Abstract
Global warming can have detrimental effects on crop production formation, but the effect of low-temperature stress on crop quality should not be ignored. Wheat is one of the main grain crops in the world, and the quality of wheat is directly related to human health. The nutritional importance of wheat in the human diet necessitates pursuing a study to collect detailed and accurate knowledge of the amino acid composition of wheat proteins under low-temperature conditions. To better understand the effect of low temperature on the composition of amino acids in mature wheat, we conducted a two-year low-temperature-controlled wheat pot experiment in artificial climate chambers with two different wheat cultivars at four low-temperature intensities during the jointing, booting, and both jointing and booting stages. Our results suggest that the contents of total amino acids, essential amino acids, and nonessential amino acids for the cold-sensitive wheat cultivar (Yangmai16) increased under the low-temperature treatments, while the contents of the cold-tolerant cultivar (Xumai30) decreased when low-temperature was applied during the jointing and double (both jointing and booting) stages. Through the amino acid score (AAS), we found that the first limiting amino acid was Lys, while the second limiting amino acid varied among Ile, Met + Cys, and Val after the low-temperature treatments. Comparing the amino acid ratio coefficients (RCs), we found that Leu and Thr in wheat grains were close to the standard protein after the low-temperature treatments, while Phe and Tyr were in a surplus, and the other essential amino acids did not meet the standard. Thus, to improve the protein quality of wheat, protective measures should be taken when low temperatures occur.
1. Introduction
In the developed and developing countries, plant-based diets have been examined as sources of protein and amino acids for humans [1]. Wheat (Triticum aestivum L.) is one of the crucial foods for humans, feeding billions of people worldwide. In addition to being a major source of ration, wheat is also an important dietary component for the majority of people in developing countries and is essential and beneficial to human health [2]. It has been reported that wheat products have contributed to more than 40% of the global protein supply in recent decades [3]. Besides, wheat grains contain more than 17 kinds of amino acids, and 8 kinds of essential amino acids cannot be synthesized by the human body [1].
The nutritional quality of protein is determined by amino acids, as they are required in the human body to regulate the growth, maintenance, repair, and replacement of tissues [1]. Studies have shown a significant positive correlation between wheat grain protein content and total amino acid content. However, cultivars with high protein content do not always possess high contents of all essential amino acids at the same time [4]. For example, previous research has shown that, with the increase in grain protein content, the content of glutamate increases significantly, while the content of phenylalanine changes slightly. In addition to the content of amino acids, protein nutritional quality is also determined by the proportion of essential amino acids, as these cannot be synthesized in the human body [5]. Shewry and Hey reported that one essential amino acid is limiting, while the other amino acids are broken down and excreted, resulting in restricted growth in humans [1]. Therefore, from a nutritional perspective, the total protein content and the contribution of essential amino acids to the total are the important factors of crop grain quality [6].
However, the amino acid content and its composition are not constant in cereals [7]. The genotype, environment, and their interactions have been found to significantly influence amino acid quality, and their environmental effects are generally larger than those of genetic factors [8,9]. Moreover, among the environmental factors, temperature is considered as a major component that has a significant effect on the quality of wheat grain [10]. Previous studies have shown that during the grain filling period, the daily temperature difference and the maximum temperature have a positive effect on most amino acids, while the daily minimum temperature and soil humidity have a negative effect. These results may be because the daily temperature difference enhances the photosynthesis during the day and weakens the respiration at night, which is conducive to nutrient accumulation and nitrogen absorption in wheat grains [11], while the lower soil humidity has a positive effect on the accumulation of nitrogen [12]. García studied 10 durum wheat cultivars under three water and temperature regimes and found that the amino acid composition showed significant variation among the 10 cultivars; the major changes in amino acid composition were caused by environmental conditions and, in particular, by temperature and water availability [13]. Moreover, tyrosine, lysine, methionine, threonine, and valine had the largest variation among environments, whereas phenylalanine, glycine, and aspartic acid had the smallest variation.
Low-temperature stress as a consequence of climate change is responsible for serious crop grain quality deterioration [14,15,16], and the intensity and frequency of low-temperature stress have been estimated to persist across each land–continent even under 21st century warming scenarios [17,18,19]. However, because of global warming, most studies focus on the effect of increasing temperature on crop yield and quality [20,21,22]; few studies focus on the low-temperature effect, especially on crop grain quality. Moreover, even in the existing studies on the effects of low temperature on crop grain quality, most of them focus more on the effects of low temperature on crop protein and starch [16,23,24]. Knowledge is still limited regarding the effect of low temperature on the amino acid quality of crop grains.
In this study, low-temperature-controlled experiments were conducted in artificial climate chambers with two different cold-sensitive wheat cultivars. The effects of low-temperature intensity and low-temperature occurrence periods on the amino acid composition were considered. In addition, to estimate the amino acid quality of wheat grain under low-temperature conditions, the amino acid scoring procedure was employed in this study. These results can form a basis to improve the nutritional quality of wheat grain and prepare a breeding objective in the future.
2. Materials and Methods
2.1. Experimental Design
Wheat pot experiments were conducted in four independent environmentally controlled artificial climate chambers at Rugao Experimental Station of the National Engineering and Technology Center for Information Agriculture (NETCIA), Jiangsu Province, China, in 2017–2019. Two winter wheat cultivars, Yangmai16 (a cold-sensitive cultivar, bred and released from Agricultural Research Institute of Lixiahe Region, Yangzhou, China, in 2004) and Xumai30 (a cold-tolerant cultivar, bred and released by Xuzhou Agricultural Institute, Xuzhou, China, in 2003) were selected as the test cultivars in these experiments. These two cultivars were widely grown in the Middle–Lower Reaches of Yangzi River Subregion (Figure 1). Ten plants per pot of each cultivar were planted in plastic pots. The sowing dates in 2017–2018 and 2018–2019 were October 26 and November 1, respectively. Four low-temperature intensities were applied during the individual (jointing or booting) and combined stages (both jointing and booting) in each growing season. According to a previous study [15], the average value of accumulated frost days from wheat stem elongation to flowering is less than 3 days in the main winter-wheat production region of China. Therefore, each low-temperature intensity lasted for 3 days. In order to eliminate other influencing factors in the ambient, such as radiation, wind, and precipitation, T1 was considered as the control (CK) in this study. The details of the treatments are summarized in Table 1. When the plants had developed enough for the treatment stages, the pots were transferred into the artificial climate chambers and exposed to low-temperature conditions. Before and after the low-temperature treatments, all the treated pots were grown under the ambient environment and surrounded by border plants. In terms of fertilization, the basal fertilizer (0.9 g of N, 0.5 g of P2O5, and 0.9 g of K2O per pot) was applied before sowing and the topdressing fertilizer (0.9 g of N per pot) was applied during the jointing stage. To avoid water stress, irrigation was carried out when the soil volumetric water content was under 75%. Other cultivation management, such as disease, pest, and weed control, was conducted following the local optimum practices.
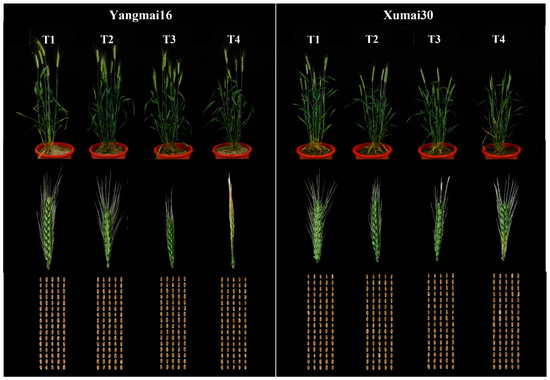
Figure 1.
The plants, spikes, and grains of Yangmai16 and Xumai30 under different low-temperature treatments.

Table 1.
Summary of the treatments.
The temperature in the artificial climate chambers was controlled precisely and was consistent with the daily changing patterns of temperature in the atmosphere during the treatment stages (Figure 2). To ensure sufficient irradiation for wheat growth, halogen lamps were employed in each chamber. The light intensity in the chambers at noon with sunshine and cloudy weather was approximately 1380 μmol m−2 s−1 and 240 μmol m−2 s−1, respectively. In addition, to keep the CO2 concentration and humidity in the chambers consistent with the ambient environment, two fans were used in each chamber.
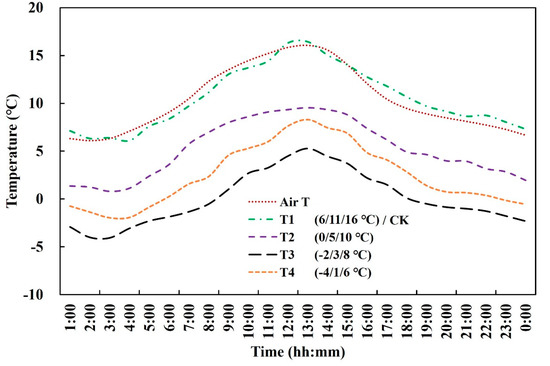
Figure 2.
Daily temperature dynamics in one environment−controlled artificial climate chamber on 8 March 2018. Air T: ambient temperature.
2.2. Sample Preparation and Measurement
The heads of 9 pots (3 pots per replication) for each treatment were harvested at maturity; then, they were air-dried for two months to obtain the grain (indehiscent fruit, named caryopsis, characteristic of Poaceae) before milling.
The amino acid compositions were measured using an amino acid analyzer (S-433D; SYKAM, Germany) according to the method by Rubin [25]. Based on whether the amino acids can be synthesized in the human body, amino acids can be divided into essential amino acids and nonessential amino acids. In this study, the essential amino acids included leucine (Leu), phenylalanine (Phe), valine (Val), isoleucine (Ile), lysine (Lys), threonine (Thr), and methionine (Met), and the nonessential amino acids included arginine (Arg), glycine (Gly), serine (Ser), tyrosine (Tyr), cystine (Cys), glutamic acid (Glu), proline (Pro), aspartic acid (Asp), alanine (Ala), and histidine (His).
2.3. Amino Acid Scoring Method
2.3.1. Amino Acid Score
The amino acid score (AAS), which refers to the ratio between the amino acid of the test protein and the amino acid of the reference protein, was calculated according to the FAO/WHO [26]. The reference protein contains various essential amino acids, and the content of each essential amino acid can completely satisfy the needs of the human body. The amino acid in the test protein that showed the lowest proportion was termed as the first limiting amino acid, and the ratio obtained was the score. The AAS equation is as follows:
2.3.2. Ratio Coefficient of Amino Acids
The ratio coefficient of amino acids (RC) is the ratio of amino acids in food equivalent to the reference amino acid, which was designed based on the theory of amino acid balance to evaluate the nutritional value of proteins. When RC = 1, it indicates that the amino acids in the test protein are consistent with the reference amino acid. If RC > 1, it indicates that the amino acid in the test protein has a relative surplus; conversely, if RC < 1, the amino acid in the test protein is relatively insufficient [6]. The RC formula is calculated as follows:
where is the average AAS for each amino acid.
2.3.3. Essential Amino Acid Index
The chemical index of essential amino acid index (EAAI) is used to evaluate the nutritional status of a protein. The EAAI is defined as the geometric mean of the egg ratio, i.e., ratios of essential amino acids in a protein to their respective amounts in the standard protein (usually egg protein). The larger the EAAI is, the more balanced the amino acid composition and the higher the protein quality and utilization [2]. The EAAI is calculated as follows:
where a, b, c, …, and x refer to the amino acid content in the test protein; A, B, C, …, and X refer to the amino acid content in the standard protein (egg protein), respectively; and n is the number of essential amino acids.
2.4. Statistical Analysis
Two years of experimental data were statistically analyzed by ANOVA. All the analysis were performed with SPSS 24.0 (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Effects of Low-Temperature on the Amino Acid Content in Wheat Grain
As shown in Figure 2, the impact of low-temperature intensities and periods on the amino acid content in wheat grain varied between the two cultivars. For the cold-sensitive cultivar, Yangmai16, the contents of total amino acids, essential amino acids, and nonessential amino acids exhibited increasing trends with the decrease in low-temperature intensity (from T4 to T1) when low-temperature treatment was applied during the jointing stage, while the amino acid contents showed the opposite trend (except for T2) when low-temperature treatment was applied during the booting stage. The contents of total amino acids, essential amino acids, and nonessential amino acids under two low-temperature treatments (during both jointing and booting stages) showed a single-peak curve distribution, with the highest content under T3 (−2/3/8 °C, Tmin/Tavg/Tmax). In general, weak low temperature (T2) had the greatest effect on total amino acids, essential acids, and nonessential acids during the jointing stage, followed by the double stage (both during the jointing and booting stages) and the booting stage. However, under medium low temperature (T3), the total amino acids, essential acids, and nonessential acids had the greatest effect during the double stage, followed by jointing and booting, while the opposite trends were found under the strong-low-temperature condition (T4) (Figure 3a–c).
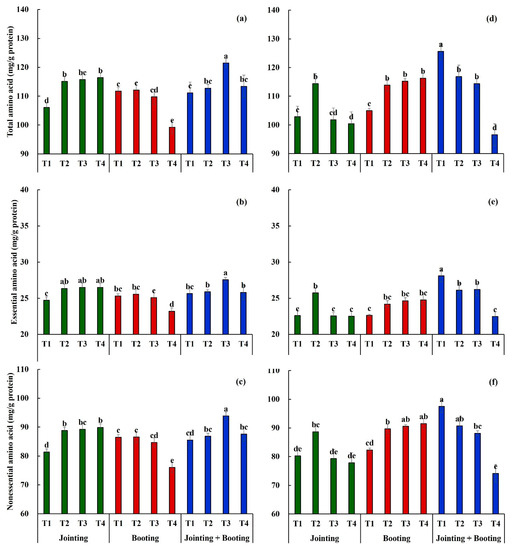
Figure 3.
The contents of total amino acids, essential amino acids, and nonessential amino acids of Yangmai16 (a–c) and Xumai30 (d–f) under different low-temperature treatments. Vertical bars represent the standard deviation of the mean. Different lowercase letters indicate significant differences at the 0.05 level.
For the cold-tolerant cultivar, Xumai30, the contents of total amino acids, essential amino acids, and nonessential amino acids also showed a single-peak curve distribution when low-temperature treatment was applied during the double stage, but with the highest content under T2 (0/5/10 °C, Tmin/Tavg/Tmax). The contents of total amino acids, essential amino acids, and nonessential amino acids exhibited increasing trends with the decrease in low-temperature intensity when low-temperature treatment was applied during the booting stage, which was opposite to the results of Yangmai16. Moreover, after double low-temperature treatments, the trends of the amino acid contents decreased with the increase in low-temperature intensity, which was also opposite to the result of Yangmai16. In general, the total amino acids, essential amino acids, and nonessential acids had the greatest effect when the weak low-temperature treatment (T2) occurred during the jointing stage, followed by the booting stage. However, when medium low temperature (T3) occurred, the amino acids were affected the most during the booting stage, followed by the double stage, while the opposite trends were detected when strong low temperature (T4) occurred (Figure 4d–f).
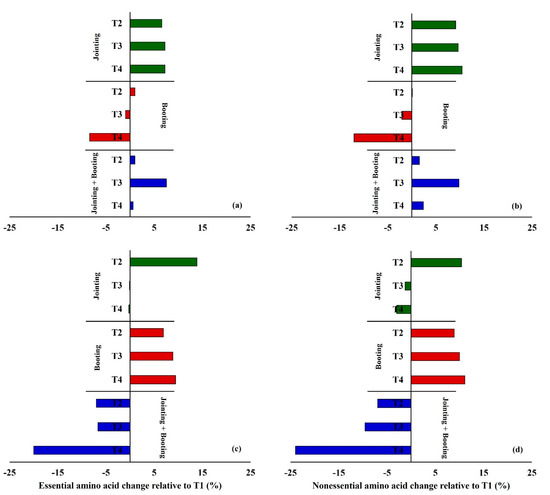
Figure 4.
Changes in essential amino acids and nonessential amino acids of Yangmai16 (a,b) and Xumai30 (c,d) relative to T1 under different low-temperature treatments.
Moreover, the nonessential amino acids were more sensitive to low temperature than the essential amino acids for both Yangmai16 (except for T2 during booting) and Xumai30 (except for T2 during jointing). For Yangmai16, compared with T1, the essential amino acids and nonessential amino acids during jointing increased by 6.62% and 9.12%, 7.24% and 9.66%, and 7.25% and 10.47% under T2, T3, and T4, respectively. The essential amino acids and nonessential amino acids during booting increased by 1.02% and 0.10%, −0.94% and −2.08%, and −8.38% and 12.01% under T2, T3, and T4, respectively, while the essential amino acids and nonessential amino acids during both jointing and booting increased by 0.98% and 1.54%, 7.53% and 9.82%, and 0.63% and 2.42%, respectively (Figure 3a,b). For Xumai30, under T2, T3, and T4, the essential amino acids and nonessential amino acids increased by 13.90% and 10.43%, −0.14% and −1.25%, and −0.29% and −3.00% during jointing; 6.89% and 8.94%, 8.89% and 10.02%, and 9.44% and 11.15% during booting; and −6.96% and −7.02%, −6.67% and 9.59%, and −20.01% and −23.94% during both jointing and booting, respectively (Figure 3c,d).
3.2. Effects of Low Temperature on the Amino Acid Composition in Wheat Grain
As shown in Figure 5, the impact of low-temperature intensities and periods on the amino acid compositions in wheat grain also varied between the cultivars and treatments. For the cold-sensitive cultivar, Yangmai16, low-temperature treatments during the jointing and double stages (both jointing and booting) increased all the amino acid compositions, except for Thr under T2 during jointing, and Ile and Pro under T2, and Val, Ile, and Pro under T4 during the double stage. Moreover, the greatest effect of low temperature during jointing on the essential and nonessential amino acid compositions was on Met under T2 and Cys under T4, with changes of 31.85% and 16.52% relative to T1. The greatest effect of low temperature during the double stage on the essential and nonessential amino acid compositions were Thr and Ser under T3, with changes of 14.75% and 11.78% relative to T1, respectively. However, low-temperature treatments during the booting stage under T4 decreased all the amino acid compositions, with the greatest effect on essential and nonessential amino acid compositions, Met (−17.90%) and Cys (−19.75%), while the effect trends of low temperature on the amino acid compositions under T2 and T3 were different, with increases observed in Phe, Lys, Thr, Gly, Ser, Tyr, Cys, Glu, and Asp under T2, and in Phe, Val, Ile, Lys, Gly, Tyr, and Asp under T3.
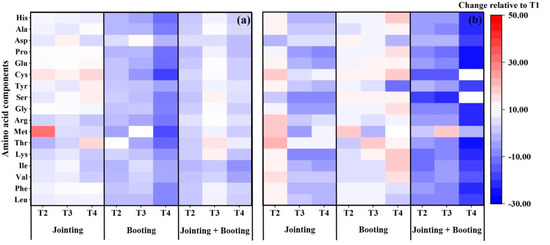
Figure 5.
The relative contents of amino acid compositions of Yangmai16 (a) and Xumai30 (b) under different low-temperature treatments.
For the cold-tolerance cultivar, Xumai30, low-temperature treatments during the booting stage increased all the amino acid compositions (except for Met under T3 and Try under T4) but decreased all the amino acid compositions when low-temperature treatments were applied during the double stage (except for Met under T2 and T3). The greatest effect of low temperature during the booting stage on the essential and nonessential amino acid compositions was on Met and Cys under T4, with changes of 52.62% and 19.43% relative to T1, respectively. The greatest effect of low temperature during the double stage on the essential and nonessential amino acid compositions was on Thr (−25.81%) and Cys (−34.41%) under T4. Moreover, the essential and nonessential amino acid compositions were increased under T2 during jointing relative to T1, with the greatest increase observed in Thr (22.65%) and Cys (17.40%), while the effect trends of the amino acid compositions under T3 and T4 were different, with increasing trends observed for Leu, Val, Thr, Arg, Tyr, Cys, Asp, and Ala under T3, and for Val, Thr, Met, Arg, Cys, Asp, and Ala under T4.
In addition, the ANOVA showed that the effects of low-temperature intensity (T) and the interaction of low-temperature intensity and stage (T × S) were not significant on the essential amino acids for any cultivars, except for the effects of T on Lys of Yangmai16 and on Ile of Xumai30, and the effect of T × S on Thr of Yangmai16 and on Val and Ile of Xumai30. However, the effects of the low-temperature stage (S) on the essential amino acids of Yangmai16 were significant except for Lys, Thr, and Met, but only Phe was significant at the 0.01 level, while the effects of the low-temperature stage (S) on Xumai30 were highly significant for Val, Ile, Thr, and Met (p < 0.01). For the nonessential amino acids, except for the low-temperature intensity (T) on Gly, Glu, and Pro of Yangmai16, and on Ser and Tyr of Xumai30, the effects of the low-temperature intensity (T) were significant for the nonessential amino acids for both cultivars. However, the effects of the low-temperature stage (S) of Yangmai16 were significant on the nonessential amino acid compositions, except for Cys, Asp, and His, and the effects of the interaction of low-temperature intensity and stage (T × S) were also significant on the nonessential amino acid compositions, except for Arg, Tyr, and Asp. For Xumai30, the effects of the low-temperature stage (S) were highly significant for Ser, Tyr, Asp, and His at the 0.01 level, while the effects of T × S were only highly significant for Ser at the 0.01 level (Table 2).

Table 2.
Effects of different low-temperature intensities (T), stages (S), and their interactions (T × S) on the amino acids of wheat.
3.3. Evaluation of the Effect of Low Temperature on the Nutritional Value of Amino Acids in Wheat Grain
3.3.1. Effects of Low Temperature on Amino Acid Score (AAS) in Wheat Grain
In general, the highest amino acid score (AAS) of each essential amino acid composition was found under T2 for both cultivars when low temperature was applied during the jointing stage. However, when low temperature was applied during the booting stage, the highest value of AAS for the cold-sensitive cultivar (Yangmai16) was found under T1, and for the cold-tolerant cultivar (Xumai30), it was found under T2. When low temperature was applied during both the jointing and booting stages, the highest AAS of each essential amino acid composition was observed under T3 for Yangmai16 and under T1 for Xumai30.
Among the essential amino acid compositions, the highest AAS was found for Phe + Tyr, and the scores in most low-temperature treatments were higher than 100, which means that the content of Phe + Tyr was higher than the standard protein amino acid composition. However, the lowest AAS was found for Lys, indicating that Lys was the first limiting amino acid under the different low-temperature treatments. Different from the first limiting amino acid, the second limiting amino acid varied among low-temperature treatments. The second limiting amino acid of Yangmai16 changed from Met + Cys to Ile under the T3 and T4 treatments during the jointing stage, while it changed from Ile to Met + Cys under the T3 and T4 treatments during the booting stage. The second limiting amino acid did not change under low-temperature treatment during the double stage, and it was always Ile. For the cold-tolerant cultivar, Xumai30, the second limiting amino acid was Met + Cys, which changed to Ile under the serious low-temperature treatment (T4) during jointing, while the second limiting amino acid was always Ile under the different low-temperature treatments during the booting stage. Moreover, the second limiting amino acid was Val under T1, T2, and T3, while it was Met + Cys under the serious low-temperature treatment (T4) when low temperature was applied during both the jointing and booting stages (Figure 6).
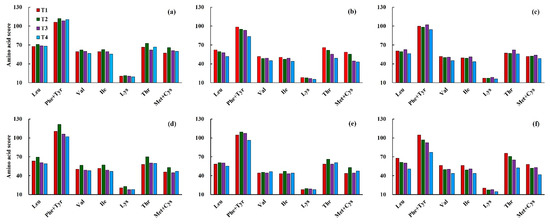
Figure 6.
Effects of different low-temperature intensities and stages on the amino acid score of Yangmai16 (a–c) and Xumai30 (d–f). (a,d) jointing stage; (b,e) booting stage; (c,f) jointing and booting stages.
3.3.2. Effects of Low Temperature on the Ratio Coefficient of Amino Acids (RC) in Wheat Grain
In general, the effects of low-temperature intensity and stage on the ratio coefficient (RC) of different amino acid compositions were not significant (Figure 7). The highest RC of each essential amino acid composition was found under T2 for the cold-sensitive cultivar of Yangmai16 (except for Phe + Tyr), and under T1 for the cold-tolerant cultivar of Xumai30 (except for Thr and Met + Cys) when low temperature was applied during the jointing stage. When low-temperature treatments occurred during the booting stage, the highest RC of each essential amino acid composition was found under T3 (Thr and Met + Cys under T1) and T2 (Val under T4 and Thr under T3) for Yangmai16 and Xumai30, respectively. However, when low-temperature treatment occurred during both the jointing and booting stages, the highest RC of each essential amino acid composition was under T3 and T4 for Yangmai16 and under T2 and T4 for Xumai30.
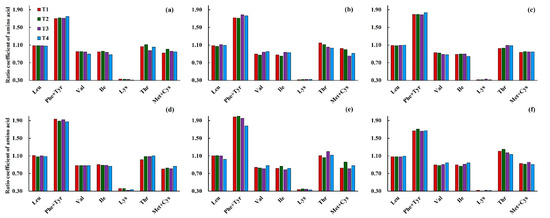
Figure 7.
Effects of different low-temperature intensities and stages on the ratio coefficient of amino acids for Yangmai16 (a–c) and Xumai30 (d–f). (a,d) jointing stage; (b,e) booting stage; (c,f) jointing and booting stages.
According to the Modern Nutrition Society, a lack of essential amino acids affects the nutritional value of proteins, while an excess of certain amino acid compositions also limits the nutritional value of proteins [27]. As shown in Figure 8, the RC values for Leu, Thr, and Phe + Tyr in both cultivars were >1 under all low-temperature treatments, especially for Phe + Tye, indicating that the contents of Leu, Thr, and Phe + Tyr were excessive in wheat grains. However, the RC values for Lys in both Yangmai16 and Xumai30 under all low-temperature treatments ranged from 0.31 to 0.36, which is very low, indicating that the contents of Lys were scarce in wheat grains and limited the nutritional value of proteins. For the other essential amino acids of Val, Ile, and Met + Cys, the RC values were closer to the standard amino acid (~1), but some of them decreased with the decrease in temperature, which means that low temperature had a negative effect on them.
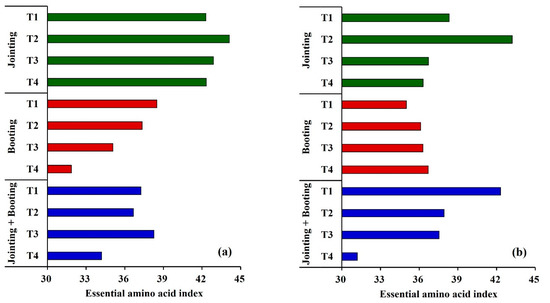
Figure 8.
Effects of different low-temperature intensities and stages on the essential amino acid index of Yangmai16 (a) and Xumai30 (b).
3.3.3. Effects of Low Temperature on the Essential Amino Acid Index (EAAI) of Wheat Grain
The larger the essential amino acid index (EAAI), the more balanced the composition of essential amino acids in the protein and the higher the quality and utilization of the protein [13]. As shown in Figure 7, the EAAI varied with the low-temperature treatments. The EAAI of Yangmai16 decreased as the temperature decreased during booting, and the EAAI of Xumai30 decreased under low-temperature treatments applied during the double stage, indicating that low temperature had negative effects on protein quality. However, the EAAI of Xumai30 increased with the decrease in temperature when low temperature was applied during the booting stage, implying that the low temperature that occurred during the booting stage might be beneficial for the improvement of protein quality for the cold-tolerant cultivar. Moreover, the highest EAAI values of Yangmai16 and Xumai30 were under T2 during jointing and under T3 for Yangmai16 during both the jointing and booting low-temperature treatment stages.
4. Discussion and Conclusions
4.1. Responses of Amino Acids in Wheat Grains to Low Temperature
The grain quality of cereal crops has a substantial influence on human health. Due to insufficient protein, mineral elements, and vitamins, nearly 2 billion people in the world suffer from malnutrition, which directly and indirectly affects human health [28]. With the development of people’s living level, the demand for high-quality crop products is increasing. The connection between food security and high quality has already extended beyond production per se [29]. From the perspective of human nutrition, protein can promote the growth and development of the human body and provide energy for human health. As the basic unit of protein, amino acids play an important role in the processes of protein synthesis [30].
Temperature, as one of the most important climatic factors, is of great significance in regulating crop growth and grain quality [8,21,31]. Previous studies focused on the effects of the increase in temperature on crop growth and the determination of yield and quality formation [9,32,33]. As a consequence of climate change, low-temperature stress is responsible for serious agricultural production losses and grain quality deterioration [15,24,34], although some areas have observed a reduction in low-temperature stress events in recent years due to global warming [35]. Most plants tend to survive and continue their life cycle by developing their tolerance ability under increasingly freezing degrees [36]. For example, in the long-term evolutionary process, cereal crops produce a large number of metabolites, such as amino acids, sugars, etc., forming their own physiological defense mechanisms to alleviate the impact of stress and adversity on them [14,37]. Studies have reported that amino acids are important small molecules for the synthesis of structural proteins and stress proteins [38]. They can be used as osmoprotectants to resist abiotic stress, and they can also adjust the nitrogen metabolism of plants by increasing the concentration of certain amino acids [39]. García found that the contents of phenylalanine, glutamate, and proline in grains increased with the increase in temperature during wheat grain filling [13]. The reasons may be that high temperature affects the upregulated expression of corresponding enzyme genes, which is conducive to the synthesis and transportation of corresponding amino acid components, such as alanine aminotransferase gene and arginine decarboxylase. In this study, the total amino acid content and essential amino acid content of Yangmai16 after low-temperature treatments during the jointing and double (both during jointing and booting) stages and of Xumai30 during the booting stage increased compared with that under T1, while the values of essential amino acids/total amino acids and essential amino acids/nonessential amino acids decreased, which is similar to the results of the studies mentioned above under temperature-increasing conditions [13]. The increase in total amino acid content and essential amino acid content indicates that when wheat is exposed to low temperature, the total amino acid content increases to reduce the damage caused by low temperature [36].
As an important osmotic adjustment substance, proline (Pro) is closely related to the cold resistance of plants. When encountering low temperature, the content of Pro rises to effectively increase the concentration of cell fluid, lower the freezing point of cells, and reduce the lethal rate of protoplasm injured by freezing under low-temperature conditions, which helps to protect the integrity and stability of cell membranes and increase the cold resistance of plants [40,41]. The Pro content of wheat in this study also showed an upward trend after low-temperature treatment in certain stages, which is consistent with the above results. However, the content of Pro in Yangmai16 after low-temperature treatment during the booting stage and in Xumai30 during the double stage decreased compared with the CK (T1), indicating that the effect of low temperature on Pro varies depending on the variety and treatment stage [42]. In addition to Pro, other amino acids may participate in the response of plants to low temperature. For example, the contents of Asp and Ser decreased after low-temperature treatment. This may be because some hydrophilic amino acids participate in the synthesis of hydrophilic proteins and bind water molecules to relieve low-temperature damage to cells [43]. Moreover, the decreasing trend of Glu in wheat grain after low-temperature treatment was found in this study, which may be because Glu is a precursor of Pro synthesis [44,45], the decrease in Glu content is used to synthesize Pro, or because Glu participates in chloroplast biosynthesis to maintain photosynthetic efficiency [46,47].
4.2. Comprehensive Evaluation of the Impact of Low Temperature on Nutritional Value of Amino Acids in Wheat
The nutritional value of proteins in food mainly depends on the quantity, type, and composition ratio of amino acids, especially essential amino acids [1]. Previous studies have shown that Lys and Thr are low in wheat and are often the first limiting amino acid and the second limiting amino acid, respectively [48]. In this study, the first limiting amino acid of wheat after different low-temperature treatments was Lys, which is consistent with previous studies [2,49,50], but the second limiting amino acid varied after the different low-temperature treatments. In general, the second limiting amino acid was Ile under most low-temperature treatments, especially for the cold-sensitive wheat cultivar, Yangmai16, implying that the impact of low temperature on Ile cannot be ignored, although we need to focus more on the impact of low temperature on Lys. Apart from amino acids insufficiency, an excess of amino acids also affects the nutritional value of proteins [1]. This study found that the content of Phe + Tyr in the two wheat varieties was obviously excessive, and the range of RC was 1.70–1.86 for Yangmai16 and 1.66–2.00 for Xumai30 among the different low-temperature treatments.
In addition to analyzing the impact of low temperature on the composition of a single essential amino acid, we considered the impact of low temperature on all essential amino acids in wheat protein. In this study, the EAAI of Yangmai16 and Xumai30 under all low-temperature treatments during jointing and of Yangmai16 under T3 during the double stage increased compared with the control (T1), indicating that low temperature might be beneficial to the compositional balance of essential amino acids. However, the EAAI of Yangmai16 decreased as the temperature decreased during booting and that of Xumai30 under two low-temperature treatments (both during the jointing and booting stages) indicated that low temperature had negative effects on protein quality. A previous study has reported that low temperature could decrease the protein accumulation rate and shorten the protein active accumulation duration, which lead to the deterioration of protein quality [24]. The present study demonstrated that the early low-temperature events or cold-hardening treatments before anthesis could increase the nutritional value of amino acids of the cold-sensitive cultivar, which may improve the quality of grain protein. However, the nutritional value of amino acids of Xumai30 was decreased after two low-temperature treatments, indicating that the protein quality of the cold-tolerant cultivar could not be enhanced by cold-hardening. In general, variety-renewal and anti-freezing measures such as cold hardening should be taken to ensure wheat grain quality.
Author Contributions
Conceptualization, L.L., J.M. and W.C.; methodology, W.Q. and B.L.; data curation, X.H., Y.C. and W.Q.; writing—original draft preparation, X.H. and L.L.; writing—review and editing, L.L., Y.Z. (Yu Zhang) and L.T.; funding acquisition, Y.Z. (Yan Zhu), L.L. and B.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Science Foundation for Distinguished Young Scholars (31725020), National Natural Science Foundation of China (31872848, 32021004, 31801260, 41961124008), and Natural Science Foundation of Jiangsu Province (BK20180523).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef]
- Jood, S.; Kapoor, A.C.; Singh, R. Amino acid composition and chemical ecaluation of protein qualtiy of cereals as affected by insect indestation. Plant Foods Hum. Nutr. 1995, 48, 159–167. [Google Scholar] [CrossRef]
- Zhong, Y.; Yang, M.; Cai, J.; Wang, X.; Zhou, Q.; Cao, W.; Dai, T.; Jiang, D. Nitrogen topdressing timing influences the spatial distribution patterns of protein components and quality traits of flours from different pearling fractions of wheat (Triticum aestivum L.) grains. Field Crops Res. 2018, 216, 120–128. [Google Scholar] [CrossRef]
- Boila, R.J.; Stothers, S.C.; Campbell, L.D. The relationship between the concentrations of individual amino acids and protein in wheat and barley grown at selected locations throughout Manitoba. Can. J. Anim. Sci. 1996, 76, 163–169. [Google Scholar] [CrossRef]
- Anjum, F.M.; Ahmad, I.; Butt, M.S.; Sheikh, M.A.; Pasha, I. Amino acid composition of spring wheats and losses of lysine during chapati baking. J. Food Compos. Anal. 2005, 18, 523–532. [Google Scholar] [CrossRef]
- Graciela, C.J.; Francisco, A.V.O.; Maria, I.G.H. Amino acid composition, score and in vitro protein digestibility of foods community consumed in Norhwest Mexico. Nutr. Hosp. 2013, 28, 365–371. [Google Scholar]
- Mladenov, N.; Przulj, N.; Hristov, N.; Djuric, V.; Milovanovic, M. Cultivar-by-environment interactions for wheat quality traits in semiarid conditions. Cereal Chem. 2011, 78, 363–367. [Google Scholar] [CrossRef]
- Peterson, C.J.; Graybosch, R.A.; Baenziger, P.S.; Grombacher, A.W. Genotype and environment effects on quality characteristics of hard red winter wheat. Crop Sci. 1992, 32, 98–103. [Google Scholar] [CrossRef]
- Malik, A.H.; Kuktaite, R.; Johansson, E. Combined effect of genetic and environmental factors on the accumulation of proteins in the wheat grain and their relationship to bread-making quality. J. Cereal Sci. 2013, 57, 170–174. [Google Scholar] [CrossRef]
- Liu, P.; Guo, W.; Jiang, Z.; Pu, H.; Feng, C.; Zhu, X.; Peng, Y.; Kuang, A.; Little, C.R. Effects of high temprature after anthesis on starch granules in grains of wheat (Triticum aestivum L.). J. Agric. Sci. 2011, 149, 159–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Y.H.; Lv, Z.Y.; Zhang, Y.; Ma, L.L.; Qin, B.Y.; Liu, Q.X.; Zhang, W.J.; Ma, S.Y.; Ma, C.X.; Huang, Z.L. Pre-anthesis night warming improves post-anthesis physiological activity and plant productivity to post-anthesis heat stress in winter wheat (Triticum aestivum L.). Environ. Exp. Bot. 2022, 197, 104819. [Google Scholar] [CrossRef]
- Xin, Z.H.; Guo, J.P.; Tan, K.Y.; Liu, K.W.; Yang, R.G.; Zhang, L.H.; Sun, Y. Relationship between amino acid quality of winter wheat and meteorological ecological factors. J. Arid Meteoro. 2020, 38, 148–156. [Google Scholar]
- García Del Moral, L.F.; Rharrabti, Y.; Martos, V.; Royo, C. Environmentally induced changes in amino acid composition in the grain of durum wheat grown under different water and temperature regimes in a mediterranean environment. J. Agr. Food Chem. 2007, 55, 8144–8151. [Google Scholar] [CrossRef] [PubMed]
- Labuschagne, M.T.; Elago, O.; Koen, E. The influence of temperature extremes on some quality and starch characteristics in bread, biscuit and durum wheat. J. Cereal Sci. 2009, 49, 184–189. [Google Scholar] [CrossRef]
- Xia, L.J.; Liu, L.L.; Asseng, S.; Xia, Y.M.; Tang, L.; Liu, B.; Cao, W.X.; Zhu, Y. Estimating spring frost and its impact on yield across winter wheat in China. Agr. Forest Meteorol. 2018, 260, 154–164. [Google Scholar] [CrossRef]
- Liu, L.L.; Song, H.; Shi, K.J.; Liu, B.; Zhang, Y.; Tang, L.; Cao, W.X.; Zhu, Y. Response of wheat grain quality to low temperature during jointing and booting stages—On the importance of considering canopy temperature. Agr. Forest Meteorol. 2019, 278, 107658. [Google Scholar] [CrossRef]
- Kodra, E.; Steinhaeuser, K.; Ganguly, A.R. Persisting cold extremes under 21st-century warming scenarios. Geophys. Res. Lett. 2011, 38, L08705. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.Q.; Ren, G.Y. Change in extreme temperature event frequency over mainland China, 1961–2008. Clim. Res. 2011, 50, 125–139. [Google Scholar] [CrossRef] [Green Version]
- Zheng, B.Y.; Chapman, S.C.; Christopher, J.T.; Frederiks, T.M.; Chenu, K. Frost trends and their estimated impact on yield in the Australian wheatbelt. J. Exp. Bot. 2015, 66, 3611–3623. [Google Scholar] [CrossRef] [Green Version]
- Asseng, S.; Foster, I.A.N.; Turner, N.C. The impact of temperature variability on wheat yields. Glob. Change Biol. 2011, 17, 997–1012. [Google Scholar] [CrossRef]
- Liu, B.; Liu, L.L.; Tian, L.Y.; Cao, W.X.; Zhu, Y.; Asseng, S. Post-heading heat stress and yield impact in winter wheat of China. Glob. Change Biol. 2014, 20, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Qiu, X.L.; Chen, J.; Cammarano, D.; Ge, Z.L.; Ruane, A.C.; Liu, L.L.; Tang, L.; Cao, W.X.; Liu, B.; et al. Impacts of 1.5 °C and 2.0 °C global warming above pre-industrial on potential winter wheat production of China. Eur. J. Agron. 2020, 120, 126149. [Google Scholar] [CrossRef]
- Triboï, E.; Martre, P.; Triboï-Blondel, A.M. Environmentally-induced changes in protein composition in developing grains of wheat are related to changes in total protein content. J. Exp. Bot. 2003, 54, 1731–1742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.H.; Gu, K.J.; Gu, D.X.; Zhang, S.M.; Wu, J.J. Quantifying the effect of low-temperature events on the grain quality formation of wheat. J. Cereal Sci. 2021, 100, 103257. [Google Scholar] [CrossRef]
- Rubin, M.; Schoonouer, D.R.; Bossard, E.H. Amino acid profiles of corn and soybean meal by a modified analysis technique. Poult Sci. J. 1975, 54, 1811–1817. [Google Scholar]
- World Health Organization. Energy and Protein Requirements; World Health Organization: Rome, Italy, 1973. [Google Scholar]
- Björck, I.; Asp, N.G.; Dahlqvist, A. Protein nutritional value of extrusion-cooked wheat flours. Food Chem. 1984, 15, 203–214. [Google Scholar] [CrossRef]
- Bailey, R.L.; West, K.P.; Black, R.E. The epidemiology of global micronutrient deficiencies. Ann. Nutr. Metab. 2015, 66, 22–33. [Google Scholar] [CrossRef]
- Altenbach, S.B. New insights into the effects of high temperature, drought and post-anthesis fertilizer on wheat grain development. J. Cereal Sci. 2012, 56, 39–50. [Google Scholar] [CrossRef]
- Khoury, C.K.; Bjorkman, A.D.; Dempewolf, H.; Ramirez-Villegas, J.; Guarino, L.; Jarvis, A.; Rieseberg, L.H.; Struik, P.C. Increasing homogeneity in global food supplies and the implications for food security. Proc. Natl. Acad. Sci. USA 2014, 111, 4001–4006. [Google Scholar] [CrossRef] [Green Version]
- Lobell, D.B.; Hammer, G.L.; Chenu, K.; Zheng, B.; McLean, G.; Chapman, S.C. The shifting influence of drought and heat stress for crops in northeast Australia. Glob. Change Biol. 2015, 21, 4115–4127. [Google Scholar] [CrossRef]
- Asseng, S.; Ewert, F.; Martre, P. Rising temperatures reduce global wheat production. Nat. Clim. Chang. 2015, 5, 143–147. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, D.Z.; Zhang, H.X.; Asseng, S.; Yin, T.W.; Qiu, X.L.; Ye, Z.; Liu, L.L.; Tang, L.; Cao, W.X.; et al. Seperating the impacts of heat stress events from rising mean temperature on winter wheat yield of China. Environ. Res. Lett. 2021, 16, 124035. [Google Scholar] [CrossRef]
- Zheng, D.X.; Yang, X.G.; Mínguez, M.I.; Mu, C.Y.; He, Q.; Wu, X. Effect of freezing temperature and duration on winter survival and grain yield of winter wheat. Agr. Forest Meteorol. 2018, 260, 1–8. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change. Global Warming of 1.5 °C: An IPCC Special Report on the Impacts Of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context Of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V., Zhai, P., Pötner, H.O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2018. [Google Scholar]
- Muhammar, A.H.; Chen, X.; Muhammad, F.; Muhammad, N.; Zhang, Y.; Xu, H.; Ke, Y.Y.; Attiogbe, K.B.; Zhang, L.L.; Li, J.C. Cold stress in wheat: Plant acclimation responses and management strateies. Front. Plant. Sci. 2021, 12, 676884. [Google Scholar]
- Frederiks, T.M.; Christopher, J.T.; Sutherland, M.W.; Borrell, A.K. Post-head emergence frost in wheat and barley: Defining the problem, assessing the damage, and identifying resistance. J. Exp. Bot. 2015, 66, 3487–3498. [Google Scholar] [CrossRef] [Green Version]
- Kovács, Z.; Simon-Sarkade, L.; Sovány, C.; Kirsch, K.; Galiba, G.; Kocsy, G. Differential effects of cold acclimation and abscisic acid on free amino acid composition in wheat. Plant Sci. 2011, 180, 61–68. [Google Scholar] [CrossRef]
- Xu, Q.; Fan, N.L.; Zhuang, L.L.; Yu, J.J. Enhanced stolon growth and metabolic adjustment in creeping bentgrass with elevated CO2 concentration. Environ. Exp. Bot. 2018, 155, 87–97. [Google Scholar] [CrossRef]
- Serpil, T.; Yasemin, E. Variation of total soluble seminal root proteins of tetraploid wild and cultivated wheat induced at cold acclimation and freezing. Acta Physiol. Plant. 2004, 26, 443–450. [Google Scholar]
- Gomes, F.P.; Oliva, M.A.; Mielke, M.S.; Almeida, A.F.; Aquino, L.A. Osmotic adjustment, proline accumulation and cell membrane stability in leaves of cocos nucifera submitted to drought stress. Sci. Hortic. 2010, 126, 379–384. [Google Scholar] [CrossRef]
- Lu, T.B.; Lv, J.Y.; Lu, H.P.; Zhang, L.S.; Wang, C.F.; Zhang, J.Q.; Zhang, X.T. The relationship of contents of protein and amino acid in winter wheat leaves with cold resistance at the turngreen stage. Acta Agric. Boreali-Occident. Sin. 2009, 18, 56–59. [Google Scholar]
- Zhao, C.X.; Zhang, R.; Niu, K.J.; Zhu, R.T.; Wang, Y.; Ma, X.; Ma, H.L. Metabolomics study of Qinghai wild Poa pratensis in response to low temperature stress. Acta Agrestia Sin. 2020, 28, 904–914. [Google Scholar]
- Fait, A.; Fromm, H.; Walter, D.; Galili, G.; Fernie, A.R. Highway or byway: The metabolic role of the GABA shunt in plants. Trends Plant Sci. 2008, 13, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Parida, A.K. Metabolomics and network analysis reveal the potential metabolites and biological pathways involved in salinity tolerance of the halophyte Salvadora persica. Environ. Exp. Bot. 2018, 148, 85–99. [Google Scholar] [CrossRef]
- Ito, H.; Takaichi, S.; Tsuji, H.; Tanaka, A. Properties of synthesis of chlorophyll a from chlorophyll b in cucumber etioplasts. J. Biol. Chem. 1994, 35, 22034–22038. [Google Scholar] [CrossRef]
- Mokochinski, J.B.; Mazzafera, P.; Sawaya, A.C.H.F.; Mumm, R.; De Vos, R.C.H.; Hall, R.D. Metabolic responses of eucalyptus species to different temperature regimes. J. Integr. Plant Biol. 2018, 60, 397–411. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Wang, C.H.; Li, F.U.; Li, K.Y.; Yang, N.; Yang, Y.E. Contents of protein and amino acid of wheat grain in different wheat production regions and their evaluation. Acta Agron. Sin. 2016, 42, 768–777. [Google Scholar] [CrossRef]
- Young, V.R.; Pellett, P.L. Plant protein in relation to human protein and amino acid nutrition. Am. J. Clin. Nutr. 1994, 59, 1203S–1212S. [Google Scholar] [CrossRef]
- Millward, D.J. The nutritional value of plant-based diets in relation to human amino acid and protein requirements. Proc. Nutr. Soc. 1999, 58, 249–260. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).