Agropyron mongolicum Keng’s Growth in Response to Nitrogen Addition Is Linked to Root Morphological Traits and Nitrogen-Use Efficiency
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Conditions and Treatments
2.2. Plant and Soil Sampling
2.3. Analysis of Biomass and Morphological Traits of the Roots
2.4. Determination of N Metabolic Enzyme Activities and N Assimilating Products
2.5. NUE Traits and Soil Available Nutrients Analysis
2.6. Statistical Analysis
3. Results
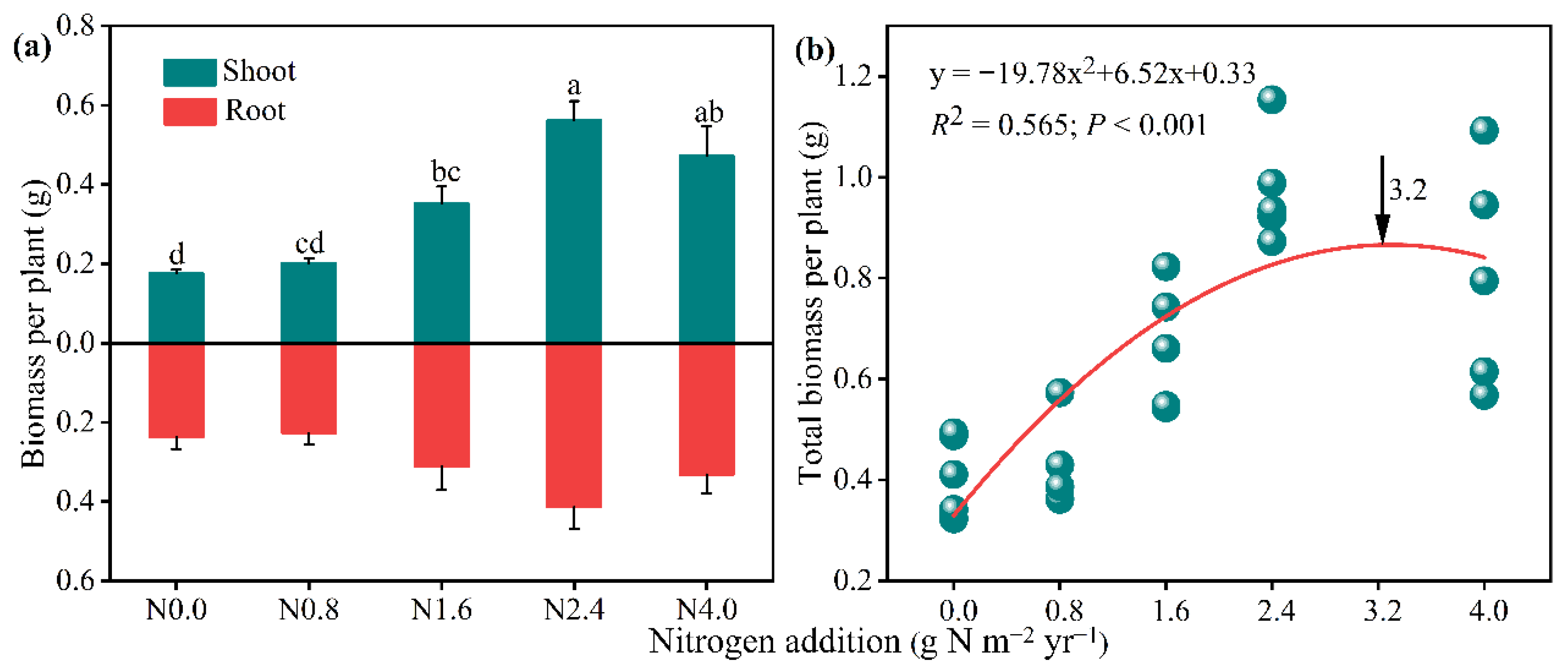
3.1. Effects of N Addition on A. mongolicum Biomass
3.2. N Addition Effects on Soil-Available Nutrient Properties and Root Morphological Traits
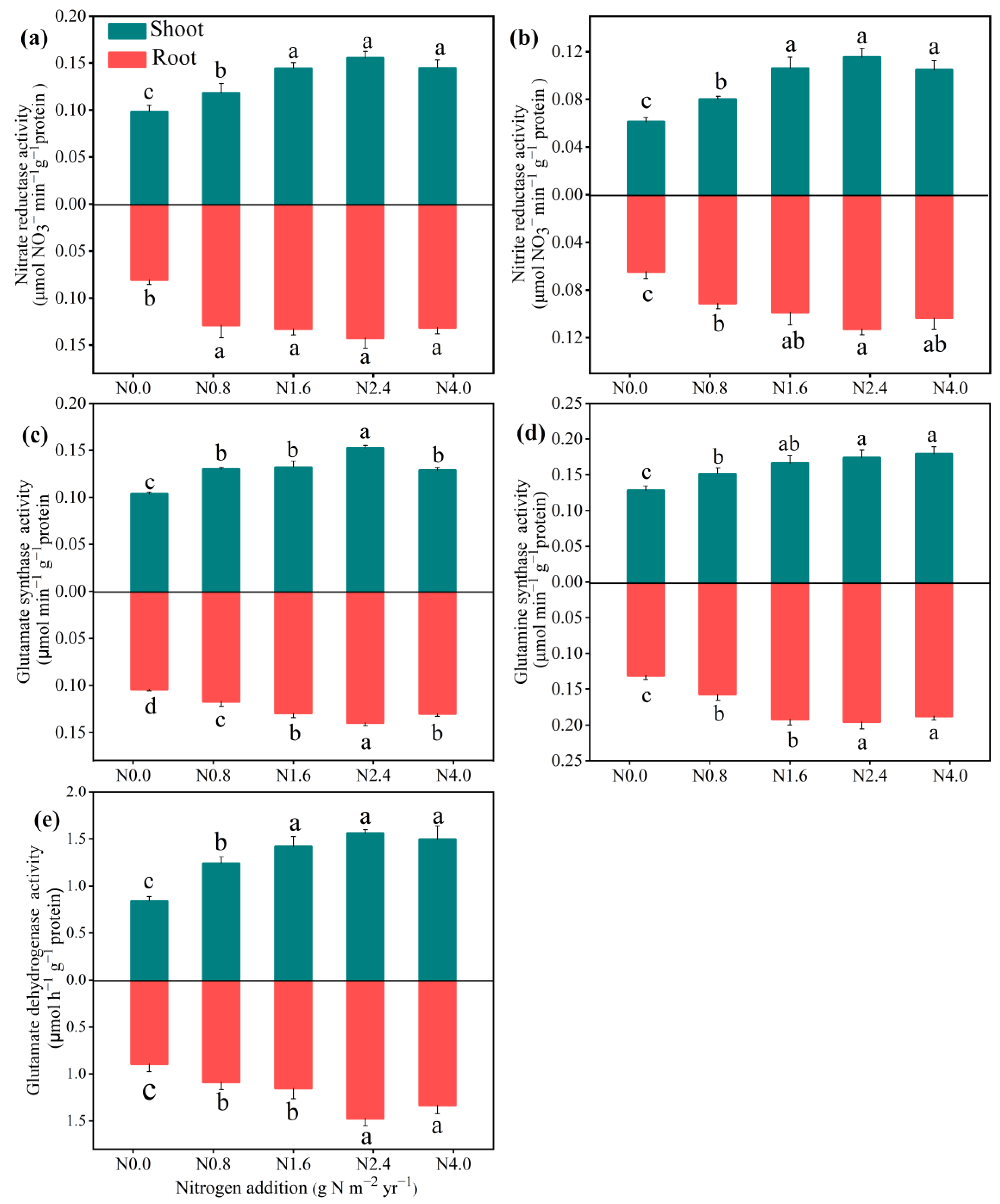
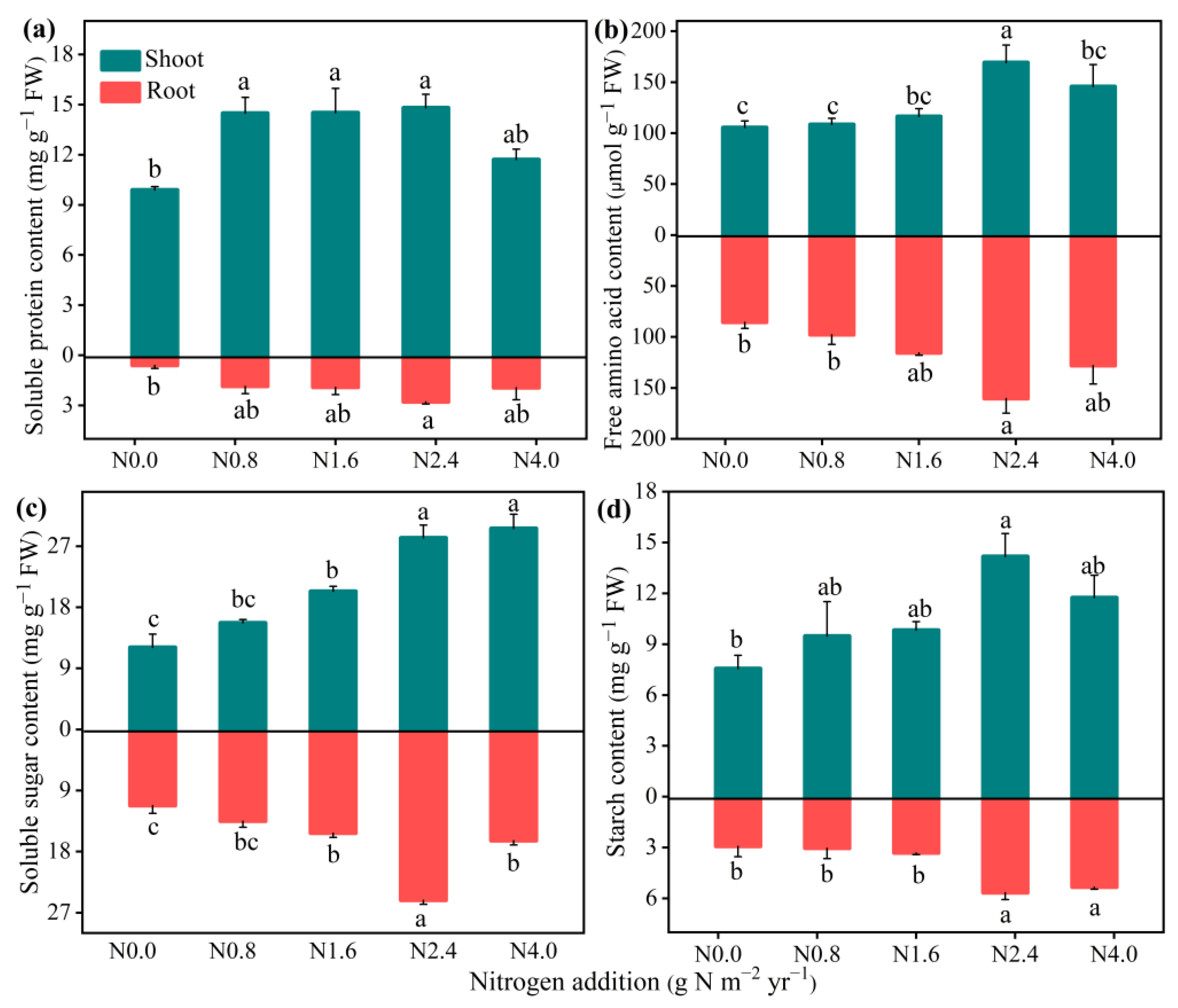
3.3. Responses of Activities of N Metabolism Enzymes and Assimilation Products in Shoot and Root to N Addition
3.4. Responses of N-Use Efficiency in A. mongolicum to N Addition
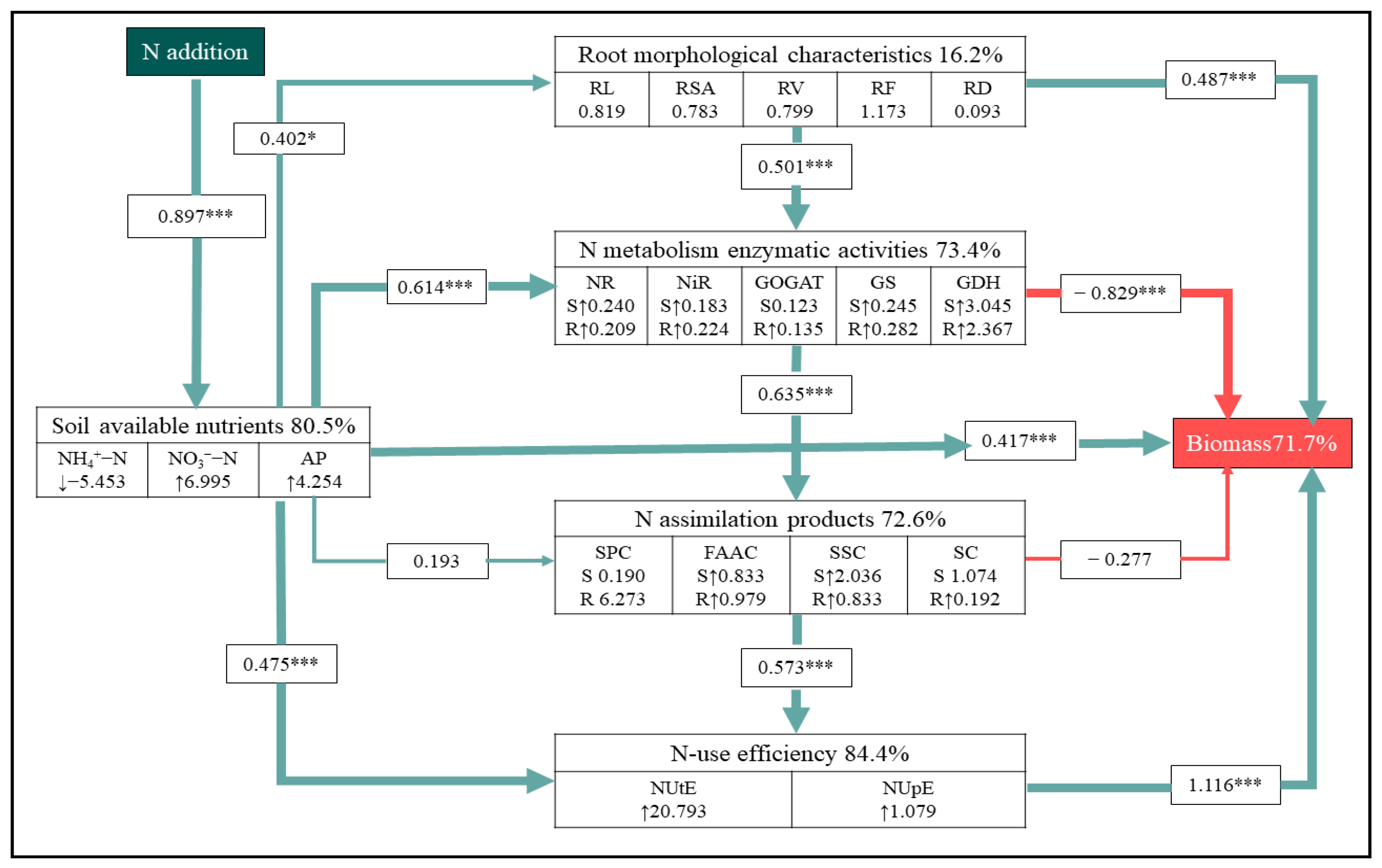
3.5. Direct and Indirect Effects of Soil-Available Nutrient and N-Use Efficiency on Biomass
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.N.; Yang, B.; Li, M.X.; Xiao, R.Q.; Rao, K.Y.; Wang, J.Q.; Zhang, T.; Guo, J.X. Community composition, structure and productivity in response to nitrogen and phosphorus additions in a temperate meadow. Sci. Total Environ. 2019, 654, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.F.; Wu, J.G.; Clark, C.M.; Naeem, S.; Pan, Q.M.; Huang, J.H.; Zhang, L.X.; Han, X.G. Tradeoffs and thresholds in the effects of nitrogen addition on biodiversity and ecosystem functioning: Evidence from inner Mongolia Grasslands. Glob. Chang. Biol. 2010, 16, 358–372. [Google Scholar] [CrossRef]
- Bai, Y.X.; She, W.W.; Zhang, Y.Q.; Qiao, Y.G.; Fu, J.; Qin, S.G. N enrichment, increased precipitation, and the effect of shrubs collectively shape the plant community in a desert ecosystem in northern China. Sci. Total Environ. 2020, 716, 135379. [Google Scholar] [CrossRef]
- Li, G.Q.; Zhao, P.; Shao, W.S.; Jin, C.Q.; Song, L.X.; Chen, Y.Y. Effect of enclosure on reproductive allocation of wheatgrass Agropyron mongolicum populations in desert steppes. Ecol. Evol. 2019, 9, 14023–14030. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Gao, C.P.; Shi, F.L.; Yun, L.; Jia, Y.S.; Wen, J.Q. Transcriptomic and proteomic analyses of drought responsive genes and proteins in Agropyron mongolicum Keng. Curr. Plant Biol. 2018, 14, 19–29. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Chen, H.Y.H. Decoupling of nitrogen and phosphorus in terrestrial plants associated with global changes. Nat. Clim. Chang. 2015, 5, 465–469. [Google Scholar] [CrossRef]
- Bowman, W.D.; Murgel, J.; Blett, T.; Porter, E. Nitrogen critical loads for alpine vegetation and soils in Rocky Mountain National Park. J. Environ. Manag. 2012, 103, 165–171. [Google Scholar] [CrossRef]
- Deng, Q.; Hui, D.F.; Dennis, S.; Reddy, K.C. Responses of terrestrial ecosystem phosphorus cycling to nitrogen addition: A meta-analysis. Glob. Ecol. Biogeogr. 2017, 26, 713–728. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, J.J.; Masclaux-Daubresse, C.; Wang, N.; Wang, H.; Zheng, B. Morphological and physiological responses to contrasting nitrogen regimes in Populus cathayana is linked to resources allocation and carbon/nitrogen partition. Environ. Exp. Bot. 2019, 162, 247–255. [Google Scholar] [CrossRef]
- Wang, X.; Guo, X.; Yu, Y.; Cui, H.; Wang, R.Q.; Guo, W.H. Increased nitrogen supply promoted the growth of non-N-fixing woody legume species but not the growth of N-fixing Robinia pseudoacacia. Sci. Rep. 2018, 8, 17896. [Google Scholar] [CrossRef]
- Yang, H.; Li, Y.; Wu, M.; Zhang, Z.H.E.; Li, L.; Wan, S. Plant community responses to nitrogen addition and increased precipitation: The importance of water availability and species traits. Glob. Chang. Biol. 2011, 17, 2936–2944. [Google Scholar] [CrossRef]
- Chen, G.T.; Tu, L.H.; Peng, Y.; Hu, H.L.; Hu, T.X.; Xu, Z.F.; Liu, L.; Tang, Y. Effect of nitrogen additions on root morphology and chemistry in a subtropical bamboo forest. Plant Soil 2016, 412, 441–451. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Wang, W.W. The decomposition of fine and coarse roots: Their global patterns and controlling factors. Sci. Rep. 2015, 5, 9940. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xue, Y.G.; Wang, Z.Q.; Yang, J.C.; Zhang, J.H. Morphological and physiological traits of roots and their relationships with shoot growth in “super” rice. Field Crops Res. 2009, 113, 31–40. [Google Scholar] [CrossRef]
- Qi, W.Z.; Liu, H.H.; Liu, P.; Dong, S.; Zhao, B.Q.; So, H.B.; Li, G.; Liu, H.D.; Zhang, J.W.; Zhao, B. Morphological and physiological characteristics of corn (Zea mays L.) roots from cultivars with different yield potentials. Eur. J. Agron. 2012, 38, 54–63. [Google Scholar] [CrossRef]
- Hu, J.; Gettel, G.; Fan, Z.B.; Lv, H.F.; Zhao, Y.M.; Yu, Y.L.; Wang, J.G.; Butterbach-Bahl, K.; Li, G.Y.; Lin, S. Drip fertigation promotes water and nitrogen use efficiency and yield stability through improved root growth for tomatoes in plastic greenhouse production. Agric. Ecosyst. Environ. 2021, 313. [Google Scholar] [CrossRef]
- Li, B.H.; Li, G.J.; Kronzucker, H.J.; Baluska, F.; Shi, W.M. Ammonium stress in Arabidopsis: Signaling, genetic loci, and physiological targets. Trends Plant Sci. 2014, 19, 107–114. [Google Scholar] [CrossRef]
- Asif, I.; Dong, Q.; Wang, Z.; Wang, X.R.; Gui, H.P.; Zhang, H.H.; Pang, N.C.; Zhang, X.L.; Song, M.Z. Growth and nitrogen metabolism are associated with nitrogen-use efficiency in cotton genotypes. Plant Physiol. Biochem. 2020, 149, 61–74. [Google Scholar] [CrossRef]
- Xu, G.H.; Fan, X.R.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef] [Green Version]
- Ju, C.X.; Buresh, R.J.; Wang, Z.Q.; Zhang, H.; Liu, L.J.; Yang, J.C.; Zhang, J.H. Root and shoot traits for rice varieties with higher grain yield and higher nitrogen use efficiency at lower nitrogen rates application. Field Crops Res. 2015, 175, 47–55. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, M.; Yao, C.S.; Zhou, X.N.; Li, W.; Zhang, Z.; Gao, Y.M.; Sun, Z.C.; Wang, Z.M.; Zhang, Y.H. Optimum Water and Nitrogen Management Increases Grain Yield and Resource Use Efficiency by Optimizing Canopy Structure in Wheat. Agronomy 2021, 11, 441. [Google Scholar] [CrossRef]
- Liu, X.J.; Zhang, Y.; Han, W.X.; Tang, A.H.; Shen, J.L.; Cui, Z.L.; Vitousek, P.; Erisman, J.W.; Goulding, K.; Christie, P.; et al. Enhanced nitrogen deposition over China. Nature 2013, 494, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Yokoyama, S.; Hiramatsu, J.I. A modified ninhydrin reagent using ascorbic acid instead of potassium cyanide. J. Biosci. Bioeng. 2003, 95, 204–205. [Google Scholar] [CrossRef]
- He, J.L.; Ma, C.F.; Ma, Y.L.; Li, H.; Kang, J.Q.; Liu, T.X.; Polle, A.; Peng, C.H.; Luo, Z.B. Cadmium tolerance in six poplar species. Environ. Sci. Pollut. Res. Int. 2013, 20, 163–174. [Google Scholar] [CrossRef]
- Yuan, X.B.; Niu, D.C.; Gherardi, L.A.; Liu, Y.B.; Wang, Y.; Elser, J.J.; Fu, H. Linkages of stoichiometric imbalances to soil microbial respiration with increasing nitrogen addition: Evidence from a long-term grassland experiment. Soil Biol. Biochem. 2019, 138, 107580. [Google Scholar] [CrossRef]
- Abenavoli, M.R.; Longo, C.; Lupini, A.; Miller, A.J.; Araniti, F.; Mercati, F.; Princi, M.P.; Sunseri, F. Phenotyping two tomato genotypes with different nitrogen use efficiency. Plant Physiol. Biochem. 2016, 107, 21–32. [Google Scholar] [CrossRef]
- Siddiqi, M.Y.; Glass, A.D.M. Utilization index: A modified approach to the estimation and comparison of nutrient utilization efficiency in plants. J. Plant Nutr. 1981, 4, 289–302. [Google Scholar] [CrossRef]
- Sánchez Rodríguez, E.; Rubio Wilhelmi, M.M.; Blasco, B.; Constán Aguilar, C.; Romero, L.; Ruiz, J.M. Variation in the use efficiency of N under moderate water deficit in tomato plants (Solanum lycopersicum) differing in their tolerance to drought. Acta Physiol. Plant. 2011, 33, 1861–1865. [Google Scholar] [CrossRef]
- Elliot George, C.; Lauchli, A. Phosphorus Efficiency and Phosphate-Iron Interaction in Maize. Agron. J. 1985, 77, 399–403. [Google Scholar] [CrossRef]
- Zong, N.; Shi, P.L.; Song, M.H.; Zhang, X.Z.; Jiang, J.; Chai, X. Nitrogen Critical Loads for an Alpine Meadow Ecosystem on the Tibetan Plateau. Environ. Manag. 2016, 57, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Ai, Z.M.; Liang, C.T.; Wang, G.L.; Liu, G.B.; Xue, S. How microbes cope with short-term N addition in a Pinus tabuliformis forest-ecological stoichiometry. Geoderma 2019, 337, 630–640. [Google Scholar] [CrossRef]
- Yuan, X.B.; Niu, D.C.; Weber-Grullon, L.; Fu, H. Nitrogen deposition enhances plant-microbe interactions in a semiarid grassland: The role of soil physicochemical properties. Geoderma 2020, 373, 114446. [Google Scholar] [CrossRef]
- Aber, J.; McDowell, W.; Nadelhoffer, K.; Magill, A.; Berntson, G.; Kamakea, M.; Fernandez, I. Nitrogen Saturation in Northern Forest Ecosystems:hypotheses revisited. BioScience 1989, 11, 921–934. [Google Scholar] [CrossRef]
- Tian, D.S.; Wang, H.; Sun, J.; Niu, S.L. Global evidence on nitrogen saturation of terrestrial ecosystem net primary productivity. Environ. Res. Lett. 2016, 11. [Google Scholar] [CrossRef] [Green Version]
- Regni, L.; Proietti, P. Effects of Nitrogen Foliar Fertilization on the Vegetative and Productive Performance of the Olive Tree and on Oil Quality. Agriculture 2019, 9, 252. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Yan, L.; Xia, J. A threefold difference in plant growth response to nitrogen addition between the laboratory and field experiments. Ecosphere 2019, 10, e02572. [Google Scholar] [CrossRef]
- Poorter, H.; Fiorani, F.; Pieruschka, R.; Wojciechowski, T.; Putten, W.H.v.d.; Kleyer, M.; Schurr, U.; Postma, J. Pampered inside, pestered outside? Differences and similarities between plants growing in controlled conditions and in the field. New Phytol. 2016, 212, 838–855. [Google Scholar] [CrossRef]
- Gong, X.Y.; Chen, Q.; Dittert, K.; Taube, F.; Lin, S. Nitrogen, phosphorus and potassium nutritional status of semiarid steppe grassland in Inner Mongolia. Plant Soil 2010, 340, 265–278. [Google Scholar] [CrossRef]
- Li, Y.; Niu, S.L.; Yu, G.R. Aggravated phosphorus limitation on biomass production under increasing nitrogen loading: A meta-analysis. Glob. Chang. Biol. 2016, 22, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Dupas, E.; Monteiro, F.A. Nitrogen and potassium, but not boron, change the morphology, production and nutrient concentration of Tanzania guineagrass roots. J. Plant Nutr. 2018, 41, 2222–2231. [Google Scholar] [CrossRef]
- Jiang, S.Y.; Sun, J.Y.; Tian, Z.W.; Hu, H.; Michel, E.J.S.; Gao, J.W.; Jiang, D.; Cao, W.X.; Dai, T.B. Root extension and nitrate transporter up-regulation induced by nitrogen deficiency improves nitrogen status and plant growth at the seedling stage of winter wheat (Triticum aestivum L.). Environ. Exp. Bot. 2017, 141, 28–40. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, C.H.; Huang, D.; Dong, Q.L.; Li, P.M.; van Nocker, S.; Ma, F.W. Physiological evaluation of nitrogen use efficiency of different apple cultivars under various nitrogen and water supply conditions. J. Integr. Agric. 2020, 19, 709–720. [Google Scholar] [CrossRef]


| Soil and Root Traits | N Addition Gradients (g N m−2 yr−1) | |||||
|---|---|---|---|---|---|---|
| N0.0 | N0.8 | N1.6 | N2.4 | N4.0 | p-Value | |
| pH | 9.07 ± 0.07 a | 9.07 ± 0.01 a | 9.06 ± 0.01 a | 8.87 ± 0.03 b | 8.88 ± 0.06 b | ** |
| NO3−-N (mg kg−1) | 1.57 ± 0.01 e | 4.26 ± 0.02 d | 6.04 ± 0.04 c | 8.08 ± 0.05 b | 9.12 ± 0.09 a | *** |
| NH4+-N (mg kg−1) | 2.52 ± 0.22 a | 2.25 ± 0.28 a | 2.1 ± 0.42 a | 1.38 ± 0.30 a | 1.55 ± 0.10 a | ns |
| AP (mg kg−1) | 2.56 ± 0.09 c | 2.66 ± 0.07 bc | 2.79 ± 0.13 bc | 2.99 ± 0.11 b | 3.39 ± 0.08 a | *** |
| N:P | 1.97 ± 0.14 e | 7.67 ± 0.17 d | 13.95 ± 0.92 c | 22.39 ± 1.0 b | 25.01 ± 0.44 a | *** |
| TRL (cm) | 2.93 ± 0.06 b | 2.98 ± 0.03 b | 3.10 ± 0.07 ab | 3.28 ± 0.06 a | 3.04 ± 0.04 b | ** |
| RSA (cm2) | 1.95 ± 0.04 b | 2.08 ± 0.04 b | 2.11 ± 0.05 b | 2.34 ± 0.04 a | 2.07 ± 0.03 b | *** |
| RV (cm3) | 0.75 ± 0.02 c | 1.22 ± 0.11 b | 1.08 ± 0.09 bc | 2.04 ± 0.12 a | 1.04 ± 0.11 bc | *** |
| RD (mm) | 0.34 ± 0.02 a | 0.35 ± 0.01 a | 0.37 ± 0.02 a | 0.39 ± 0.01 a | 0.38 ± 0.02 a | ns |
| RF | 3.27 ± 0.06 c | 3.36 ± 0.08 bc | 3.55 ± 0.06 ab | 3.69 ± 0.06 a | 3.45 ± 0.06 abc | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, A.; Wang, X.; Wang, X.; Xu, D.; Cao, B. Agropyron mongolicum Keng’s Growth in Response to Nitrogen Addition Is Linked to Root Morphological Traits and Nitrogen-Use Efficiency. Agronomy 2022, 12, 1146. https://doi.org/10.3390/agronomy12051146
Xu A, Wang X, Wang X, Xu D, Cao B. Agropyron mongolicum Keng’s Growth in Response to Nitrogen Addition Is Linked to Root Morphological Traits and Nitrogen-Use Efficiency. Agronomy. 2022; 12(5):1146. https://doi.org/10.3390/agronomy12051146
Chicago/Turabian StyleXu, Aiyun, Xing Wang, Xiaojia Wang, Dongmei Xu, and Bing Cao. 2022. "Agropyron mongolicum Keng’s Growth in Response to Nitrogen Addition Is Linked to Root Morphological Traits and Nitrogen-Use Efficiency" Agronomy 12, no. 5: 1146. https://doi.org/10.3390/agronomy12051146
APA StyleXu, A., Wang, X., Wang, X., Xu, D., & Cao, B. (2022). Agropyron mongolicum Keng’s Growth in Response to Nitrogen Addition Is Linked to Root Morphological Traits and Nitrogen-Use Efficiency. Agronomy, 12(5), 1146. https://doi.org/10.3390/agronomy12051146





