Progeny Selection to Develop a Sustainable Arabica Coffee Cultivar
Abstract
1. Introduction
2. Materials and Methods
2.1. Statistical Analysis
2.1.1. Genetic Parameter Estimates
2.1.2. Correlation between Productivity Analysis Strategies
2.1.3. Correlation between Traits
2.1.4. Selection Strategies
3. Results
3.1. Genetic Parameter Estimates
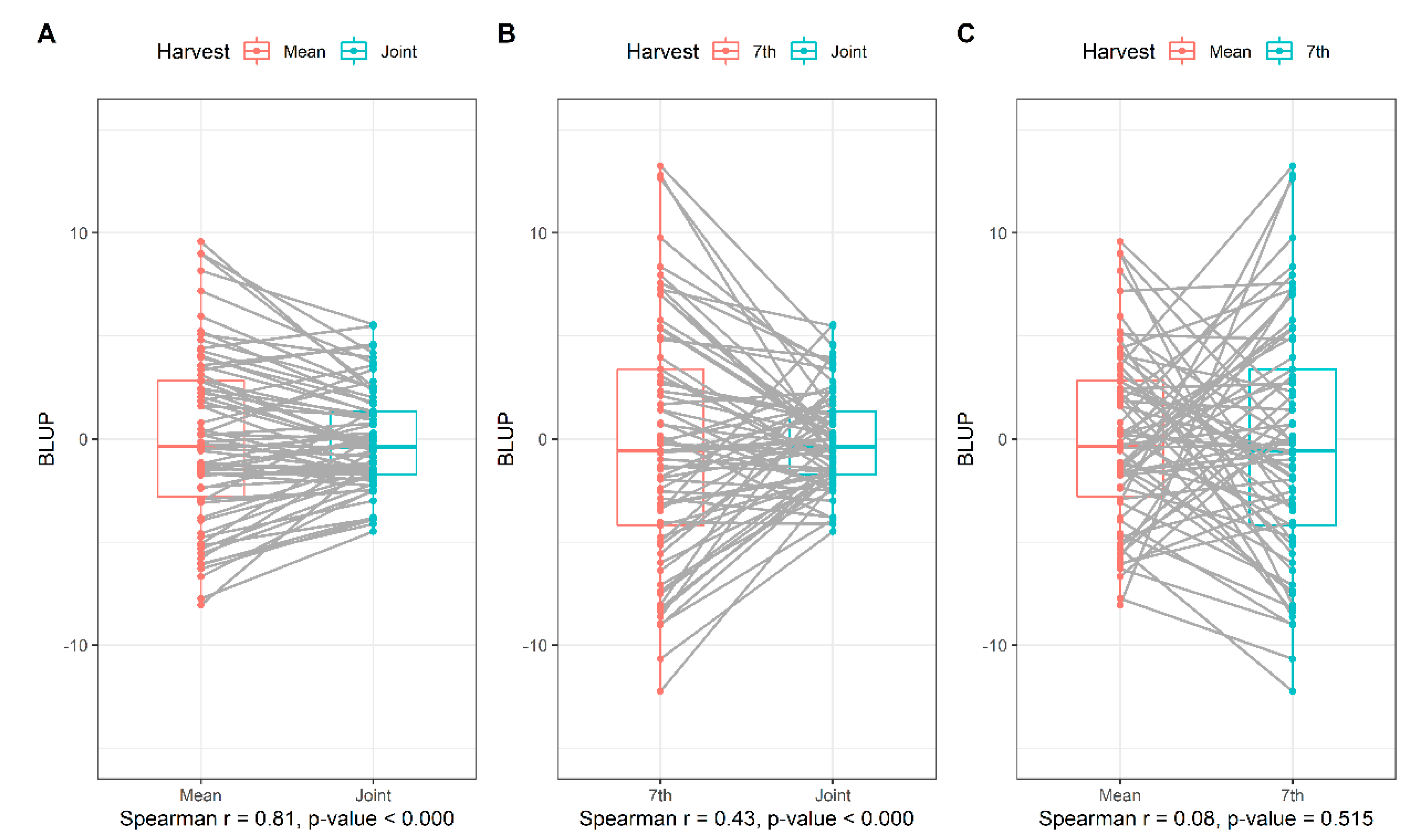
3.2. Correlation between Productivity Analysis Strategies
3.3. Correlation between Traits
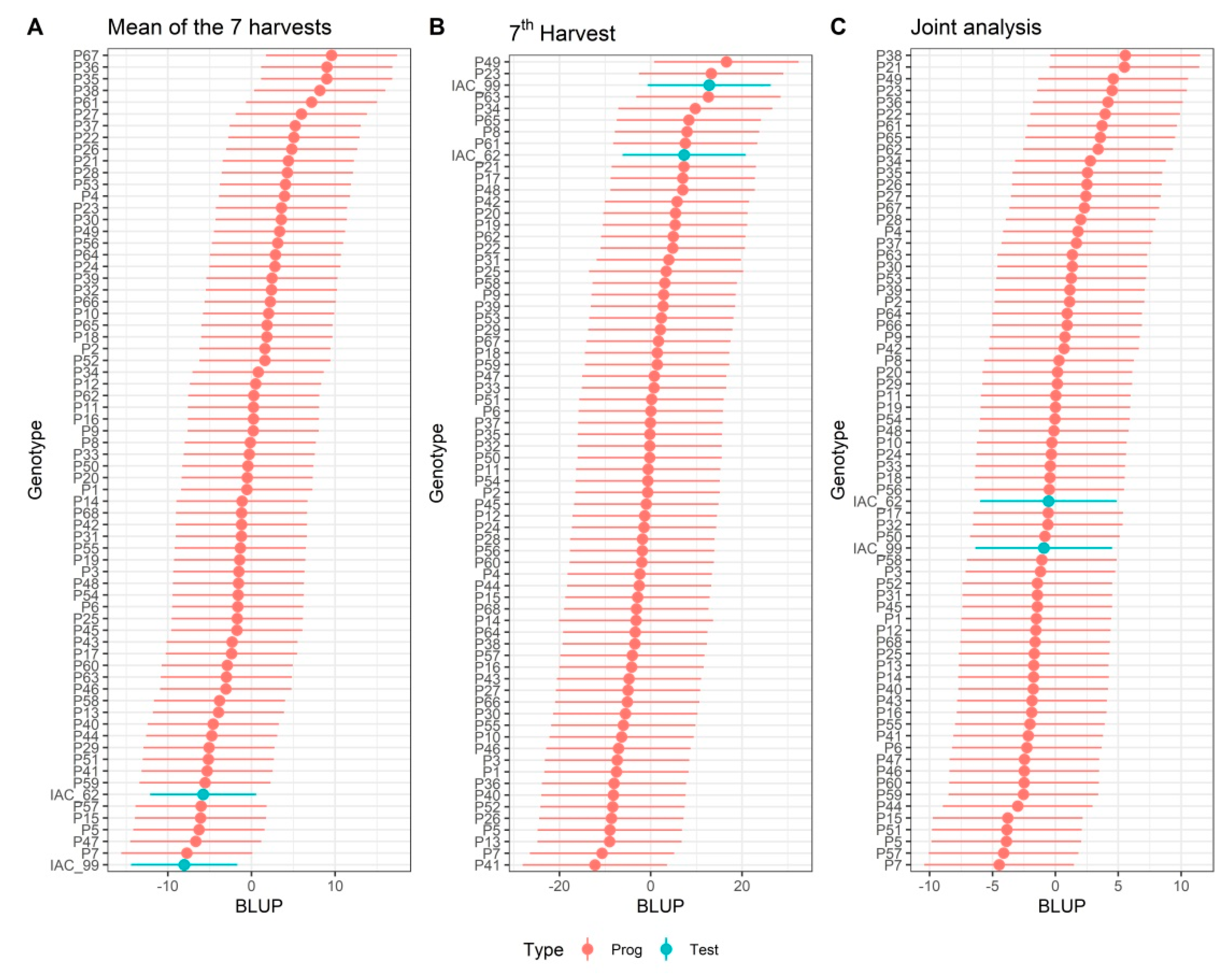
3.4. Selection Strategies
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statements
Conflicts of Interest
References
- Talhinhas, P.; Batista, D.; Diniz, I.; Vieira, A.; Silva, D.N.; Loureiro, A.; Tavares, S.; Pereira, A.P.; Azinheira, H.G.; Guerra-Guimarães, L.; et al. The Coffee Leaf Rust Pathogen Hemileia vastatrix: One and a Half Centuries around the Tropics: Coffee Leaf Rust Caused by Hemileia vastatrix. Mol. Plant Pathol. 2017, 18, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- De Souza, A.F.; Zambolim, L.; de Jesus Júnior, V.C.; Cecon, P.R. Chemical Approaches to Manage Coffee Leaf Rust in Drip Irrigated Trees. Australas. Plant. Pathol. 2011, 40, 293–300. [Google Scholar] [CrossRef]
- Zambolim, L. Current Status and Management of Coffee Leaf Rust in Brazil. Trop. Plant Pathol. 2016, 41, 1–8. [Google Scholar] [CrossRef]
- Vale, P.A.S.; Resende, M.L.V.; Botelho, D.M.d.S.; Pozza, E.A.; Ogoshi, C.; Monteiro, A.C.A.; Costa, B.H.G.; Vasconcelos, V.A.M. Temperature, Incubation Time and Virulence of Cercospora coffeicola in the Production of Cercosporin. J. Phytopathol. 2019, 167, 371–379. [Google Scholar] [CrossRef]
- Eskes, A.; Hoogstraten, J.G.J.; Toma Braghini, M.; Carvalho, A. Race-Specificity and Inheritance of Incomplete Resistance to Coffee Leaf Rust in Some Icatu Coffee Progenies and Derivatives of Hibrido de Timor. Euphytica 1990, 47, 11–19. [Google Scholar] [CrossRef]
- Botelho, D.M.S.; de Resende, M.L.V.; Andrade, V.T.; Pereira, A.A.; Patricio, F.R.A.; Junior, P.M.R.; Ogoshi, C.; de Rezende, J.C. Cercosporiosis Resistance in Coffee Germplasm Collection. Euphytica 2017, 213, 117. [Google Scholar] [CrossRef]
- Falconer, D.S.; Mackay, T.F.C. Introduction to Quantitative Genetics, 4th ed.; Prentice Hall: Harlow, UK, 1996; ISBN 9780582243026. [Google Scholar]
- Andrade, V.T.; Gonçalves, F.M.A.; Nunes, J.A.R.; Botelho, C.E. Statistical Modeling Implications for Coffee Progenies Selection. Euphytica 2016, 207, 177–189. [Google Scholar] [CrossRef]
- Pereira, T.B.; Carvalho, J.P.F.; Botelho, C.E.; de Resende, M.D.V.; de Rezende, J.C.; Mendes, A.N.G. Eficiência Da Seleção de Progênies de Café F4 Pela Metodologia de Modelos Mistos (REML/BLUP). Bragantia 2013, 72, 230–236. [Google Scholar] [CrossRef][Green Version]
- Henderson, C.R. Applications of Linear Models in Animal Breeding; Univ. of Guelph: Guelph, ON, Canada, 1984; ISBN 9780889550308. [Google Scholar]
- Chavarría-Perez, L.M.; Giordani, W.; Dias, K.O.G.; Costa, Z.P.; Ribeiro, C.A.M.; Benedetti, A.R.; Cauz-Santos, L.A.; Pereira, G.S.; Rosa, J.R.B.F.; Garcia, A.A.F.; et al. Improving Yield and Fruit Quality Traits in Sweet Passion Fruit: Evidence for Genotype by Environment Interaction and Selection of Promising Genotypes. PLoS ONE 2020, 15, e0232818. [Google Scholar] [CrossRef]
- Botelho, C.E.; Mendes, A.N.G.; Carvalho, S.P.; Carvalho, G.R.; Gonçalves, F.M.A.; Carvalho, A.M. Avaliação de Progênies de Café Obtidas por Cruzamentos das Cultivares Icatu e Catimor. Coffee Sci. 2007, 2, 10–19. [Google Scholar]
- Botelho, C.E.; Mendes, A.N.G.; Carvalho, G.R.; Bartholo, G.F.; Carvalho, S.P. Seleção de Progênies F4 de Cafeeiros Obtidas Pelo Cruzamento de Icatu Com Catimor. Rev. Ceres 2010, 57, 274–281. [Google Scholar] [CrossRef]
- Antunes Filho, H.; Carvalho, A. Melhoramento Do Cafeeiro: VII—Ocorrência de Lojas Vazias Em Frutos de Café “Mundo Novo”. Bragantia 1954, 13, 165–179. [Google Scholar] [CrossRef][Green Version]
- Brasil, Ministério da Agricultura. Pecuária e Abastecimento Instrução Normativa n. 8, 11 de Junho de 2003. Available online: https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/insumos-pecuarios/alimentacao-animal/arquivos-alimentacao-animal/legislacao/instrucao-normativa-no-9-de-27-de-junho-de-2003.pdf (accessed on 10 December 2021).
- Capucho, A.S.; Zambolim, E.M.; Freitas, R.L.; Haddad, F.; Caixeta, E.T.; Zambolim, L. Identification of Race XXXIII of Hemileia Vastatrix on Coffea Arabica Catimor Derivatives in Brazil. Australas. Plant Dis. Notes 2012, 7, 189–191. [Google Scholar] [CrossRef]
- De Custódio, A.A.P.; Pozza, E.A.; Guimarães, S.d.S.C.; Koshikumo, É.S.M.; Hoyos, J.M.A.; de Souza, P.E. Comparison and Validation of Diagrammatic Scales for Brown Eye Spots in Coffee Tree Leaves. Ciênc. Agrotecnologia 2011, 35, 1067–1076. [Google Scholar] [CrossRef]
- Shaner, G.; Finney, R.E. The Effect of Nitrogen Fertilization on the Expression of Slow-Mildewing Resistance in Knox Wheat. Phytopathology 1977, 77, 1051. [Google Scholar] [CrossRef]
- Box, G.E.P.; Cox, D.R. An Analysis of Transformations. J. R. Stat. Soc. 1964, 26, 211–252. [Google Scholar] [CrossRef]
- Ripley, B.; Venables, B.; Bates, D.M.; Hornik, K.; Gebhardt, A.; Firth, D. Support Functions and Datasets for Venables and Ripley’s MASS. R Package Version 7.3–53.1. 2021. Available online: https://cran.r-project.org/web/packages/MASS/MASS.pdf (accessed on 10 December 2021).
- de Resende, M.D.V.; Duarte, J.B. Precisão e controle de qualidade em experimentos de avaliação de cultivares. Pesqui Agropecu Trop. 2007, 37, 182–194. [Google Scholar]
- Gilmour, A.R.; Thompson, R.; Cullis, B.R. Average information REML: An efficient algorithm for variance parameter estimation in linear mixed models. Biometrics 1995, 51, 1440–1450. [Google Scholar] [CrossRef]
- Mulamba, N.N.; Mock, J.J. Improvement of Yield Potential of the ETO Blanco Maize (Zea mays L.) Population by Breeding for Plant Traits [Mexico]. Egypt. J. Genet. Cytol. 1978, 7, 40–45. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Soft. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Revelle, W.; Elleman, L.G.; Hall, A. Statistical Analyses and Computer Programming in Personality. In The Cambridge University Press Handbook of Personality; Corr, P.J., Ed.; Cambridge University Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Wickham, H. Advanced R; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Verran, J.A.; Ferketich, S.L. Testing linear model assumptions: Residual analysis. Nurs. Res. 1987, 36, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Zambolim, L.; Caixeta, E.T. An Overview of Physiological Specialization of Coffee Leaf Rust–New Designation of Pathotypes. Int. J. Curr. Res. 2021, 13, 15479–15490. [Google Scholar]
- Carvalho, G.R.; Botelho, C.E.; Bartholo, G.F.; Pereira, A.A.; Nogueira, Â.M.; de Carvalho, A.M. Comportamento de Progênies F4 Obtidas Por Cruzamentos de “Icatu” Com “Catimor”. Ciênc. Agrotecnologia 2009, 33, 47–52. [Google Scholar] [CrossRef]
- Bernardo, R.N. Breeding for Quantitative Traits in Plants, 2nd ed.; Stemma Press: Woodbury, NY, USA, 2010; ISBN 9780972072410. [Google Scholar]
- Pereira, F.A.C.; de Carvalho, S.P.; Rezende, T.T.; Oliveira, L.; Maia, D.R.B. Selection of Coffea arabica L. Hybrids Using Mixed Models with Different Structures of Variance-Covariance Matrices. Coffee Sci. 2018, 13, 304–311. [Google Scholar] [CrossRef]
- Krishnan, S.; Matsumoto, T.; Nagai, C.; Falconer, J.; Shriner, S.; Long, J.; Medrano, J.F.; Vega, F.E. Vulnerability of Coffee (Coffea spp.) Genetic Resources in the United States. Genet. Resour. Crop Evol. 2021, 68, 2691–2710. [Google Scholar] [CrossRef]
- Waller, J.M. Coffee Rust—Epidemiology and Control. Crop Prot. 1982, 1, 385–404. [Google Scholar] [CrossRef]
- Eastburn, D.M.; McElrone, A.J.; Bilgin, D.D. Influence of Atmospheric and Climatic Change on Plant-Pathogen Interactions: Climatic Change and Host-Pathogen Interactions. Plant Pathol. 2011, 60, 54–69. [Google Scholar] [CrossRef]
- Camargo, Â.P.D.; Camargo, M.B.P.D. Definição e Esquematização Das Fases Fenológicas Do Cafeeiro Arábica Nas Condições Tropicais Do Brasil. Bragantia 2001, 60, 65–68. [Google Scholar] [CrossRef]
- Bernardes, T.; Moreira, M.A.; Adami, M.; Giarolla, A.; Rudorff, B.F.T. Monitoring Biennial Bearing Effect on Coffee Yield Using MODIS Remote Sensing Imagery. Remote Sens. 2012, 4, 2492–2509. [Google Scholar] [CrossRef]
- Da Silva, F.M.; de Alves, M.C.; Souza, J.C.S.; de Oliveira, M.S. Efeitos Da Colheita Manual Na Bienalidade Do Cafeeiro Em Ijaci, Minas Gerais. Ciênc. Agrotecnologia 2010, 34, 625–632. [Google Scholar] [CrossRef]
- Sera, G.H.; Machado, A.C.Z.; Ito, D.S.; Shigueoka, L.H.; da Silva, S.A.; Sera, T. IPR 106: New Arabica Coffee Cultivar, Resistant to Some Meloidogyne Paranaensis and M. Incognita Nematode Populations of Paraná. Crop Breed. Appl. Biotechnol. 2020, 20, e305520317. [Google Scholar] [CrossRef]
- Alkimim, E.R.; Caixeta, E.T.; Sousa, T.V.; Pereira, A.A.; de Oliveira, A.C.B.; Zambolim, L.; Sakiyama, N.S. Marker-Assisted Selection Provides Arabica Coffee with Genes from Other Coffea Species Targeting on Multiple Resistance to Rust and Coffee Berry Disease. Mol. Breed. 2017, 37, 6. [Google Scholar] [CrossRef]
- Avelino, J.; Cristancho, M.; Georgiou, S.; Imbach, P.; Aguilar, L.; Bornemann, G.; Läderach, P.; Anzueto, F.; Hruska, A.J.; Morales, C. The Coffee Rust Crises in Colombia and Central America (2008–2013): Impacts, Plausible Causes and Proposed Solutions. Food Sec. 2015, 7, 303–321. [Google Scholar] [CrossRef]



| CLS 1 | BEIn 1 | CLIn 1 | BES 1 | YI | |
|---|---|---|---|---|---|
| µ | 2.55 a | 2.68 a | 893.62 a | 1849.36 a | 513.11 |
| 1.41 * | 0.07 * | 58.37 * | 200.18 * | 0.00 ns | |
| 0.50 | 0.50 | 0.74 | 0.58 | 0.30 | |
| 342.08 | 14.39 | 39.19 | 39.00 | 0.07 | |
| 329.29 | 20.28 | 34.83 | 65.18 | 0.00 | |
| 1.02 | 0.60 | 1.01 | 0.71 | 0.64 | |
| MK | HS | FG | Hr7 | PROD | |
| µ | 23.72 | 26.50 | 9.77 | 53.17 | 34.82 |
| 20.15 ** | 39.62 ** | 19.33 ** | 103.18 * | 14.81 ** | |
| 0.61 | 0.71 | 0.61 | 0.37 | 0.38 | |
| 18.92 | 23.76 | 45.02 | 19.10 | 17.05 | |
| 25.87 | 25.70 | 62.51 | 41.11 | 51.73 | |
| 0.73 | 0.92 | 0.72 | 0.46 | 0.21 |
| Rank | Progeny | IMM |
|---|---|---|
| 1 | P35 [H 29-1-8-5 (III.5) plant 2] | 17.00 |
| 2 | P26 [H 32-11-17-4 (I.4) plant 2] | 20.37 |
| 3 | P12 [H 32-11-17-4 (III.4) plant 5] | 22.25 |
| 4 | P41 [H 136-1-19-7 (III.28) plant 4] | 22.25 |
| 5 | P1 [H 29-1-9-8 (II.7) plant 1] | 22.75 |
| 6 | P25 [H 32-3-15-20 (I.13) plant 5] | 23.37 |
| 7 | P7 [H 136-1-14-14 (II.23) plant 1] | 23.37 |
| 8 | P36 [H 29-1-8-5 (III.5) plant 3] | 23.62 |
| 9 | P46 [H 136-1-14-10 (I.22) plant 1] | 23.62 |
| 10 | P3 [H 29-1-8-5 (I.5) plant 4] | 23.75 |
| 11 | P44 [H 29-1-8-16 (III.16) plant 3] | 24.12 |
| 12 | P50 [H 32-3-15-20 (II.13) plant 1] | 24.62 |
| 13 | P53 [H 32-3-15-20 (II.13) plant 5] | 26.00 |
| 14 | P10 [H 32-11-17-4 (III.4) plant 1] | 26.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, P.C.; de Rezende Abrahão, J.C.; Porto, A.C.d.M.; Nadaleti, D.H.S.; Gonçalves, F.M.A.; Carvalho, G.R.; Botelho, C.E. Progeny Selection to Develop a Sustainable Arabica Coffee Cultivar. Agronomy 2022, 12, 1144. https://doi.org/10.3390/agronomy12051144
Moreira PC, de Rezende Abrahão JC, Porto ACdM, Nadaleti DHS, Gonçalves FMA, Carvalho GR, Botelho CE. Progeny Selection to Develop a Sustainable Arabica Coffee Cultivar. Agronomy. 2022; 12(5):1144. https://doi.org/10.3390/agronomy12051144
Chicago/Turabian StyleMoreira, Priscila Carvalho, Juliana Costa de Rezende Abrahão, Antonio Carlos da Mota Porto, Denis Henrique Silva Nadaleti, Flávia Maria Avelar Gonçalves, Gladyston Rodrigues Carvalho, and Cesar Elias Botelho. 2022. "Progeny Selection to Develop a Sustainable Arabica Coffee Cultivar" Agronomy 12, no. 5: 1144. https://doi.org/10.3390/agronomy12051144
APA StyleMoreira, P. C., de Rezende Abrahão, J. C., Porto, A. C. d. M., Nadaleti, D. H. S., Gonçalves, F. M. A., Carvalho, G. R., & Botelho, C. E. (2022). Progeny Selection to Develop a Sustainable Arabica Coffee Cultivar. Agronomy, 12(5), 1144. https://doi.org/10.3390/agronomy12051144






