar]
- Amand, P.C.S.; Wehner, T.C. Eight isolates of Didymellabryoniae from geographically diverse areas exhibit variation in virulence but not isolate by cultivar interaction on Cucumis sativus. Plant Dis. 1995, 79, 1136–1139. [Google Scholar] [CrossRef]
- Hu, F.Y.; Mo, J.Y.; Wei, J.G.; Guo, T.X.; Huang, S.P.; Pan, C.B. Preliminary study of the variation of watermelon and melon gummy stem blight pathogen. China Veg. 2012, 25, 9–12, (In Chinese with English Title and Abstract). [Google Scholar]
- Zhang, J.-X.; Bruton, B.D.; Biles, C.L. Cell wall-degrading enzymes of Didymella bryoniae in relation to fungal growth and virulence in cantaloupe fruit. Eur. J. Plant Pathol. 2014, 139, 749–761. [Google Scholar] [CrossRef]
- Zhang, N.; Bi, Y.-F.; Guo, J.; Xu, B.-H.; Qian, C.-T.; Chen, J.-F. Changes of PAL, PPO and POD activites in melon with different resistances after inoculated with Didymellabryoniae. Plant Physiol. J. 2016, 52, 1169–1175, (In Chinese with English Title and Abstract). [Google Scholar]
- Zhou, X.-H.; Wolukau, J.N.; Li, Y.; Chen, J.-F. Relationships between activity changes of superoxide dismutases, catalase, peroxidase and resistance to gummy stem blight in melon. China Veg. 2007, 2, 4–6, (In Chinese with English Title and Abstract). [Google Scholar]
- Guo, Q.-W.; Wang, H.-Y.; Li, J.; Mbira, K.G.; Liu, J.; Chen, J.-F. Effects of BTH on activities of POD and PPO in resistant and susceptible melon seedlings challenged with gummy stem blight. China Veg. 2013, 26, 7–10, (In Chinese with English Title and Abstract). [Google Scholar]
- Cohen, S.; Cohen, Y. Genetics and nature of resistance to race 2 of Sphaerothecafuliginea in Cucumis melo PI 124111. Genetics 1986, 76, 1165–1167. [Google Scholar]
- Bi, Y.-F.; Xu, B.-H.; Qian, C.-T.; Chen, J.-F. Research progress in melon resistance breeding to gummy stem blight. China Veg. 2013, 20, 10–16, (In Chinese with English Title and Abstract). [Google Scholar]
- Wehner, T.C.; Amand, P.C.S. Field tests for cucumber resistance to gummy stem blight in North Carolina. HortScience 1993, 28, 327–329. [Google Scholar] [CrossRef]
- Amand, P.C.S.; Wehner, T.C. Greenhouse detached-leaf and field testing methods to determine cucumber resistance to gummy stem blight. J. Am. Soc. Hortic. Sci. 1995, 120, 673–680. [Google Scholar] [CrossRef]
- Yao, X.-F.; Xu, J.-H.; Li, P.-F.; Zhang, M.; Ren, R.-S.; Liu, G.; Yang, X.-P. Comparison of different identifying methods for oriental pickling melon (Cucumis melo var. conomon) resistance to Didymella bryoniae at the seedling stage. China Veg. 2017, 30, 11–14, (In Chinese with English Title and Abstract). [Google Scholar]
- Kenigsbuch, D.; Cohen, Y. Inheritance of resistance to downy mildew in Cucumis melo PI 124112 and commonality of resistance genes with PI 124111F. Plant Dis. 1992, 76, 615–617. [Google Scholar] [CrossRef]
- Boyhan, G.E.; Norton, J.D.; Abrahams, B.R. Screening for resistance to anthracnose (race 2), gummy stem blight, and root knot nematode in watermelon germplasm. Cucurbit Genet. Coop. Rep. 1994, 17, 106–110. [Google Scholar]
- Keinath, A.P. Differential susceptibility of nine cucurbit species to the foliar blight and crown canker phases of gummy stem blight. Plant Dis. 2014, 98, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Keinath, A.P. Survival of Didymella bryoniae in infested muskmelon crowns in South Carolina. Plant Dis. 2008, 92, 1223–1228. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Keinath, A.P. Differential sensitivity to boscalid in conidia and ascospores of Didymellabryoniae and frequency of boscalid-insensitive isolates in South Carolina. Plant Dis. 2012, 96, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Melvin, R.H.; Summer, D.R. Variable resistance to gummy stem blight in watermelon PI 271778. HortScience 1998, 33, 599. [Google Scholar]
- Frantz, J.D.; Jahn, M.M. Five independent loci each control monogenic resistance to gummy stem blight in melon (Cucumis melo L.). Theor. Appl. Genet. 2004, 108, 1033–1038. [Google Scholar] [CrossRef]
- Wolukau, J.N.; Zhou, X.H.; Li, Y.; Zhang, Y.B.; Chen, J.F. Resistance to gummy stem blight in melon (Cucumis melo L.) germplasm and inheritance of resistance from plant introductions 157076, 420145, and 323498. HortScience 2007, 42, 215–221. [Google Scholar] [CrossRef]
- Santos, L.; Candido, W.; Rabelo, H.; Marin, M.V.; Braz, L.T. Reaction of melon genotypes to Didymella bryoniae (Fuckel) Rehm. Chil. J. Agric. Res. 2017, 77, 71–77. [Google Scholar] [CrossRef][Green Version]
- Meer, Q.P.V.D.; Bennekom, J.L.V.; Giessen, A.C.V.D. Gummy stem blight resistance of cucumbers (Cucumis sativus L.). Euphytica 1978, 27, 861–864. [Google Scholar] [CrossRef]
- Wyszogrodzka, A.J.; Williams, P.H.; Peterson, C.E. Search for resistance to gummy stem blight (Didymellabryoniae) in cucumber (Cucumis sativus L.). Euphytica 1986, 35, 603–613. [Google Scholar] [CrossRef]
- Wehner, T.C.; Shetty, N.V. Screening the cucumber germplasm collection for resistance to gummy stem blight in North Carolina field tests. HortScience 2000, 35, 1132–1140. [Google Scholar] [CrossRef]
- Sowell, G.J.R. Additional sources of resistance to gummy stem blight of muskmelon. Books Abroad 1981, 65, 253–254. [Google Scholar] [CrossRef]
- Mcgrath, D.J.; Vawdrey, L.L.; Walker, I.O. Resistance to gummy stem blight in muskmelon. HortScience 1993, 28, 113–121. [Google Scholar] [CrossRef]
- Zhang, Y.-P.; Kyle, M.; Anagnostou, K.; Zitter, T.A. Screening melon (Cucumis melo) for resistance to gummy stem blight in the greenhouse and field. HortScience 1997, 32, 117–121. [Google Scholar] [CrossRef]
- Sakata, Y.; Wako, T.; Sugiyama, M.; Morishita, M. Screening melons for resistance to gummy stem blight. Acta Hortic. 2000, 1, 171–178. [Google Scholar] [CrossRef]
- Santos, G.R.; Neto, M.C.; Ramos, L.N.; Café-Filho, A.C.; Reis, A.; Momenté, V.G.; Pelúzio, J.M.; Ignácio, M. Reaction of melon genotypes to the gummy stem blight and the downy mildew. Hortic. Bras. 2009, 27, 160–165. [Google Scholar] [CrossRef]
- Bi, Y.-F.; Xu, B.-H.; Qian, C.-T.; Guo, J.; Zhang, Y.-B.; Yi, H.-P.; Chen, J.-F. Pyramiding disease resistance genes and variety improvement by molecular marker-assisted selection in melon (Cucumis melon L.). Sci. Agric. Sin. 2015, 48, 523–533, (In Chinese with English Title and Abstract). [Google Scholar]
- Gu, W.-H.; Yang, H.J.; Ma, K.; Jin, H.J.; Zheng, H.J.; Dai, F.M.; Lu, J.P.; Wei, C.M. Evalution on gummy stem blight resistance of watermelon germplasm and application. Acta Agric. Shanghai 2004, 20, 65–67, (In Chinese with English Title and Abstract). [Google Scholar]
- Gusmini, G.; Song, R.; Wehner, T.C. New sources of resistance to gummy stem blight in watermelon. Crop Sci. 2005, 45, 582–588. [Google Scholar] [CrossRef]
- Song, R.-H.; Gusmini, G.; Wehner, T.C. Screening the watermelon germplasm collection for resistance to gummy stem blight. Int. Hortic. Congr. Adv. Veg. Breed. 2004, 637, 63–68. [Google Scholar] [CrossRef]
- Song, R.-H.; Yang, H.-J.; Ma, K.; Gu, W.-H. Identification and evaluation on gummy stem blight resistance of watermelon germplasm resources. J. Plant Genet. Resour. 2007, 8, 72–75, (In Chinese with English Title and Abstract). [Google Scholar]
- Song, R.-H.; Dai, F.-M.; Yang, H.-J.; Gu, W.-H.; She, J.-H. Resistance identification of watermelon germplasms to the Fusarium wilt and gummy stem blight. Plant Prot. 2009, 35, 117–120, (In Chinese with English Title and Abstract). [Google Scholar]
- Santos, G.; Leão, E.U.; Garcia, M.M.V.; Maluf, W.R.; Cardon, C.H.; Gonçalves, C.G.; Nascimento, I.R. Reaction of experimental watermelon genotypes to gummy stem blight. Hortic. Bras. 2013, 31, 540–548. [Google Scholar] [CrossRef][Green Version]
- Li, Y. Biological Characteristics of Gummy Stem Blight on Cucurbits and Screening of Resistance Resources on. Cucumber.Ph.D. Thesis, Nanjing Agricultural University, Nanjing, China, 2007. (In Chinese with English Title and Abstract). [Google Scholar]
- Lou, L.-N.; Wang, H.-Y.; Qian, C.-T.; Liu, J.; Bai, Y.-L.; Chen, J.-F. Genetic mapping of gummy stem blight (Didymellabryoniae) resistance genes in Cucumis sativus-hystrix introgression lines. Euphytica 2013, 192, 359–369. [Google Scholar] [CrossRef]
- Wang, X.-D.; Li, G.-Y. Producing pycinidia and varidty susceptibility of gummy stem bight fungus of muskmelon. Xinjiang Agric. Sci. 2004, 41, 341–344, (In Chinese with English Title and Abstract). [Google Scholar]
- Bhardwaj, D.R.; Gautam, K.K.; Saha, S.; Singh, B. Mining the source of resistance for downy mildew and gummy stem blight in bottle gourd (Lagenaria siceraria) accessions. Indian J. Agric. Sci. 2018, 88, 746–750. [Google Scholar]
- Keinath, A.P.; Somai, B.M.; Dean, R.A. Method of Diagnosing Gummy Stem Blight in Plants Using a Polymerase Chain Reaction Assay; Clemson University: Clemson, SC, USA, 2001. [Google Scholar]
- Dai, F.-M.; Liu, S.-H.; Ren, X.-J.; Lu, J.-P.; Ni, X.-H.; Xu, J.-Y. PCR technique for rapid diagnosis of Didymellabryoniae on watermelon. Acta Phytopathol. Sin. 2006, 36, 439–445, (In Chinese with English Title and Abstract). [Google Scholar]
- Zhou, X.-H.; Wolukau, J.N.; Li, Y.; Zhang, Y.-B.; Wu, M.-Z.; Chen, J.-F. Screening and RAPD analysis of gummy stem blight resistance in melon germplasm. Acta Hortic. Sin. 2007, 34, 1201–1206, (In Chinese with English Title and Abstract). [Google Scholar]
- Zhang, S.-P.; Liu, S.-L.; Miao, H.; Shi, Y.-X.; Wang, M.; Wang, Y.; Li, B.-J.; Gu, X.-F. Inheritance and QTL mapping of resistance to gummy stem blight in cucumber stem. Mol. Breed. 2017, 37, e49. [Google Scholar] [CrossRef]
- Amand, P.C.S.; Wehner, T.C. Generation means analysis of leaf and stem resistance to gummy stem blight in cucumber. J. Am. Soc. Hortic. Sci. 2001, 126, 95–99. [Google Scholar] [CrossRef]
- Gusmini, G.; Rivera-Burgos, L.A.; Wehner, T.C. Inheritance of resistance to gummy stem blight in watermelon. HortScience 2017, 52, 1477–1482. [Google Scholar] [CrossRef]
- Rivera-Burgos, L.A.; Silverman, E.; Sari, N.; Wehner, T.C. Evaluation of resistance to gummy stem blight in a population of recombinant inbred lines of watermelon x citron. HortScience 2021, 56, 380–388. [Google Scholar] [CrossRef]
- Ren, H.-Y.; Li, G.; Fang, L.; Ru, S.-J.; Wang, H.-R. Relationship between the resistance to gummy stem blight in the different varieties of melon and the transcript profile of ascorbate peroxidase gene. Acta Phytopathol. Sin. 2012, 42, 306–310, (In Chinese with English Title and Abstract). [Google Scholar]
- Xu, B.-H.; Qian, C.-T.; Wang, H.-Y.; Bi, Y.-F.; Lou, Q.-F.; Zhang, Y.-B.; Yi, H.-P.; Chen, J.-F. The expression analysis of defence genes in the genes pyramided melon (Cucumis melo L.) resistance to gummy stem blight. J. Nanjing Agric. Univ. 2014, 37, 63–68, (In Chinese with English Title and Abstract). [Google Scholar]
- Lu, X.-M.; Zhang, N.; Xia, M.-L.; Qian, C.-T.; Chen, J.-F. Changs of endogenous hormone contents and expression analysis of related genes in melon with different resistance after inoculated with Didymellabryoniae. J. Nanjing Agric. Univ. 2018, 41, 248–255, (In Chinese with English Title and Abstract). [Google Scholar]
- Hu, Z.-Y.; Deng, G.-C.; Mou, H.-P.; Xu, Y.-H.; Li, C.; Yang, J.-H.; Zhang, M.-F. A re-sequencing-based ultra-dense genetic map reveals a gummy stem blight resistance-associated gene in Cucumis melo. DNA Res. 2018, 25, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.R.; Zhang, Y.B.; Zhou, X.H.; Chen, J.F. SSR marker linked to gummy stem blight resistance gene Gsb-1 in melon and its allelism with resistance gene from PI 420145. China Veg. 2009, 5, 1–4, (In Chinese with English Title and Abstract). [Google Scholar]
- Zhang, Y.-B.; Chen, J.-F.; Yi, H.-P.; Qian, C.-T.; Wu, M.-Z. ISSR marker linked to gene Gsb-2 resistant to gummy stem blight in melon (Cucumis melo). J. Fruit Sci. 2011, 28, 296–300, (In Chinese with English Title and Abstract). [Google Scholar]
- Zhang, X.-J.; Zhang, Y.-B.; Zhang, Y.; Wang, D.-M.; Yi, H.-P. ISSR markers linked to Gsb-3 resistance of gummy stem blight (Didymellabryoniae) in melon. Acta Bot. Sin. Northwest China 2013, 33, 261–265, (In Chinese with English Title and Abstract). [Google Scholar]
- Wang, H.-Y.; Qian, C.-T.; Lou, L.-N.; Lou, Q.-F.; Zhang, Y.-B.; Yi, H.-P.; Wu, M.-Z.; Chen, J.-F. A SSR marker linked to Gsb-4 loci resistance to gummy stem blight in melon. Acta Hortic. Sin. 2012, 39, 574–580, (In Chinese with English Title and Abstract). [Google Scholar]
- Zhang, N.; Xu, B.-H.; Bi, Y.-F.; Lou, Q.-F.; Chen, J.-F.; Qian, C.-T.; Zhang, Y.-B.; Yi, H.-P. Development of a muskmelon cultivar with improved resistance to gummy stem blight and desired agronomic traits using gene pyramiding. Czech J. Genet. Plant Breed. 2017, 53, 23–29. [Google Scholar] [CrossRef]
- Wolukau, J.N.; Zhou, X.; Chen, J.F. Identification of amplified fragment length polymorphism markers linked to gummy stem blight (Didymellabryoniae) resistance in melon (Cucumis melo L.) PI 420145. HortScience 2009, 44, 32–34. [Google Scholar] [CrossRef]
- Hassan, M.Z.; Rahim, M.A.; Natarajam, S.; Robin, A.H.K.; Kim, H.T.; Park, J.I.; Nou, I.S. Gummy stem blight resistance in melon: Inheritance pattern and development of molecular markers. Int. J. Mol. Sci. 2018, 19, 2914. [Google Scholar] [CrossRef] [PubMed]
- Ha, K.-W.; Zhang, H.-L.; Liu, J.-L.; Liu, P.; Wang, J.-S. Molecular location of Didymella bryoniae resistant gene carried by 4G21 on Cucumis melo L. Acta Hortic. Sin. 2010, 7, 1079–1084, (In Chinese with English Title and Abstract). [Google Scholar]
- Ren, H.-Y.; Fang, L.; Ru, S.-J.; Wang, H.-R. A preliminary investigation of a mutant melon plant edr 2 on resistance to gummy stem blight. Sci. Agric. Sin. 2009, 42, 3131–3138, (In Chinese with English Title and Abstract). [Google Scholar]
- Bi, Y.-F.; Guo, J.; Xu, B.-H.; Zhang, Y.-B.; Yi, H.-P.; Qian, C.-T.; Chen, J.-F. Resistance evaluation of melon (Cucumis melo L.) to gummy stem blight and expression of PAL gene. Acta Bot. Boreal.-Occident. Sin. 2015, 35, 239–244, (In Chinese with English Title and Abstract). [Google Scholar]
- Zuniga, T.L.; Jantz, J.P.; Zitter, T.A.; Jahn, M.K. Monogenic dominant resistance to gummy stem blight in two melon (Cucumis melo) accessions. Plant Dis. 1999, 83, 1105–1107. [Google Scholar] [CrossRef]
- Liu, S.-L.; Shi, Y.-X.; Miao, H.; Wang, M.; Li, B.-J.; Gu, X.-F.; Zhang, S.-P. Genetic analysis and QTL mapping of resistance to gummy stem blight in Cucumis sativus seedling stage. Plant Dis. 2017, 101, 1145–1152. [Google Scholar] [CrossRef]
- Liu, J. Fine Mapping of QTLs for Resistant Gummy Stem Blight in Cucumis sativus/hystrix Introgression Line HH1-8-1-2. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2013. (In Chinese with English Title and Abstract). [Google Scholar]
- Liu, L.-Z.; Qu, W.-Q.; Chen, Y.-L.; Chen, Y.-Y.; Zhu, W.-M. QTL molecular marker location of gummy stem blight resistance in melon (Cucumis melo). J. Fruit Sci. 2013, 30, 748–752, (In Chinese with English Title and Abstract). [Google Scholar]
- Zhang, X.; Xu, J.; Li, J.; Lou, Q.-F.; Chen, J.-F. QTL mapping and identification of candidate gene for resistance to gummy stem blight in Cucumis sativus/hystrix introgression line ‘IL77’. Acta Hortic. Sin. 2018, 45, 2141–2152, (In Chinese with English Title and Abstract). [Google Scholar]
- Ren, R.-S.; Xu, J.-H.; Zhang, M.; Liu, G.; Yao, X.-F.; Zhu, L.-L.; Hou, Q. Identification and molecular mapping of a gummy stem blight resistance gene in wild watermelon (Citrullus amarus) germplasm PI 189225. Plant Dis. 2019, 104, 16–24. [Google Scholar] [CrossRef]
- Gimode, W.; Bao, K.; Fei, Z.; Mcgregor, C. QTL associated with gummy stem blight resistance in watermelon. Theor. Appl. Genet. 2021, 134, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Kim, D.S.; Sang, G.K.; Huh, Y.C.; Lee, J. QTL mapping for gummy stem blight resistance in watermelon (Citrullus spp.). Plants 2021, 10, 500. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.Z.; Rahim, M.A.; Jung, H.J.; Park, J.I.; Park, J.I.; Nou, I.S.; Nou, I.S. Genome-wide characterization of NBS-encoding genes in watermelon and their potential association with gummy stem blight resistance. Int. J. Mol. Sci. 2019, 20, 902. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wu, S.; Bai, Y.; Sun, H.-H.; Jiao, C.; Guo, S.-G.; Zhao, K.; Blanca, J.; Zhang, Z.-H.; Huang, S.-W.; et al. Cucurbit Genomics Database (CuGenDB): A central portal for comparative and functional genomics of cucurbit crops. Nucleic Acids Res. 2018, 10, e1093. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.-G.; Zhang, J.-G.; Sun, H.-H.; Salse, J.; Lucas, W.J.; Zhang, H.-Y.; Zheng, Y.; Mao, L.-Y.; Ren, Y.; Wang, Z.-W.; et al. The draft genome of watermelon (Citrullus lanatus) and resequencing of 20 diverse accessions. Nat. Genet. 2013, 45, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, X.; Reddy, U.; Sun, H.; Bao, K.; Gao, L.; Mao, L.; Patel, T.; Ortiz, C.; Abburi, V.L. Genome of ‘Charleston Gray’, the principal American watermelon cultivar, and genetic characterization of 1,365 accessions in the U.S. National Plant Germplasm System watermelon collection. Plant Biotechnol. J. 2019, 12, 2246–2258. [Google Scholar] [CrossRef]
- Zhao, Q.; Wu, J.-Z.; Zhang, L.-Y.; Yan, C.; Jiang, S.-K.; Li, Z.-G.; Sun, D.-Q.; Lai, Y.-C.; Gong, Z.-P. Genome-scale analyses and characteristics of putative pathogenicity genes of Stagonosporopsiscucurbitacearum, a pumpkin gummy stem blight fungus. Sci. Rep. 2020, 10, e18065. [Google Scholar] [CrossRef]
- Branham, S.; Levi, A.; Wechter, P. QTL mapping identifies novel source of resistance to Fusarium wilt race 1 in Citrullus amarus. Plant Dis. 2018, 103, 984–989. [Google Scholar] [CrossRef]
- Branham, S.E.; Levi, A.; Katawczik, M.L.; Wechter, W.P. QTL mapping of resistance to bacterial fruit blotch in Citrullus amarus. Theor. Appl. Genet. 2019, 132, 146–1471. [Google Scholar] [CrossRef]
- Wang, F.; Qi, J.; Tian, M.; Gao, Y.; Li, D. Genome sequence resource for Stagonosporopsiscucurbitacearum, a cause of gummy stem blight disease of watermelon. Mol. Plant-Microbe Interact. 2021, 34, 977–980. [Google Scholar] [CrossRef]
- Chen, J.; Lu, X.; Ren, Q.; Sun, Y. Identification of circRNA and their target genes related to resistance to gummy stem blight in melon. J. Nanjing Agric. Univ. 2020, 43, 629–636. [Google Scholar]
- Keinath, A.P. Soil amendment with cabbage residue and crop rotation to reduce gummy stem blight and increase growth and yield of watermelon. Plant Dis. 1996, 80, 564–570. [Google Scholar] [CrossRef]
- Hu, F.-Y.; Mo, J.-Y.; Wei, J.-G.; Guo, T.-X.; Huang, S.-P. Research progresses in occurrence and control of watermelon and melon gummy stem blight. China Veg. 2011, 24, 40–44, (In Chinese with English Title and Abstract). [Google Scholar]
- Zhou, X.-G.; Everts, K.L. Anthracnose and gummy stem blight are reduced on watermelon grown on a no-till hairy vetch cover crop. Plant Dis. 2012, 96, 431–436. [Google Scholar] [CrossRef]
- Dos Santos, G.R.; Sousa, S.C.R.; Juliatti, F.C.; Rodrigues, A.C.; Dalcin, M.S.; Bonifacio, A. Control of gummy stem blight in watermelon through different manament systems. Biosci. J. 2016, 32, 371–377. [Google Scholar] [CrossRef]
- Utkhede, R.S.; Koch, C.A. Evaluation of biological and chemical treatments for control of gummy stem blight on cucumber plants grown hydroponically in greenhouses. J. Int. Organ. Biol. Control 2004, 49, 109–117. [Google Scholar] [CrossRef]
- Dalcon, M.S.; Tschoeke, P.H.; Aguiar, R.W.S.; Fidelis, R.R.; Didinet, J.; Santos, G.R. Severity of gummy stem blight on melon in relation to cultivars, use of fungicides and growing season. Hortic. Bras. 2017, 35, 483–489. [Google Scholar] [CrossRef]
- Crino, P.; Bianco, C.L.; Rouphael, Y.; Colla, G.; Saccardo, F. Evaluation of rootstock resistance to fusarium wilt and gummy stem blight and effect on yield and quality of a grafted ‘inodorus’ melon. HortScience 2007, 42, 521–525. [Google Scholar] [CrossRef]
- Keinath, A.P. Susceptibility of cucurbit rootstocks to Didymella bryoniae and control of gummy stem blight on grafted watermelon seedlings with fungicides. Plant Dis. 2013, 97, 1018–1024. [Google Scholar] [CrossRef][Green Version]
- An, J.; Kumar, P.; Sharma, P.; Kumar, S.; Thakur, V. Rootstocks and grafting interactionstudies for gummy stem blight (Didymellabryoniae) and horticultural traits in parthenocarpic cucumber. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 3808–3817. [Google Scholar]
- Ito, L.A.; Charlo, H.C.O.; Castoldi, R.; Braz, L.T.; Camargo, M. Rootstocks selection to gummy stem blight resistance and their effetion on the yield of melon ‘Bnusn 2’. Rev. Bras. Frutic. 2009, 31, 262–267. [Google Scholar] [CrossRef]
- Gasparotto, F.; Oliveira, R.R.D.; Penharbel, M.P.; Tessmann, D.J.; Nascimento, J.F.; Vida, J.B. Effect of grafting on the control of gummy stem blight in muskmelons. Semin. Ciênc.Agrár. 2016, 37, 2859–2866. [Google Scholar] [CrossRef][Green Version]
- Nofal, M.A.; EI-Naggar, M.A.A.; Ismail, B.R. Biological control of gummy stem blight (Mycosphaerellamelonis L. passerini) on cantaloupe plants under protected cultivations. Acta Hortic. 1996, 434, 169–176. [Google Scholar] [CrossRef]
- Dong, H.-Q.; Liu, F.; Mu, W.; Zhu, T.-S. Selection of antagonistic bacteria for suppression of cucumber gummy stem blight disease. Acta Agric. Boreal. Occident. Sin. 2008, 17, 205–209, (In Chinese with English Title and Abstract). [Google Scholar]
- Chaudhary, S. Evaluation of Streptomycetes for Biological Control of Gummy Stem Blight Disease of Cantaloupe. Ph.D. Thesis, The University of Texas-Pan American, Edinburg, TX, USA, 2009. [Google Scholar]
- Nga, N.T.T.; Giau, N.T.; Long, N.T.; Lu¨ beck, M.; Shetty, N.P.; Neergaard, E.; Thuy, T.T.T.; Kim, P.V.; Jørgense, H.J.L. Rhizobacterially induced protection of watermelon against Didymellabryoniae. J. Appl. Microbiol. 2010, 109, 567–582. [Google Scholar] [CrossRef]
- Zhao, J.; Xue, Q.-H.; Du, J.-Z.; Yu, Y.-H.; Xue, L. Biocontrol effect of broad-specytum antagonistic actinomycete on melon gummy stem blight. J. Northwest Agric. For. Univ. 2012, 40, 65–71, (In Chinese with English Title and Abstract). [Google Scholar]
- Bai, R.-X.; Zeng, H.-W.; Fan, Q.; Yin, J.; Sui, Z.-M.; Yuan, L. Effects of Ceriporialacerata on gummy stem blight control, growth promotion and yield increase of cucumbers. Sci. Agric. Sin. 2019, 52, 2604–2615, (In Chinese with English Title and Abstract). [Google Scholar]
- Lu, M.; Qi, J.; Xu, C.-J.; Hu, W.-Q.; Zhang, L.; Yu, J.-X.; Wang, X.-J.; Duan, M.-Y.; Jin, Y.-Z. Effects of Trichoderma spp. on growth of melon seedlings and inhibiting effect of the pathogen growth of mycospharellamelonis. Anhui Agric. Sci. Bull. 2019, 25, 23–27, (In Chinese with English Title and Abstract). [Google Scholar]
- Nuangmek, W.; Aiduang, W.; Kumla, J.; Lumyong, S.; Suwannarach, N. Evaluation of a newly identified endophytic fungus, Trichoderma phayaoense for plant growth promotion and biological control of gummy stem blight and wilt of muskmelon. Front. Microbiol. 2021, 12, 634772. [Google Scholar] [CrossRef]
- Geng, M.-M.; Jia, R.-L.; Sui, Z.-M.; Huang, J.-G. Effect of biopesticide Pythium oligandrum broth on gummy stem blight in cucumber seedlings, animal health and plant growth. Acta Microbiol. Sin. 2016, 56, 1159–1167, (In Chinese with English Title and Abstract). [Google Scholar]
- Keinath, A.P. Polyoxin D and other biopesticides reduce gummy stem blight but not anthracnose on melon seedlings. Plant Health Prog. 2016, 17, 177–181. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Q.; Tan, G.-F.; Ma, Y.-Q.; Meng, P.-H.; Zhang, J. Cucurbitaceous Vegetables’ Gummy Stem Blight Research. Agronomy 2022, 12, 1283. https://doi.org/10.3390/agronomy12061283
Luo Q, Tan G-F, Ma Y-Q, Meng P-H, Zhang J. Cucurbitaceous Vegetables’ Gummy Stem Blight Research. Agronomy. 2022; 12(6):1283. https://doi.org/10.3390/agronomy12061283
Chicago/Turabian StyleLuo, Qing, Guo-Fei Tan, Yi-Qiao Ma, Ping-Hong Meng, and Jian Zhang. 2022. "Cucurbitaceous Vegetables’ Gummy Stem Blight Research" Agronomy 12, no. 6: 1283. https://doi.org/10.3390/agronomy12061283
APA StyleLuo, Q., Tan, G.-F., Ma, Y.-Q., Meng, P.-H., & Zhang, J. (2022). Cucurbitaceous Vegetables’ Gummy Stem Blight Research. Agronomy, 12(6), 1283. https://doi.org/10.3390/agronomy12061283








