The study was conducted to establish the nutritional balance of 12 macro- and micro-nutrients in ‘Rojo Brillante’ persimmon, PDO ‘Kaki Ribera del Xúquer’ Spain). The high-yielding population was made up of 13 of the 58 orchards, with yields from 46.38 t·ha−1 to 81.32 t·ha−1.
3.1. DRIS Norms
The orchards with yields higher than 46.38 t·ha−1 were considered high-yielding, and they represented 22.41% of the entire dataset.
The mean nutrient ratios and SDs of the high-yielding population are shown in
Table 1.
The variance ratios of all the selected dual ratios of the low- vs. high-yielding population were higher than 1, which indicates a relatively higher variance for the low-yielding population (data not shown).
Table 1 shows a wide range between the obtained ratios, from values per 10
3 to values of 10
−3. This is because a decision was made to maintain macronutrients as percentages and micronutrients as parts per million, which are frequently used units. This was performed to confer more practicality to the use of the norms obtained by farmers in the future. After obtaining DRIS norms, the equations to determine the DRIS indices of the selected nutrients were as follows (
Table 2):
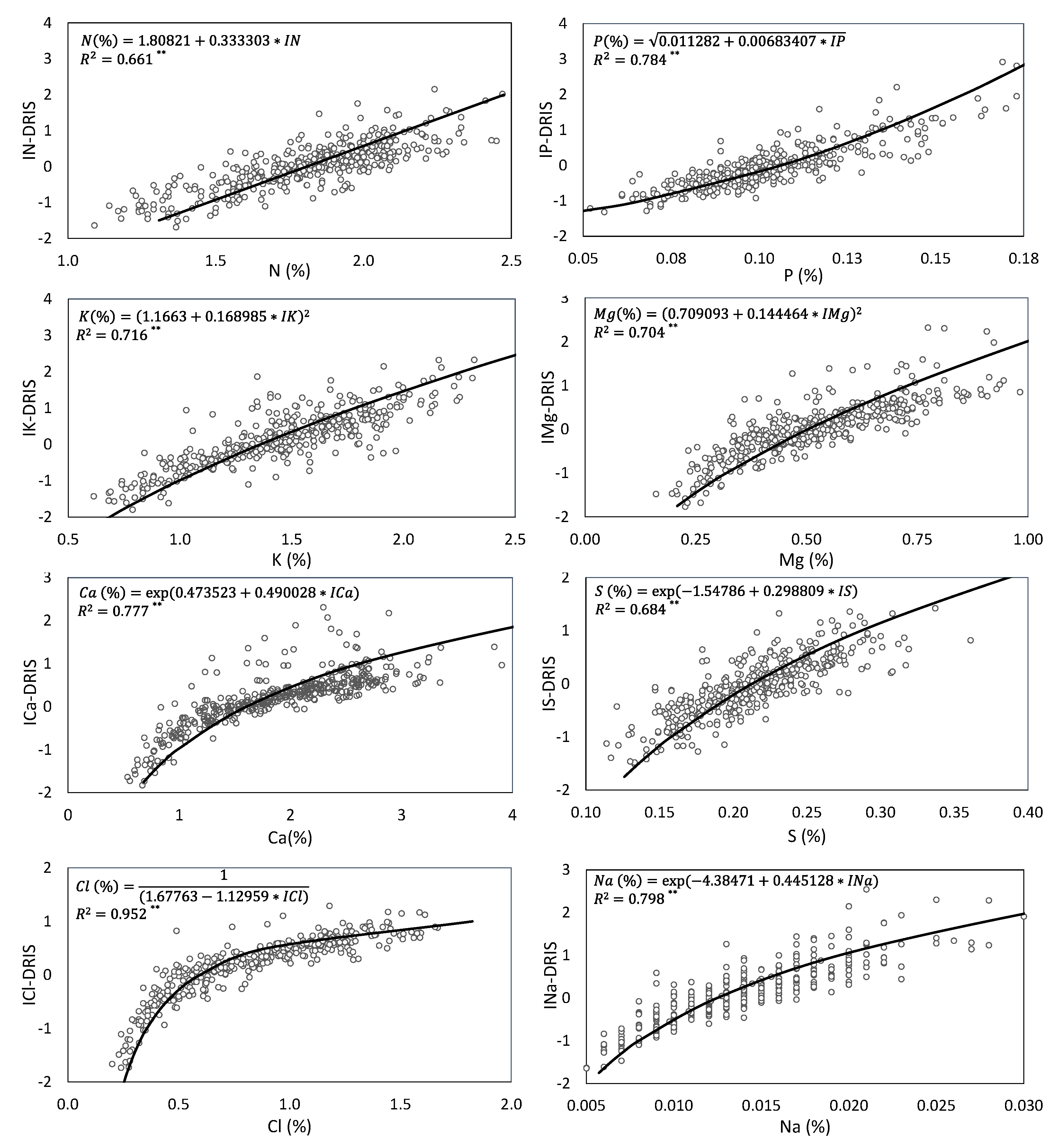
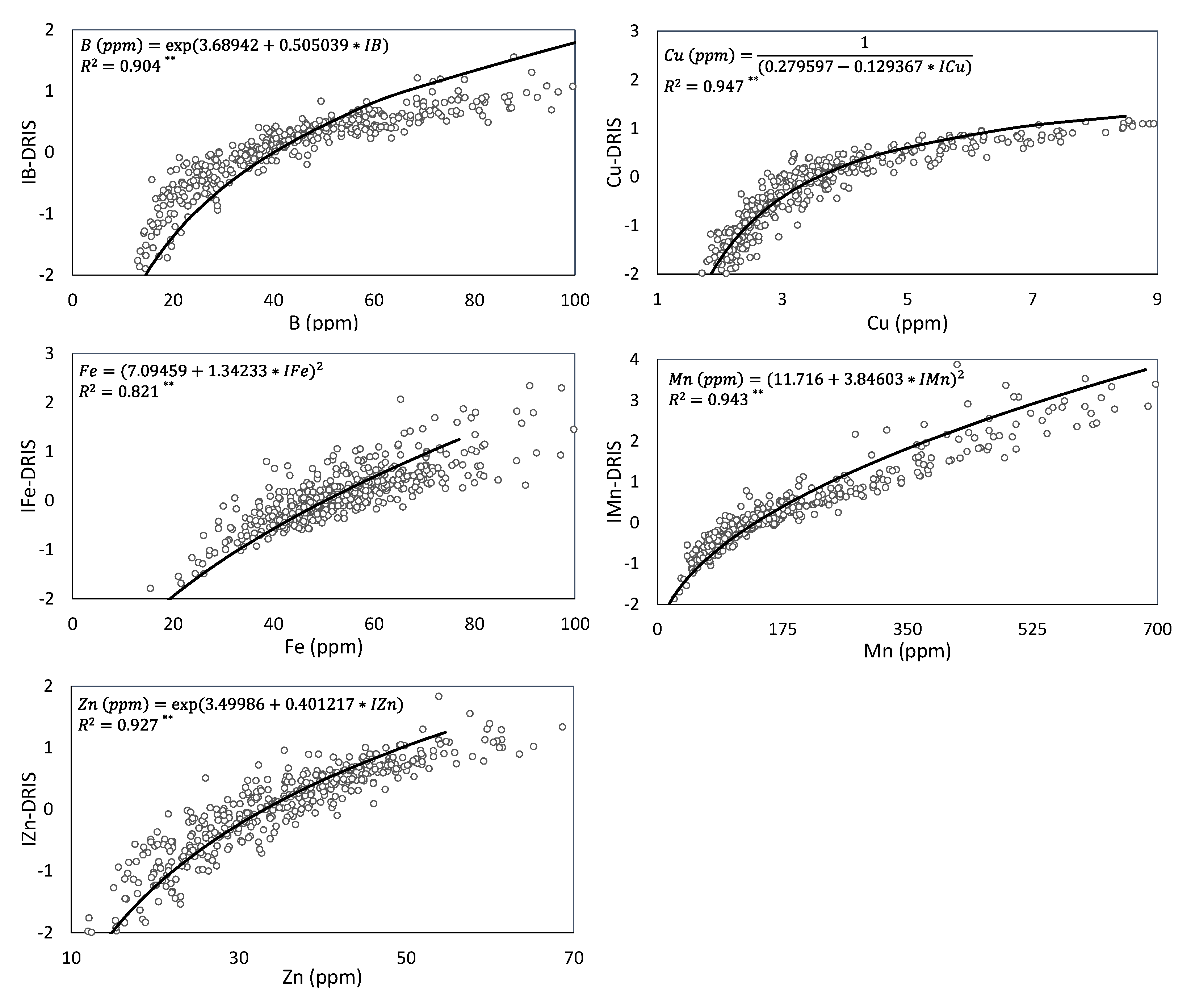
Figure 1 and
Figure 2 show the relation between the leaf nutrient concentrations of the high-yielding population and DRIS indices, estimated by regression analysis. In all cases, the lower the DRIS index, the lower the nutrient concentration. This increases the reliability of DRIS norms because approaches correctly indicate nutritional limitations [
16]. For each relation, the regression models that were best adapted to data evolution were fitted depending on the percentage of variation of the dependent variable that was collectively explained by all the independent variables (R2). In DRIS studies, linear regression models are often fitted to the relation between the DRIS index and leaf nutrient concentration [
13,
35]. Other authors have established that linear, quadratic and logarithmic are the best fitting regression models [
16,
36,
37]. Nevertheless, in this study, a wide range of regression models was selected as each relationship between the nutrient concentration and its DRIS index differently evolved depending on how the plant vegetative cycle advanced. Thus a single linear regression model was used for N, while non-linear regression models were selected for the other nutrients. Of the non-linear models, five exponential regression models (Ca, S, Na, B and Zn), four square root-X (K, Mg, Fe and Mn), two inverse Y (Cl and Cu), and one Y quadratic (P) were obtained.
The DRIS index values well explained the leaf nutrient concentrations because all the obtained regression equations had an R
2 > 0.6 (
p < 0.01). These results confirm that the DRIS method effectively diagnoses the nutritional status of persimmon crops. The DRIS indices of micronutrients, especially Cu and Mn (R
2 > 0.94), more clearly explained the leaf nutrient concentrations than the DRIS indices of macronutrients. The higher R
2 in micronutrients than in macronutrients has been previously reported in ‘Gigante’ cactus pear and cotton [
16,
38]. Of the macronutrients, N and S showed the weakest correlation with their DRIS indices (R
2 < 0.7). In other crops, a low correlation of the N and S concentrations with their respective DRIS indices has been observed in grass, apple and corn. This implies that other important factors play a role in the nutrient balance [
36,
39,
40]. Aliyu et al. [
35] have also observed this in corn, who attributed the low R
2 in the relation of the N concentration to the IN with N imbalance, instead of N deficiency, provided the S and Zn additions in their study.
Based on the relation between the DRIS indices and nutrient contents in the leaf samples of the high-yielding population, it was possible to determine NORs. A plant is nutritionally balanced when DRIS indices come close to zero [
11]. NORs can be obtained when the regression models of the relation of nutrient content and indices move towards this nutritional balance point (BP) [
13].
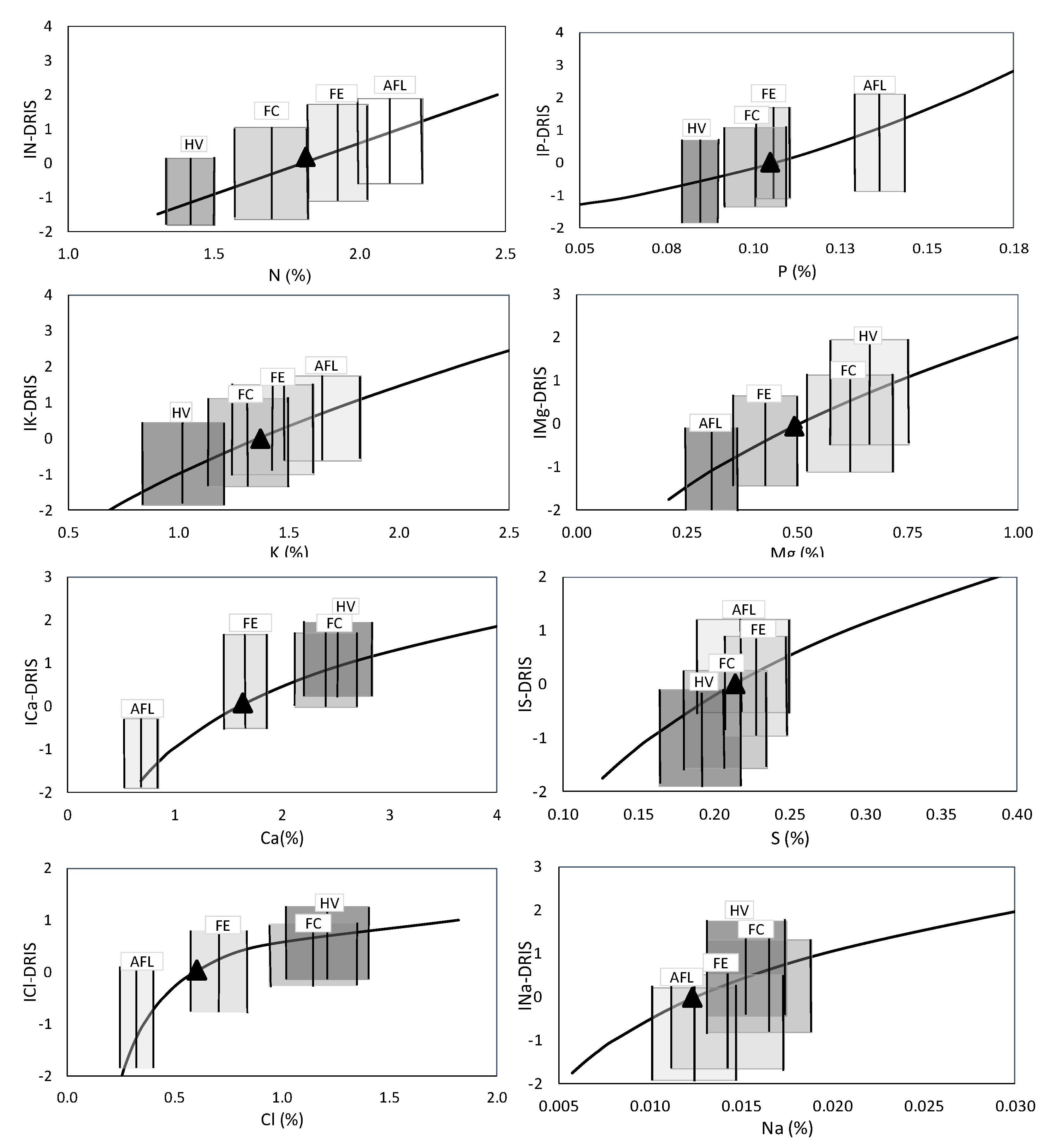
For this study,
Figure 3 and
Figure 4 represent the optimal range of each nutrient in relation to the estimated DRIS index in the different evaluated phenological stages (AF, FE, FC, HV) and the BP when regression equations equal zero.
As the value of each ratio is added to one index subtotal and subtracted from another before averaging, all the DRIS indices are balanced at around zero [
11]. Accordingly, having obtained the I
DRIS equations, the equilibrium point of each nutrient was determined by setting each I
DRIS equation to equal zero. In most cases, the BP did not fall within the optimal range of all the phenological stages. Only for S and Zn was the BP within the optimal range of the concentration of these nutrients in all four studied phenological stages. Despite not finding the BP in all the phenological stages in the other nutrients, the triangle was found in the phenological FE stage in them all. Only for N was the BP not located in FE, but it was on the vertical line, which indicates the optimal lower range of this stage.
These results indicated that FE could be the best-recommended stage for carrying out leaf analyses to correct fertilisation programmes (nutrient doses). However, provided the importance of knowing the optimal foliar sampling time, an in-depth study was carried out, as mentioned in the subsequent section.
Moreover, to evaluate any statistical differences among the different sampling times, the mean nutrient value for ‘Rojo Brillante’ persimmon was taken from the samples of the high-yielding population in each determined phenological stage (AFL, FE, FC, HV) (
Table 3).
When comparing the different phenological stages, the Ca, Mg, B, Fe, Na and Cl concentrations rose, and N, P, K, S, and Cu lowered as the study period advanced. These results are similar to other previously reported leaf nutrient concentrations for persimmon fruit [
41,
42]. Mn was the only element to decrease and then increase again. Clark and Smith [
41] also observed this behaviour in ‘Fuyu’ persimmon. Similar to other fruit trees grown in calcareous soil, persimmon is sensitive to Mn and Zn deficiency. The correction of these compounds is usually made by foliar applications to cover needs [
7]. Therefore, Mn and Zn showed no clear increasing or decreasing trend because the foliar applications of these nutrients were not applied homogeneously in all the plots. In addition, Mn is one of the active principles of Mancozeb [
43], the main fungicide used in the Valencian Community for controlling circular leaf spots (CLS) in persimmon caused by
Mycosphaerella nawae Hiura & Ikata. Since August 2008, CLS has become the most important persimmon foliar disease in Spain and leads to severe yield losses upon harvest [
44]. This fungicide is applied mainly in spring when
M.Nawae ascospores are released from leaf litter [
45].
Table 3 shows significant differences in the concentration of most nutrients among the phenological stages during the study period. A bigger difference was obtained between the mean N and K values, with significant differences noted in each phenological state. Nutrients Ca, Mg, Cu, Fe and Zn, and those related to salinity (Na and Cl), remained constant from the FC stage.
The NOR in other crops has been previously established by the DRIS method taking leaves from a specific phenological stage. Guimaraes et al. [
46] sampled ratoon sugarcane at harvest, and Serra et al. [
16] also did during cotton crop flowering. Nevertheless, these results reveal that establishing a single NOR for the whole vegetative cycle will lead to wide ranges and, therefore, these ranges will not often cover the specificity of each phenological stage. Thus, the differences observed among different phenological stages reveal the need to establish distinct NOR reference tables for each studied phenological time (
Table 4,
Table 5,
Table 6 and
Table 7). The main optimum ranges of leaves at FE coincide with the nutrient concentration observed by Pomares et al. [
7] in July, which is when the FE of the PDO ‘Kaki Ribera del Xúquer’ takes place. Nevertheless, the NORs for FE and HV were slightly lower than the ranges reported by George et al. [
21], whose results might be lower because they employed a different cultivar, cv ‘Fuyu’ compared to ‘Rojo Brillante’, and edaphic conditions.
3.2. Selecting the Sampling Period
In order to determine the optimum sampling time to know the more balanced nutritional period of persimmon plants, production was taken into account by separating data into two groups based on the total average production. With an average of 40 t·ha
−1, a block of 28 high-yielding orchards and a block of 30 low-yielding orchards were established. The leaf analysis not only depends on correct crop stage selection but also on correctly choosing leaf type or knowing the influence of cultivation conditions, such as irrigation type [
47]. Data were divided according to irrigation system type, flood (FL) or drip (D), and the sprout origin, vegetative (V) or floral (F).
The NBI can be applied to identify deficiencies, excesses or nutritional balances of macronutrients (N, P, K, Ca, Mg, S) and micronutrients (Fe, Mn, Zn, Cu, Mo, B) in leaf tissue [
48]. Thus, to establish the optimum sampling time, the DRIS method tool of the NBI was used. In this study, the NBI was determined in the four different phenological stages after taking into account yield, sprout type and irrigation (
Table 8). The data showed that only the samples from the vegetative sprouts of the drip-irrigated orchards with yields over 40 t·ha
−1 have no significant differences among phenological stages. NBIs were lower in all cases during FE, with significant differences compared to the other phenological groups. The second most balanced phenological stage was FC, which had no significant differences with FE, except for the samples from the flood-irrigated orchards from vegetative sprouts with yields above 40 t·ha
−1. The most unbalanced stage was AF. As AF was sampled in May, the highest NBI was obtained because the period from March to June is characterized by the high nutrient requirements needed for sprouting, flowering and fruit set [
7].
Regarding the subgroups into which data were divided, the sprout location of samples was a determinant in the AF stage. The leaves from vegetative sprouts were more unbalanced. In all cases, the content of nutrients in the leaves from fruiting shoots was lower (data not shown). This could be because fruiting shoots have started fruit set, which induces leaves to change from being a sink to a source of nutrients and photosynthates [
49]. Nevertheless, sprout types showed no significant differences in the other phenological stages. This agrees with Clark and Smith [
42] because these authors did not observe any major differences between the nutrient concentrations measured in the leaves sampled from fruiting or non-fruiting shoots. This finding suggests that the leaves in close proximity to fruit are not subjected to disproportionate nutrient stress during fruit development.
Approximately in the FE stage, and despite NBIs being low in all cases, the yield had a significant effect. Upon sampling (July), the high-yielding persimmon trees (>40 t·ha−1) had greater nutrient mobility than the low-yielding trees. These trees had higher nutrient requirements because they had a larger fruit set.
To know the order of the most unbalanced nutrients in each phenological stage, and bearing in mind that sprout was a determinant of AF and yield in FE,
Figure 5 shows the mean DRIS indices of the total population for each nutritional status. Nutrient excess or deficiency varies in order and severity terms depending on sampling time. As observed in the different NBIs, the greatest nutrient excesses and deficiencies are for AF and HV.
At the AF sampling time, and similar to the results in
Table 8, the leaves from vegetative sprouts exhibited greater nutrient imbalances than those from floral sprouts. Nevertheless, in both cases, the lowest indices were for Ca, Cl and Mg. This does not imply that they are limiting for crops; rather, leaves did not have enough time to uptake these nutrients.
Of these elements, Ca is a highly immobile element [
50,
51]. Mg is the central atom in the chlorophyll molecule that is needed for its biosynthesis. It increases throughout the vegetative cycle because it is known that chlorophyll content in young leaves is lower than in old leaves [
52]. Regarding Cl, although its deficiency is presented, it appears in excess in the other phenological stages, which verifies this crop’s salinity sensitivity.
Conversely, a larger quantity of Mn appears in AF and suggests it is excessive. Despite this nutrient occupying intermediate positions at FE and FC, Mn once again appeared in excessive amounts at HV. As indicated above, this is because persimmon trees are foliar-sprayed with Mn at different vegetative cycle times. With the obtained results, it can be established that the first Mn application is necessary because the Mn level is not excessive as harvest advances. However, an attempt should be made to reduce the last treatment as much as possible. The growing cycle ends with an excessive Mn concentration and, as persimmon is a deciduous crop, leaves fall off when the cycle ends, and part of the immobile elements present in leaves are nutrients that return to the soil. It could induce excessive Mn concentrations in new plant tissue that can alter several processes, such as enzyme activity, absorption, translocation and utilisation of other mineral elements (Ca, Mg, Fe and P), which cause oxidative stress [
53,
54].
During FE, apart from the differences observed between the NBIs of the high-yielding population vs. the low-yielding population,
Figure 5 shows that the order of deficiency or excess is presented by I
DRIS changes. While Cu was deficient only in the low-yielding orchards, B was deficient only in the high-yielding orchards. Both these elements could be determinants for knowing final ‘Rojo Brillante’ persimmon yields. S, P and K appeared as limiting deficiency elements in both populations. Of these, P was limited from FE and S in all the studied nutritional stages. In the last two phenological stages, nutrients were unbalanced and not limiting for the period from July to September, as this period requires considerable nutrients to cover full fruit development. Because of this, these nutrients were already used by fruit sets. Nevertheless, when paying attention to K, and despite persimmon crops usually being fertilised with this nutrient during FE, plants presented K deficiency. Thus, K fertilisation could be advisable to improve persimmon production. However, K fertilisation must be cautiously carried out because it can decrease Ca uptake and subsequently affect fruit firmness or fruit conservation [
7].
Both P and S are limiting nutrients from FE, which is the more balanced sampling period, until the end of the study period. Increasing P deficiency from flowering to harvest has also been observed by Gosavi et al. [
47], who attributed it to not only developing fruit (cell division), demanding more P, but also to limiting P supply in soil because of high pH and calcareousness.