Abstract
Biochar adsorption and microbial remediation have great potential in the field of soil remediation, but since both are stressed by high concentrations of toxic heavy metals when applied alone, combining the two may become an effective remediation method. In this study, the application effect of phosphorus-solubilizing bacteria (PSB) combined with rice husk biochar on the remediation of Pb/Cd-mixed pollution and the form differences of toxic metal were studied qualitatively and quantitatively. Compared with the contaminated soil, the combined remediation of biochar and PSB significantly increased the pH, carbon and phosphorus by 9.0%, 299.4% and 157.0%. Meanwhile, combined remediation increased the total microbial, bacterial and fungal biomass by 92.11%, 103.13% and 138.10%. This confirmed that the addition of biochar increased the soil nutrients and provided good conditions for PSB or native microorganisms to flourish. The extraction results showed that the stable form of Pb/Cd with biochar + PSB was better than that with biochar/PSB alone. Combined remediation significantly increased the acid-soluble and non-bioavailable fraction of Pb/Cd by 5/15 times and 14/5.8 times in contaminated soil. The acid-soluble and non-bioavailable fractions are the main fraction of toxic metals after combined remediation (>80%). The acid-soluble and non-bioavailable fractions were mainly carbonates and phosphate-based Pb/Cd minerals (XRD analysis). PCA and a GWB model further confirmed that the release of pH and phosphorus was the key to the passivation of Pb/Cd in a short time. Meanwhile, the combination of the biochar (phosphorus supply guarantee) and PSB (acid-soluble phosphorus function) can reduce soil acidification and improve soil nutrients, thus increasing microbial abundance in contaminated soil, even more than that in non-contaminated soil.
1. Introduction
Rapid urbanization and industrialization have led to an increase in toxic heavy metal pollution in soils worldwide. Since heavy metals are transportable and have difficulty degrading in the environment, they are easily concentrated by plants and animals, and then amplified by the biological chain, thereby threatening human health [1,2]. In China, lead (Pb) and cadmium (Cd) are some of the most serious toxic heavy metal pollutants, according to the National Soil Pollution Status Survey Bulletin [3]; the over-the-standard rate of total pollution detection points in 2014 was 16.1%, of which the pollution detection point over-standard rate of Cd was 7.0% and of Pb was 1.5%. Pb poisoning can affect the nerve center, hematopoietic function, the liver and kidney system and lead to serious diseases [4,5]. High levels of Cd can cause kidney toxicity, osteoporosis, cardiovascular disease and other diseases in humans [6,7]. Common heavy metal remediation technologies include physical adsorption, precipitation, solidification stability and bio-sorption [8,9,10,11]. However, the high migration and biological toxicity of heavy metal ions in soil are important factors for their harmfulness. Therefore, in order to prevent Pb or Cd from being absorbed by plants, especially crops, toxic heavy metal stabilization and immobilization in the soil have become the predominant remediation methods.
Microbial remediation technology is one of the most promising technologies for existing-soil remediation. It has superiorities in terms of non-pollution, simple operation, low cost, sustainability, etc. [12]. Some specific functional microorganisms (phosphate-solubilizing bacteria and fungi) have been widely used in the remediation of toxic heavy metal pollution [10,13]. Phosphorus-solubilizing bacteria (PSB) can remediate toxic metal pollution via adsorption, dissolution and chelation [11,14]. In particular, PSB can decompose phosphorus and combine it with heavy metals to form phosphate-stable minerals [10]. However, toxic heavy metals are highly stressful to microorganisms. When the concentration of toxic heavy metals exceeds the threshold level of microorganisms, it will produce a variety of toxic effects (physiological, biochemical and genetic) on the microorganisms, thus inhibiting cell membrane synthesis and cell division and reducing enzyme activity such that the microorganisms are unable to perform their ecological functions [9,15]. For example, previous studies have shown that although phosphorus-solubilizing Enterobacteriaceae (Enterobacter sp., CGMCC17428) have good remediation effects on Pb and Cd, they can only tolerate 200 mg/L of Cd and 500 mg/L of Pb in solution [10,16]. Therefore, we need a kind of carrier that can both protect microorganisms and aggregate toxic metals from the environment to assist microorganisms in remediation.
Biochar can improve the physical properties of soil and regulate soil environmental factors. Most biochar can create a benign environment for microorganisms, promoting the colonization and activity of soil microorganisms [17,18,19,20]. In addition, biochar is often used as a soil remediation agent that can adsorb various heavy metals from contaminated soils [21]. However, the adsorption performance of biochar can be affected by environmental factors such as acid rain and wet and dry alternation, and therefore, there is a risk of secondary pollution [22]. The porous structure of biochar can act as a habitat for microorganisms and protect them from external biotic and abiotic stresses [23]. Moreover, biochar can be used as a food source to provide nutrients for microorganisms, which directly or indirectly affects the composition and structure of the soil microbial community [24]. In addition, biochar can also affect the microbial activity (enzyme activity) in soil by changing the pH value and nutrient content of soil [25]. Therefore, biochar, in combination with phosphate-solubilizing microorganisms, has a very high potential to remediate toxic heavy metals. Our previous studies have demonstrated that biochar can be used in combination with PSB to remediate Pb or Cd in solution [11,23], but the mechanism of biochar combined with PSB for the remediation of toxic heavy metals and the improvement of microbial communities has not been deeply investigated in the case of mixed contaminated soil.
As an organic solid waste, rice husk can easily pollute the environment. Converting it into biochar for soil improvement can reduce environmental pollution and improve the resource utilization of agricultural solid waste. The utilization of agricultural organic solid waste, combined with functional microorganisms in soil remediation, will be a new application of agricultural solid waste. The aim of this study is to use biochar and PSB to passivate and remediate the mixed pollution of Pb and Cd in soil, and to investigate the performance differences between the application of biochar or PSB alone and the co-application of both biochar and PSB. The X-ray diffraction (XRD) method, attenuated total reflection infrared spectroscopy (ATR-IR) and a four-step extraction method were used to analyze the changes of toxic heavy metal speciation both qualitatively and quantitatively. The Geochemist’s Workbench (GWB) model was used to further confirm the types of Pb/Cd minerals finally formed by biochar combined with PSB in soil, as well as the differences between the various treatments. The principal component analysis method was used to study the mechanism of biochar combined with PSB on soil physical and chemical properties, biological properties and toxic metals’ speciation. This study connects physical remediation methods with bioremediation methods to remediate toxic metal pollution, which may bring new inspiration to the achievement of the sustainable and environmentally friendly production of agricultural soils.
2. Materials and Methods
2.1. Preparation of Materials
An alkaline rice husk biochar (rice biochar = RB) was selected from the Institute of Soil Science, Chinese Academy of Sciences (Nanjing), Chinese Academy of Sciences. The biochar was produced via the pyrolysis of a mixture of rice husk (70 wt%) and pig manure (30 wt%) at 450 °C, which is an industrial biochar. The soil was obtained from a local yellow-brown earth (Dystriccambisols) in Nanjing (118°85′ E, 32°04′ N); the soil texture was loam with moderate voids. A total of 5 cm of topsoil was stripped with a wooden shovel, and then 5–15 cm of soil was excavated for testing. After the soil was taken from the outdoors, the plant roots, insects, stones and other debris were removed from the soil samples; then, the soils were placed indoors in a cool and ventilated place to dry naturally, and were crushed and ground with a wooden stick. In the end, the samples taken below 50 mesh sieves (0.28 mm) were stored in a self-sealing bag. The basic properties of the soil and biochar are shown in Table 1. Phosphate-solubilizing bacteria (PSB, Enterobacter sp., strain conservation number: CGMCC17428) were isolated from local soybean roots at the College of Resources and Environmental Science, Nanjing Agricultural University, Nanjing, China. The Enterobacter sp. is one of the efficient PSB; it can fully release phosphorus in the environment by secreting acids and enzymes [11]. Under the condition of sufficient phosphorus in the environment, the phosphorus (soluble total phosphorus)-dissolved amount of PSB can reach ~45 mg/L in three days. Bacterial liquid: PSBs were activated by a 3-day culture on a solid-medium plate (beef peptone and agar); then, the 6 × 6 mm inoculum plugs (solid) were cultured in 100 mL beef peptone medium and incubated for 24 h in a thermostatic shaker (37 °C, 180 rpm). Then, the bacteria were propagated in LB medium (1000 mL distilled water containing 10.0 g tryptone, 5.0 g yeast extract and 10.0 g NaCl, pH = 7) [11], and the experiment was carried out after 5 days. The number of Enterobacter sp. cells is 2.6 × 108 cells/mL; the content of phosphate (PO4) in the bacterial solution was 0.171 mg/L (molybdenum blue method) (Supplementary Information). In the sterile console, a pipette gun was used to suck 3 mL of bacterial solution, transfer it to the simulated polluted soil, and then stir it evenly with a sterile wooden stick.

Table 1.
The properties of the materials. Part of the data comes from our previous studies (-: below the detection line) (Chen et al., 2019; 2020).
2.2. Experimental Methods for Toxic Heavy Metal Remediation
In this experiment, four treatments were set up (Table 2). We first simulate heavy metal pollution in soil, and then use biochar and bacteria for joint or separate remediation. In the sterile console, all experimental materials were added to a 150 mL culture flask, following the steps below. All toxic heavy metal solutions (5 mL of a mixture of 500 mg/L Pb2+ and 271.24 mg/L Cd2+, pH = 5.3) were added to the soil (20 g) and mixed well and then placed in an artificial climate chamber (27 °C, alternating light and dark for 12 h) for 7 days to simulate the pollution of the soil environment by toxic metals (soil moisture = 50–55%). Then, biochar (1 g) and bacterial solution (3 mL the cultured PSB bacterial solution) were added to simulate the short-term emergency remediation after toxic metal pollution in soil. Destructive sampling was performed after 30 days of incubation, with three replicates for each treatment. All culture flasks were incubated in an artificial climate chamber (RDN-300C-4, Ningbo Southeast Instruments) at 30 °C with 27% humidity and 12 h of alternating light and dark. The soil humidity was adjusted to 60% of the saturated soil water content every three days, which was also the initial soil water content of the experiment. After 30 days of incubation, destructive sampling was carried out and the samples were divided into two parts. One part was stored in a refrigerator at −20 °C for a short period of time to determine the biomass of the microorganisms in the soil (PLFA), and the other part was used for the analysis of the soil physical and chemical properties and toxic metal morphology (four-step sequential extraction).

Table 2.
Composition of all the treatments (RB—rice husk biochar, PSB—Enterobacter sp.).
2.3. Soil Sample Measurements
2.3.1. Soil Chemical and Physical Properties
Soil total nitrogen (TN) was determined by the micro-Kjeldahl method [26]. Soil total carbon (TC) was titrated by the combustion oxidation titration method [27]. Soil available phosphorus (AP) was determined by sodium bicarbonate leaching and the molybdenum antimony anti-colorimetric method [28]. Available potassium (AK) was extracted by NH4OAc and determined by the flame photometry method. Soil moisture was analyzed by an analytical balance (Denver, T214). Soil pH was measured using a portable pH meter (IQ 150 United States, soil: deionized water = 1:2). The drying bulk density (DBD), saturated bulk density (SBD), water holding capacity (WHC) and air-filled porosity (AFP) were measured as described by Handreck and Black [29]. The DBD and SBD were determined by dividing the weight of saturated substrate and the dry substrate by the volume.
The crystal structure of the soil samples was analyzed by X-ray diffraction (XRD, D8ADVANCE, Bruker, Karlsruhe, Germany), with a scanning angle of 10~80°. Based on the measured XRD spectrograms, the crystal structure, physical phase composition and crystalline state of the samples were analyzed. Attenuated total reflection infrared spectroscopy (ATR-IR) analysis was applied to the Nicolet iS5 Fourier-transform infrared spectrometer (ThermoFisher Scientific Inc., Madison, WI, USA) and Thermo Scientific OMNIC software (ThermoFisher Scientific Inc., Madison, WI, USA) to determine the functional groups of the soil before and after remediation.
2.3.2. Microbial Community Determination
In this experiment, the soil microbial community abundance was analyzed by the phospholipid fatty acids method (PLFA) [30,31]. Briefly, PLFA method detection is divided into four steps, as follows. (1) Extraction: 8.0 g of freeze-dried soil sample (−70 °C) was weighed in a triangular flask; then, it was extracted with Bligh–Dyer extract (chloroform, methanol, citric acid buffer in the volume ratio of 1:2:0.8 v/v/v) in a dark environment. (2) Purification: the sample in the previous step was dissolved in chloroform and then added to the silica gel column. Then, different doses of chloroform, acetone and methanol were added in turn for extraction. After drying with nitrogen, the sample was frozen at −20 °C. (3) Methylation: 1 mL toluene and methanol (1:1, v/v) and 1 mL 0.2 M KOH were added to the sample in the previous step, then stirred, heated in a water bath (30 min) and the pH was adjusted to neutral (about 7). A total of 5 mL hexane and chloroform (4:1, v/v) and 3 mL ultrapure water were added to the solution, which was shaken and centrifuged. The supernatant was aspirated into a GC bottle, blown with nitrogen until dry and stored in dark conditions at −20 °C. (4) Measure: the methylated sample was put into the GC bottle and dissolved by hexane (2 × 100 μL, vortex) and then transferred to the interpolation tube. The phospholipid fatty acid profile was measured on the GC-MS machine (Agilent Technologies, Palo Alto, CA, USA). The detailed experimental method is shown in the Supplementary Information. Based on the differences in the properties of the phospholipid fatty acids, the different types and contents of PLFAs in the phospholipid fatty acid profiles were used to identify the bacterial abundance and fungal abundance, as well as the total microbial biomass. The PLFAs i14:0, i15:0, a15:0, i16:0, 10Me16:0, i17:0, a17:0, 10Me 17:0, 10Me 18:0, 16:1 ω7c, 16:1 ω9c, 17:1 ω8c, cy17:0, 18:1 ω7c and cy19:0 were used as bacterial biomarkers [32,33,34]. The PLFAs 16:1 ω5c, 18:2 ω6c and 18:1 ω9c were used as fungal biomarkers [35].
2.3.3. Speciation and Bioavailability of Toxic Heavy Metals
The four-step sequential extraction method was used to indicate the speciation and bioavailability of heavy metals in soil samples [36]. The four-step extraction method corresponds to four types of heavy metals: water soluble fraction; exchangeable fraction; acid-soluble fraction; non-bioavailable fraction. ① Each soil sample (0.1 g dried soil sample) was mixed with 20 mL deionized water and shaken in a constant-temperature shaker (200 rpm/min) for 24 h at 20 °C in order to extract the water soluble fraction. ② A total of 8 mL of 0.5 M MgCl2 (adjusted to pH 7.0 using NaOH or HCl) was added into the tube with the solid residue from step one and shaken in a constant-temperature shaker (200 rpm/min) for 24 h at 20 °C. This step was used to extract the exchangeable fraction in the samples. ③ A total of 8 mL of 1 M NaOAc (adjusted to pH 5.0 with HOAc) was added to the solid residue from step two and vibrated under the same conditions (200 rpm/min, 24 h, 20 °C) to obtain the acid-soluble fraction in the sample. ④ The solid residue from the third step was put into the digestion tube with 9 mL of 36% HCl and 3 mL of 70% HNO3 for 16 h, and then heated at 95 °C for 2 h. This step was used to extract the non-bioavailable fraction in the samples. All steps were performed only once. The supernatant was collected and filtered through a 0.45 µm filter before analysis with an inductively coupled plasma optical emission spectrometer (ICP-OES) (Perkin-Elmer, 7000DV) to determine the Pb2+ and Cd2+ concentration.
2.3.4. Data Analysis
We analyzed the relationship between the remediation reagents (biochar and PSB), the soil physical and chemical properties, the biological characteristics and the speciation and bioavailability of the heavy metals (PCA: principal component analysis). The ANOVA method (Tukey test, SPASS 22.0, probability level: p < 0.05) was used to analyze the differences in nutrient element content and microbial biomass between all treatments. All data were tested for normal distribution, and the results compounded the inspection requirements (Supplementary Information, Table S1). The Geochemist’s Workbench (GWB 12, Aqueous Solutions LLC.) model was applied to simulate the final products of Pb and Cd minerals under a range of H2PO4− concentrations and pH values at a fixed Pb (500 mg/L) and Cd (271.24 mg/L) concentration. The results are shown as assemblage diagrams calculated by the Phase2 module. Carbonate was balanced with atmosphere (~10−3.5 fugacity) in the medium where Na+ and NO3− were employed as balance species. The calculation conditions are shown in Table S2 (Supplementary Information) and in our previous research [37]. The vertical axis was set as the activity of H2PO4− from 0 to 120, while the transverse axis was set as pH values from 6 to 9. The final minerals formed by different treatments are displayed in the mixed system of Pb and Cd.
3. Results
3.1. Changes in Soil Properties
The soil water content and pH changes are shown in Table 3. The lowest soil water content was found in the co-application of PSB and biochar (Soil + RB + PSB + Pb/Cd), which was 17.04% lower than that of the heavy metal-contaminated soil (Soil + Pb/Cd), 6.67% lower than that of the soil with PSB alone (Soil + PSB + Pb/Cd), and 31.29% lower than that of the soil with biochar alone (Soil + RB + Pb/Cd). These results indicate that the addition of biochar contributes to the moisture retention during the incubation process, while the bacteria have significant water consumption due to reproduction.

Table 3.
The changes of soil water content in a 3-day interval and the soil pH of each treatment interval over 30 days (RB—rice husk biochar, PSB—Enterobacter sp.).
The pH results indicate that the heavy metal pollution made the soil significantly acidified, and the pH of the contaminated soil was about 13.10% lower than the initial soil (pH = 7.94). Compared with the toxic metal-contaminated soil, the soil pH was 4.78% lower with the addition of PSB, while the soil pH increased by 15.36% with the addition of biochar. The pH of the soil with the co-application of biochar and PSB was lower than that of biochar alone, which might have been caused by the organic acids secreted during the rapid multiplication of microorganisms. However, the pH of the soil with biochar and PSB was still 10.00% higher than that of the toxic metal-contaminated soil.
The TC, TN, AP and AK contents of the different treatments are shown in Figure 1. The soil with the application of biochar had the highest TN content, and the addition of PSB (5.87%) or biochar (10.20%) alone can increase the TN content of the toxic metal-contaminated soil (Figure 1A). There was no significant difference in TC content between Soil + PSB + Pb/Cd and Soil + Pb/Cd. The addition of biochar can cause a significant increase in soil TC, but the TC content of the soil with the co-application of biochar and PSB was 12.29% lower than the soil with the application of biochar only (Figure 1B). Compared with the toxic metal-contaminated soil, the addition of PSB, biochar and PSB + biochar increased the AP content by 22.44%, 67.63% and 157.02% in contaminated soil, respectively (Figure 1C). The addition of biochar significantly increased the content of AK in soil, biochar and PSB + biochar increased the AK content by 163.4%, 203.2%. PSB also can promote the release of AK in soil (Figure 1D).
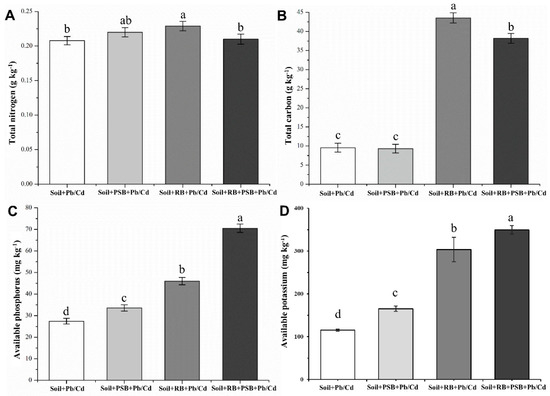
Figure 1.
Nutrient element contents in soil with different treatments ((A): Total nitrogen, (B): Total carbon, (C): Available phosphorus, (D): Available potassium, RB—rice husk biochar, PSB—Enterobacter sp.). Different letters indicate significant differences among treatments.
3.2. Analysis of Bacterial, Fungal and Total Microbial Biomass in the Soil
The bacterial, fungal and total microbial biomass in the soil after 30 days of remediation were measured by the PLFA technique (Figure 2). Toxic metal pollution produces serious stress on the reproduction and growth of microorganisms in the soil, and the results of microbial abundance showed that the bacterial biomass, fungal biomass and total microbial biomass in toxic metal-contaminated soil were 42.86%, 53.33% and 44.93% lower than those in non-contaminated soil. Compared with the toxic metal-contaminated soil, the application of PSB resulted in a significant increase in the total microbial biomass (55.26%), bacterial biomass (62.50%) and fungal biomass (90.48%) in toxic metal-contaminated soil. The application of biochar also significantly increased the abundance of microorganisms in the contaminated soil; the total microorganisms’ biomass, the bacterial biomass and the fungal biomass were increased by 44.74%, 43.75% and 58.73%, respectively. In the case of the combined remediation of biochar and PSB, the total microbial biomass increased by 92.11%, the total bacteria biomass increased by 103.13% and the total fungal biomass increased by 138.10%. The improvement of the fungal biomass by PSB was significantly better than that by bacterial biomass, while there was little difference between the improvement of fungal biomass and bacterial biomass by biochar. The improvement of bacterial biomass in polluted soil by the combination of biochar and PSB was significantly better than that of fungal biomass. In addition, the total biomass of bacteria and microorganisms in soil after combined remediation was even higher than that in non-contaminated soil.
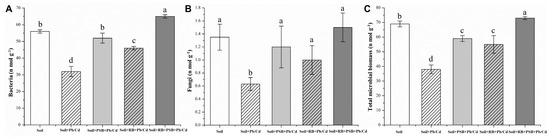
Figure 2.
Microbial biomass of microorganisms in soils with different treatments ((A): Bacteria biomass, (B): Fungi biomass, (C): Total microbial biomass, RB—rice husk biochar, PSB—Enterobacter sp.). Different letters indicate significant differences among treatments.
3.3. Speciation Analysis of Toxic Heavy Metals
The results of the toxic metal morphology extraction are shown in Figure 3. The morphology of both Pb and Cd in the contaminated soil was dominated by the water-soluble fraction (Pb/Cd = 9.40%/56.55%) and the exchangeable fraction (Pb/Cd = 80.40%/37.79%). In contrast to the Pb and Cd-contaminated soil, the application of PSB increased the water-soluble fraction, the acid-soluble fraction and the non-bioavailable fraction of Pb by 59.04%, 296.28% and 342.86%, respectively, but the exchangeable fraction decreased by 45.76%. Meanwhile, the water-soluble fraction of Cd was significantly decreased (by 52.49%), and the exchangeable fraction, acid-soluble fraction and non-bioavailable fraction were increased by 41.91%, 208.10% and 385.16%, respectively. After the addition of biochar, the water-soluble fraction and exchangeable fraction of Pb2+ decreased by 42.21% and 60.11%; the acid-soluble fraction and non-bioavailable fraction increased significantly, by 464.94% and 683.36%. However, the water-soluble fraction of Cd2+ decreased significantly (by 98.23%), the exchangeable fraction increased significantly (by 32.86%), the acid-soluble fraction increased significantly (by 949.83%), and the non-bioavailable fraction increased by 27.70%.
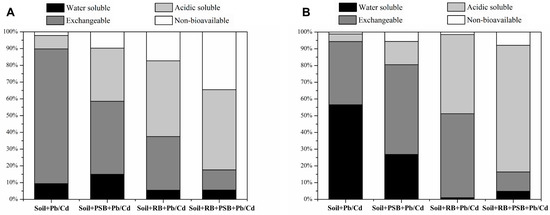
Figure 3.
Speciation of toxic heavy metals Pb (A) and Cd (B) in soils (RB—rice husk biochar, PSB—Enterobacter sp.).
The combined repair of biochar and PSB makes the form of toxic metals (Pb/Cd) more stable in contaminated soil. The water-soluble fraction (41.00%/91.49%) and exchangeable fraction (85.01%/69.33%) of Pb/Cd decreased. The acid soluble fraction and non-bioavailable fraction of Pb/Cd increased significantly, by 5/15 times and 14/6 times, respectively. The improvement of the non-bioavailable fraction of Pb/Cd in soil remediation by biochar combined with PSB was higher than the sum of the two alone.
3.4. XRD and ATR-IR Analysis
The results of XRD showed that there were obvious toxic heavy metal minerals in the soil after remediation (Figure 4). The peaks at 44.35°, 47.73° and 67.12° and the peaks at 19.11°, 29.74° and 41.60° in Soil + PSB + Pb/Cd confirmed that Pb and Cd existed primarily as phosphates [11]. The characteristic vibrational sorption peaks of Pb carbonate (27.54°, 33.21°, 47.95°) and Cd carbonate (45.22°, 52.57°, 72.98°) were observed in the Soil + RB + Pb/Cd and Soil + RB + PSB + Pb/Cd [22]. The characteristic peaks of Pb and Cd minerals in the soils remediated by biochar and PSB were significantly more numerous and more clearly defined than those of biochar or PSB applied alone. In the infrared spectrogram, it is difficult to find the characteristic peaks of Pb/Cd minerals produced after the remediation of toxic metals by PSB because the soil makes up the majority of the sample (Supplementary Information, Figure S2).
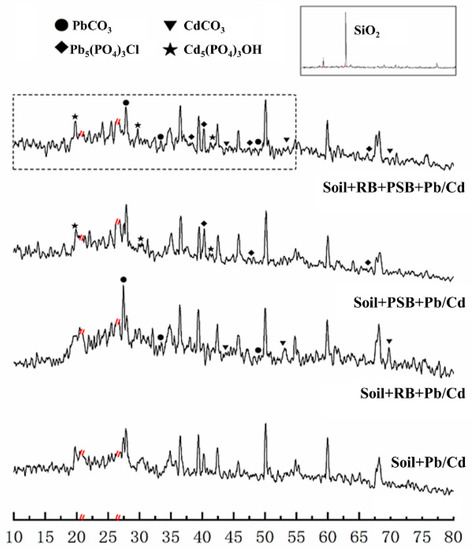
Figure 4.
XRD patterns of various treatments (RB—rice husk biochar, PSB—Enterobacter sp.). All the patterns have been baseline-corrected and no pattern smoothing was performed. In order to avoid the strong quartz peak affecting the existence of other Pb-mineral peaks, we removed the range of the quartz peak at 20.6–21.9° and 26.5–26.9° (quartz at 21.3° and 26.7°).
3.5. GWB Model Analysis
The GWB model can simulate the final form of heavy metal minerals in the environment. The mineral assemblage diagrams, as a function of pH and H2PO4− activity, revealed that mineral species of Pb or Cd are theoretically present in the phosphate–carbonate system (Figure 5). The range of different treatment methods in the model can prove the difference between different treatments on the formation of Pb/Cd-coexisting minerals. Soil + Pb/Cd was in interval 6 or 9, the dominant minerals include Pb3(CO3)2(OH)2 and CdCO3. Soil + PSB + Pb/Cd was in interval 6, and PbCO3, Pb5(PO4)3Cl and CdCO3 were its main minerals. The decrease in pH and the increase in P content by PSB are conducive to the formation of Pb-phosphate. Soil + RB + Pb/Cd was in interval 9 or 10, including four coexisting minerals (Pb5(PO4)3Cl, Pb3(CO3)2(OH)2, CdCO3 and Pb(OH)2). Soil + RB + PSB + Pb/Cd was in interval 9, of which Pb5(PO4)3Cl and CdCO3 were the most important minerals. The H2PO4− content of Soil + RB + PSB + Pb/Cd was higher than that of Soil + RB + Pb/Cd, and the pH was lower than that of Soil + RB + Pb/Cd, which made the minerals that formed in Soil + RB + PSB + Pb/Cd dominated by Pb5(PO4)3Cl and CdCO3. In the mixed system of Pb and Cd, there is obvious competition between Pb and Cd, and the formation of Pb-phosphate is obviously better than Cd-phosphate. According to a single-phase diagram, CdCO3 was the main mineral, forming at 5.5 < pH < 8.7 and H2PO4− > 0 mg/L. As the P concentration increases, Cd5(PO4)3OH might occur when pH > 8.5 and with more P (>40 mg/L). However, reactions in the system trended to form less Pb5(PO4)3Cl with increasing pH when the initial P concentration was fixed (see Pb in Figure 5). This means that when the soil pH is 5–8, Pb is more likely to preferentially form minerals. Meanwhile, when P is sufficient, the minerals formed by Pb are more stable. Although the effect of pH on the stability of Pb and Cd minerals is opposite in our reaction system, the higher the P content, the more conducive the final minerals are to form Pb/Cd-phosphate, which indicates that the P content is the key factor for determining the final formation of stable minerals.
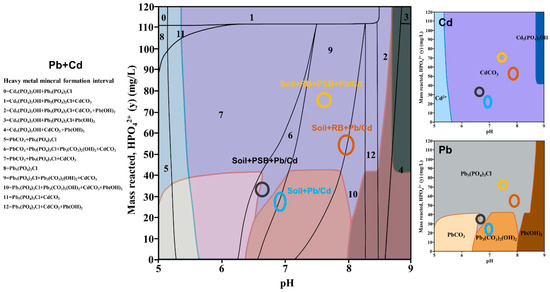
Figure 5.
Final minerals (Pb + Cd) formed by different treatments in the GWB model (RB—rice husk biochar, PSB—Enterobacter sp.).
3.6. PCA (Principal Component Analysis)
As shown by the plot of the PCA (Figure 6), the total explanation of the two axes is 92.19%, and PC1 and PC2 explained 82.06% and 10.13%, respectively, indicating a good correlation between the environmental and microbial factors. The contaminated soil (Soil + Pb/Cd treatment) is the farthest from the soil sample of the control treatment, indicating that the toxic heavy metal Pb/Cd significantly affected the basic soil properties. The Soil + PSB + Pb/Cd treatment was closer to the contaminated soil than the Soil + RB + Pb/Cd treatment, indicating that adding biochar is an easier way to improve the toxic metal pollution in soil than PSB.
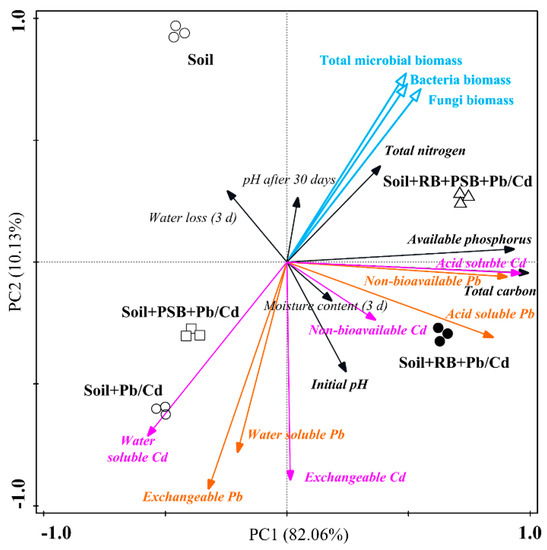
Figure 6.
The principal component analysis (PCA) shows the relationships among the soil physical properties, soil nutrients, biological characteristics and speciation of toxic heavy metals in the different treatments (RB—rice husk biochar, PSB—Enterobacter sp.).
Based on the principle that the larger loading coefficients of variables in the PCA plot indicate relatively greater influence of variables on PC, it means that PC1 mainly responds to some biological activity-related properties and PC2 mainly responds to some chemical–physical-related properties. The environmental factor analysis results reveal that most environmental factors were negatively correlated with the Soil + Pb/Cd and Soil + PSB + Pb/Cd treatments. The total carbon, available phosphorus and total nitrogen and microbial factors (bacteria, fungi and total microorganisms) were the most correlated with the Soil + RB + PSB + Pb/Cd treatment. Meanwhile, the positive correlation and location between the Soil + RB + PSB + Pb/Cd treatment and environmental factors were significantly stronger than those between the non-contaminated soil and environmental factors, which confirmed that the soil remediated with PSB and biochar could recover the soil biological characteristics in a short time, and that even the physical and chemical characteristics were better than non-contaminated soil.
The mineral forms of toxic metals in soil are obviously affected by environmental and biological factors. The water-soluble fraction and exchangeable fraction of Pb2+/Cd2+ were relatively concentrated; they were closest to the position of the Soil + Pb/Cd and Soil + PSB + Pb/Cd treatments, and showed a strong positive correlation (acute angle). The non-bioavailable Cd and acid-soluble Pb were most strongly correlated with the Soil + RB + Pb/Cd treatment. The positive correlation between the soil initial pH (7.94) and the non-bioavailable fraction of Cd was stronger than that between the initial pH and non-bioavailable fraction of Pb. In addition, there was a positive correlation between moisture content (3d) and the acid-soluble fraction of Pb and the non-bioavailable fraction of Cd. The regulation of pH and water helps to indirectly promote the formation of toxic metal minerals.
The acid-soluble fraction of Cd and the non-bioavailable fraction of Pb showed obvious aggregation, which had a strong correlation with the available P and the total C, which means that these two elements have an important influence on the formation of stable forms of toxic metals. In particular, the strong correlation between TC and the acid-soluble fraction of Cd was inseparable from the formation of Cd-carbonate, which was consistent with our extraction results. The stable forms of the four toxic metals were concentrated in the fourth quadrant, which were closest to the Soil + RB + PSB + Pb/Cd and Soil + RB + Pb/Cd treatments. In addition, microbial factors were positively correlated with the acid-soluble fraction and the non-bioavailable fraction of toxic metals, while the water-soluble fraction and the exchangeable fraction of toxic metals were negatively correlated, indicating that more water-soluble toxic metals would inhibit the reproduction of microorganisms.
4. Discussion
4.1. Effect of Phosphorus-Solubilizing Bacteria and Biochar on the Physicochemical Properties of Toxic Heavy Metal-Contaminated Soil
Soil pH, nutrients and moisture are the main factors affecting the survival and reproduction of soil microorganisms. In toxic metal-contaminated soil, the addition of PSB alone increased soil water loss by 9.23% and decreased soil pH by 4.78%. Some studies have shown that the metabolism and secretion of acidic substances by the microorganisms themselves can lead to decreases in pH in the environment [38,39]. However, the acidic environment it created promoted the decomposition and migration of toxic metal minerals; therefore, we speculate that this increased the stress received by the microorganisms. The addition of rice husk biochar was able to significantly adjust the overall physicochemical properties of the soil, where the pH and water content of the soil were greatly improved (Table 1). In this study, rice husk biochar was found to increase soil pH by 25.80% (Table 1), which was due to the fact that biochar contains a certain number of alkaline substances, thus causing the soil pH to increase [40,41]. Meanwhile, the loose and porous structure of biochar itself improved the porosity of the soil (RB addition reduced the soil bulk density by 8.5%), while the hydrophobic surface of biochar reduced the infiltration capacity of water in the soil and prevented water evaporation [31,42,43]. In addition, the adsorption of soil particles and the enhancement of soil aggregates by biochar can effectively stabilize soil properties and reduce the impact of environmental changes on soil [44], which means that biochar can reduce soil erosion well and maintain a stable soil environment.
Our results also showed that the addition of biochar led to an increase in water retention capacity of the soil (water loss reduced by 21.77–31.36%). Combined with our previous research, the water retention provided by biochar prolonged the time of water loss [31], thus providing sufficient water and resistance time for microbial colonization. In addition, this study also found that rice husk biochar significantly increased the nutrient content of the soil (Figure 2). Compared with toxic metal-contaminated soils, biochar was able to adsorb/retain nutrient elements from the environment in addition to providing both efficient nitrogen and phosphorus itself (Table 1) [45,46]. Therefore, biochar can provide a favorable environment for microbial reproduction and development in the practical applications, especially for beneficial microorganisms that are less resistant to environmental stresses. In addition, the adsorption and complexation of toxic metal ions by biochar can fix toxic metal ions from the environment, so as to reduce the contact between microorganisms and toxic metal ions and reduce the entry of metal ions into microbial cells.
4.2. Effect of PSB and Biochar on Microbial Properties in Toxic Heavy Metal-Polluted Soils
Toxic heavy metal pollution leads to strong stress on microorganisms in soil. In this study, it was found that toxic metal stress led to a decrease in total microbial biomass (44.93%) compared with unpolluted soil. The total microbial biomass of the Soil + PSB + Pb/Cd treatment was higher than that of the Soil + Pb/Cd treatment, but lower than that of the non-contaminated soil. This was because PSB have a certain toxic metal resistance and can reproduce and expand in a stressful environment. In addition, PSB can fix toxic metals onto dead cells through cell adsorption and division, which makes the increase in fungal biomass in the PSB repair treatment more obvious. Although it would reproduce largely in a short time, it will gradually be limited by the nutrient content. Therefore, compared with the co-remediation of biochar and PSB, the total microbial biomass of the PSB treatment was still less in the contaminated soil (Figure 2). In contrast, the biochar addition alone improved fungal biomass (58.73%) more significantly than bacteria (43.75%), probably because the biochar addition affected the ratio of fungi/bacteria in the soil by changing soil C/N (Figure 2A,B), which is similar to the results of Farrell’s (2013) study [47]. This phenomenon is because fungi use carbon more efficiently than bacteria while requiring less nitrogen for the same unit [48,49]. When biochar is combined with PSB for remediation, biochar could not only be a sustainable supply of nutrients for PSB [50], but can also provide habitat for some beneficial microorganisms with weak stress resistance, thus ensuring that different microbial communities in the soil could be well colonized. Meanwhile, the increase in total microbial biomass in the combined remediation of biochar and PSB may benefit from the increase in bacterial biomass (PSB biomass). When PSB are well colonized, they are able to immobilize toxic metal ions directly (adsorption) or indirectly (mineral formation), thus reducing their own stress [11,23]. This facilitates the treatment and remediation of toxic metal ions of Pb and Cd in the soil.
4.3. Reduction in Riskiness of Toxic Heavy Metal-Polluted Soil by PSB and Biochar
In toxic heavy metal-pollution stress, most microorganisms are not resistant to 500 mg/L of Pb2+ and 275 mg/L of Cd2+, and Cd2+ especially is highly toxic to microorganisms. Therefore, increasing the ratio of stable forms of toxic metals is the main way of protecting soil biological systems. According to the results of the four-step extraction method and the XRD detection, toxic metals were predominantly present in the water-soluble fraction and the exchangeable fraction (total: 89.80% for Pb and 94.34% for Cd) in the soil (Figure 3), and very few were present in the acid-soluble and non-bioavailable fraction (acid-soluble fraction: 8.00% for Pb and 4.51% for Cd; non-bioavailable fraction: 2.20% for Pb and 1.16% for Cd). This result indicates that, in short-term toxic metal pollution, the soil itself is not able to immobilize toxic metal ions well, and that toxic metal ions are mainly in an unstable form when combined with organic matter and are easily utilized by microorganisms or changed to the free state of toxic metal ions in a slightly acidic environment, thereby greatly increasing the toxic metal migration rate and stress effects. In contrast, the content of toxic metals in the exchangeable fraction (Cd increased the most), the acid-soluble fraction (Pb increased the most) and the non-bioavailable fraction (Cd increased the most) increased greatly with the addition of PSB, but the XRD results showed there was small amounts of Pb or Cd minerals, which implies that the biosorption function (major role) and mineralization of PSB can immobilize toxic metals, thus reducing the migration of toxic metals and the stress on other microorganisms. In addition, the increase in pH will inhibit the formation of water-soluble and exchangeable heavy metals (Figure 5). The reduction in soil pH by PSB may increase the dissolution of heavy metals and increase the potential risk of heavy metals in the short term. Therefore, it is necessary to use biochar to improve soil pH, so as to make up for the deficiency of PSB alone. Not only that, since biosorption immobilization is also susceptible to the environment, the remediation of toxic metals by PSB can only maintain short-term stability, and there is still a risk of secondary pollution. Moreover, microorganisms have to prioritize their own survival when resisting toxic metal stress [23], and thus, they may not be able to ensure the sufficient release of soil C and P elements to achieve the effect of passivating and immobilizing toxic metals.
Toxic heavy metal forms in soils with biochar addition alone were mainly in the acid-soluble fraction (45.20% for Pb and 47.32% for Cd) and in the exchangeable fraction (32.07% for Pb and 50.20% for Cd), which confirms that biochar can improve the immobilization of toxic metals through its own adsorption, cation exchange and carbon element release, etc., in the presence of biochar addition [51]. Biochar addition significantly increased the acid-soluble fraction of toxic metals (Pb: increased by 464.94%; Cd: increased by 949.83%) compared to the contaminated soil. XRD results confirmed that these insoluble minerals in contaminated soil are mainly carbonate, which is because the CO32− and alkaline environment created by biochar increased the production of carbonates, especially for Cd [23]. In addition, there was a significant increase in the non-bioavailable fraction of Pb (686.36%) and a smaller increase in Cd (27.70%) after biochar remediation. This result is because the mineral precipitation of Pb phosphate (Ksp = 8.0 × 10−43) is more stable than that of the Cd phosphate (Ksp = 1 × 10−30) class [11,22,52,53], thus confirming that the P element contained in biochar can serve as a means of immobilizing toxic metal ions.
In the case of the combined remediation of biochar and PSB, the content of toxic metals in the non-bioavailable fraction increased significantly; the increase in Pb2+ (1468.77%), especially, was more obvious than that of Cd2+ (582.00%). There are two main reasons for this result: 1. PSB were attached to the biochar for reproduction and growth, and dissolved organic or inorganic phosphorus sources by secreting enzymes and organic acid solubilization, which allowed for the release of insoluble phosphorus on the biochar and, thus, the probability of toxic metal phosphate formation was increased. This phenomenon is consistent with the results of XRD; 2. Although toxic metal minerals of Pb and Cd were generated, the Pb phosphate minerals are more stable than the Cd phosphate minerals, which leads to the preferential formation of Pb phosphate minerals (especially the Pb5(PO4)3(OH,F,Cl)) in the environment. This is also the main reason for why there is more Pb than Cd in the non-bioavailable fraction. Moreover, the extraction results of this study also showed that, in the mixed Pb and Cd-contaminated soil, biochar combined with PSB could repair the toxic metals mainly by forming toxic metal phosphorus minerals in a short time (30 days), so as to reduce the stress of toxic metals on soil microorganisms and improve the recovery ability of the soil ecosystem.
Meanwhile, we verified, by the GWB model, that the presence of biochar (P supply) and PSB (organic acid secretion) was beneficial to the formation of heavy metal minerals, and that, eventually, the mineral formation tended to the most stable form (Pb-phosphate and Cd-carbonate). In the mixed system, Pb and Cd have obviously phenomenon of competitive mineralization. In the model, the mineral formation types of Cd are less than those of Pb. At the same time, the model shows that biochar can change the minerals formed by heavy metals into a more stable form (phosphate). When the concentration of P is constant, the content of Pb phosphate increases with the decrease in pH, but the content of Cd phosphate changes on the contrary. Because heavy metal pollution will lead to soil acidification, Pb is more likely to be preferentially passivated as a stable mineral in the mixed Pb/Cd soil-polluted environment (pH < 8). In addition, when the pH is 8.5 and the P concentration is >100 mg/L, Pb5(PO4)3Cl and Cd5(PO4)3OH will coexist in large quantities, indicating that these two environmental factors will determine the simultaneous stabilization of Pb and Cd. However, there are relatively few highly alkaline soils (>9) in the real environment, which is not conducive to crop growth. Therefore, the content of P will be the guarantee for the passivation and remediation of heavy metals (Pb and Cd). We further confirmed, with the GWB model, that Cd5(PO4)3OH can be formed even if the pH is less than 8 when the P concentration is sufficient (>170 mg/L) (Table S1). Therefore, in the combined remediation, the acidification caused by PSB can be compensated for by the alkalinity of biochar itself, so as to form a stable mineralization reaction system in the microenvironment (biochar surface or content). To sum up, the most important thing in repairing mixed heavy metal pollution is to block and stabilize. The adsorption capacity of biochar and the mineralization capacity of phosphorus-dissolving bacteria have excellent application prospects on the basis of preventing the diffusion of pollution sources.
5. Conclusions
In the face of sudden Pb/Cd pollution, the combined remediation of biochar and bacteria can have a very good immobilization effect in a short time and effectively reduce the diffusion of toxic heavy metals. The physical/chemical and biological properties of the original soil were significantly reduced under high concentrations of toxic metal stress, such as pH (decreased by 11.31%) and total microbial biomass (decreased by 44.93%). The addition of PSB and biochar, respectively, both partially improved the properties of the contaminated soil. PSB can reduce the pH of contaminated soil by 4.78% through acid secretion, reduce the water content by 1.50% via their own reproduction and reduce the highly hazardous toxic metal form of Cd in the water-soluble fraction by 52.49%. The addition of biochar increased the soil pH by 15.4%, the total N by 10.20% and the available P by 67.63%, but the bacterial biomass improvement was not as good as the addition of PSB alone; both of them cannot restore the contaminated soil to the original soil properties in a short period of time. The combination of PSB and biochar can not only restore the basic properties of the soil in the short time, but can even surpass the non-contaminated soil in some biological properties, such as bacteria (by 16.07%). Compared with the toxic metal-contaminated soil, the combined remediation can reduce the unstable toxic metal forms (water-soluble fraction: Pb decreased by 41.00% and Cd decreased by 91.49%; exchangeable fraction: Pb decreased by 85.01% and Cd decreased by 69.33%) and increase the stable toxic metal forms (acid-soluble fraction: Pb increased by 5 times and Cd increased by 15 times; non-bioavailable fraction: Pb increased by 14 times and Cd increased by 6 times), so the effect of the combined remediation of both was much better than the single remediation. This study shows that the combination of biochar and PSB remediated the mixed pollution of Pb and Cd in soil, mainly by forming two toxic metal minerals, Pb phosphate and Cd carbonate, in the short term (30 days), and thereby reduced the stress of toxic metals on soil microorganisms so that the microbial biomass of polluted soil can be restored to its original state in the short term.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy12051003/s1, Table S1: Test results of normal distribution of index data, Table S2: Parameters in Act2 program module of the GWB software, Figure S1: The soluble reactive phosphate (PO4) of bacterial liquid and medium during five-day culture. Figure S2: Final minerals (Pb + Cd) formed at high P concentration in the GWB model. Refs [30,31,34,35,54] are cited in the supplementary materials.
Author Contributions
Conceptualization, H.C. and Y.W. (Yanyi Wu); Data curation, C.Z., L.P. and Y.L.; Methodology, W.L. and Y.D.; Validation, Y.W. (Yuhong Wang); Writing—original draft, H.C. and W.L.; Writing—review & editing, H.C., Z.L. and Y.W. (Yuhong Wang). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China project (Number 42007105).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
We have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Shen, Z.T.; Hou, D.Y.; Zhao, B.; Xu, W.D.; Ok, Y.S.; Bolan, N.S.; Alessi, D.S. Stability of heavy metals in soil washing residue with and without biochar addition under accelerated ageing. Sci. Total Environ. 2018, 619, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Wu, X.J.; Yao, L.G.; Chen, Z.J. Heavy metal-immobilizing bacteria combined with calcium polypeptides reduced the uptake of Cd in wheat and shifted the rhizosphere bacterial communities. Environ. Pollut. 2020, 267, 115432. [Google Scholar] [CrossRef] [PubMed]
- National Soil Pollution Status Survey Bulletin. Ministry of Ecological and Environment of the People’s Republic of China. Available online: http://www.mee.gov.cn/gkml/sthjbgw/qt/201404/t20140417270670wh.htm (accessed on 17 April 2014).
- Manzetti, S.; van der Spoel, E.R.; van der Spoel, D. Chemical Properties, Environmental Fate, and Degradation of Seven Classes of Pollutants. Chem. Res. Toxicol. 2014, 27, 713–737. [Google Scholar] [CrossRef] [PubMed]
- Kalia, K.; Flora, S.J.S. Strategies for safe and effective therapeutic measures for chronic arsenic and lead poisoning. J. Occup. Health 2005, 47, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Chen, H.P.; Kopittke, P.M.; Kretzschmar, R.; Zhao, F.J.; Wang, P. The Voltaic Effect as a Novel Mechanism Controlling the Remobilization of Cadmium in Paddy Soils during Drainage. Environ. Sci. Technol. 2021, 55, 1750–1758. [Google Scholar] [CrossRef]
- Godt, J.; Scheidig, F.; Grosse-Siestrup, C.; Esche, V.; Brandenburg, P.; Reich, A.; Groneberg, D.A. The toxicity of cadmium and resulting hazards for human health. J. Occup. Med. Toxicol. 2006, 11, 22. [Google Scholar] [CrossRef] [Green Version]
- Melvin, S.S.; Selvarajan, E.; Sarswat, A.; Muthukumar, H.; Jacob, J.M.; Mukesh, M.; Pugazhendhi, A. Nanomaterials as adsorbents for As(III) and As(V) removal from water: A review. J. Hazard. Mater. 2022, 424, 127572. [Google Scholar]
- Melvin, S.S.; Selvarajan, E.; Chidambaram, R.; Patel, H.; Brindhadevi, K. Clean approach for chromium removal in aqueous environments and role of nanomaterials in bioremediation: Present research and future perspective. Chemosphere 2021, 284, 131368. [Google Scholar]
- Li, Z.; Su, M.; Duan, X.F.; Tian, D.; Yang, M.Y.; Guo, J.Y.; Wang, S.M.; Hu, S.J. Induced biotransformation of lead II by Enterobacter sp in SO4-PO4-Cl-Para solution. J. Hazard. Mater. 2018, 357, 491–497. [Google Scholar] [CrossRef]
- Chen, H.M.; Zhang, J.W.; Tang, L.Y.; Su, M.; Tian, D.; Zhang, L.; Li, Z.; Hu, S.J. Enhanced Pb immobilization via the combination of biochar and phosphate solubilizing bacteria. Environ. Int. 2019, 127, 395–401. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Zhao, X.; Yuan, J.; Li, M.; Li, T. Phosphate functionalized iron based nanomaterials coupled with phosphate solubilizing bacteria as an efficient remediation system to enhance lead passivation in soil. J. Hazard. Mater. 2021, 419, 126433. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.D.; Shao, W.; Zhang, K.Y.; Huo, Y.Q.; Zhu, J.; Li, M. Pb biosorption by Leclercia adecarboxylata: Protective and immobilized mechanisms of extracellular polymeric substances. Chem. Eng. J. 2019, 375, 122113. [Google Scholar] [CrossRef]
- Giller, K. Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: A review. Soil Biol. Biochem. 1998, 30, 1389–1414. [Google Scholar] [CrossRef]
- Jiang, Z.Q.; Jiang, L.; Zhang, L.; Su, M.; Tian, D.; Wang, T.; Sun, Y.L.; Nong, Y.; Hu, S.J.; Wang, S.M.; et al. Contrasting the Pb II and Cd II tolerance of Enterobacter sp. via its cellular stress responses. Environ. Microbiol. 2020, 22, 1507–1516. [Google Scholar] [CrossRef]
- Steiner, C.; Das, K.C.; Garcia, M.; Forster, B.; Zech, W. Charcoal and smoke extract stimulate the soil microbial community in a highly weathered xanthic Ferralsol. Pedobiologia 2008, 51, 359–366. [Google Scholar] [CrossRef]
- Liang, B.Q.; Lehmann, J.; Sohi, S.P.; Thies, J.E.; O’Neill, B.; Trujillo, L.; Gaunt, J.; Solomon, D.; Grossman, J.; Neves, E.G.; et al. Black carbon affects the cycling of non-black carbon in soil. Org. Geochem. 2010, 41, 206–213. [Google Scholar] [CrossRef]
- Smith, J.L.; Collins, H.P.; Bailey, V.L. The effect of young biochar on soil respiration. Soil Biol. Biochem. 2010, 42, 2345–2347. [Google Scholar] [CrossRef]
- Zhu, X.M.; Chen, B.L.; Zhu, L.Z.; Xing, B.S. Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: A review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef]
- Lian, F.; Xing, B.S. Black Carbon Biochar in Water/Soil Environments: Molecular Structure, Sorption, Stability, and Potential Risk. Environ. Sci. Technol. 2017, 51, 13517–13532. [Google Scholar] [CrossRef]
- Shen, Z.T.; Tian, D.; Zhang, X.Y.; Tang, L.Y.; Su, M.; Zhang, L.; Li, Z.; Hu, S.J.; Hou, D.Y. Mechanisms of biochar assisted immobilization of Pb2+ by bioapatite in aqueous solution. Chemosphere 2018, 190, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Tang, L.Y.; Wang, Z.J.; Su, M.; Tian, D.; Zhang, L.; Li, Z. Evaluating the protection of bacteria from extreme Cd II stress by P-enriched biochar. Environ. Pollut. 2020, 263, 114483. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Y.; Zhang, L.; Riaz, M.; Xia, H.; Jiang, C.C. Biochar amendment improved fruit quality and soil properties and microbial communities at different depths in citrus production. J. Clean. Prod. 2021, 292, 126062. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Liu, Y.D.; Zhan, W.H.; Zheng, K.X.; Wang, J.N.; Zhan, C.S.; Chen, R.H. Stabilization of heavy metal-contaminated soils by biochar: Challenges and recommendations. Sci. Total Environ. 2020, 729, 139060. [Google Scholar] [CrossRef] [PubMed]
- Helmke, P.A.; Sparks, D.L. Methods of Soil Analysis, Part 3: Chemical Methods; ASA, CSSA, SSSA Books: Burlington, MA, USA, 1996; ISBN 0891188258. [Google Scholar]
- Nelson, D.W. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis: Part 3 Chemical Methods; ASA, CSSA, SSSA Books: Madison, CT, USA, 1996; Volume 9, pp. 961–1010. [Google Scholar] [CrossRef]
- Watanabe, F.S.; Olsen, S.R. Test of an Ascorbic Acid Method for Determining Phosphorus in Water and NaHCO3 Extracts from Soil. Soil Sci. Soc. Am. J. 1965, 291, 677–678. [Google Scholar] [CrossRef]
- Handreck, K.; Black, N. Growing Media for Ornamental Plant and Turf; University of New South Wales Press: Sydney, Australia, 2005. [Google Scholar]
- Frostegård, A.; Bååth, E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol. Fertil. Soils 1996, 221–222, 59–65. [Google Scholar] [CrossRef]
- Chen, H.M.; Ma, J.Y.; Wei, J.X.; Gong, X.; Yu, X.C.; Guo, H.; Zhao, Y.W. Biochar increases plant growth and alters microbial communities via regulating the moisture and temperature of green roof substrates. Sci. Total Environ. 2018, 635, 333–342. [Google Scholar] [CrossRef]
- Denef, K.; Roobroeck, D.; Wadu, M.C.M.; Lootens, P.; Boeckx, P. Microbial community composition and rhizodeposit-carbon assimilation in differently managed temperate grassland soils. Soil Biol. Biochem. 2009, 41, 144–153. [Google Scholar] [CrossRef]
- Frostegard, A.; Tunlid, A.; Baath, E. Use and misuse of PLFA measurements in soils. Soil Biol. Biochem. 2011, 43, 1621–1625. [Google Scholar] [CrossRef]
- Wan, X.H.; Huang, Z.Q.; He, Z.M.; Yu, Z.P.; Wang, M.H.; Davis, M.R.; Yang, Y.S. Soil C:N ratio is the major determinant of soil microbial community structure in subtropical coniferous and broadleaf forest plantations. Plant Soil 2015, 387, 103–116. [Google Scholar] [CrossRef]
- Swallow, M.; Quideau, S.A.; MacKenzie, M.D.; Kishchuk, B.E. Microbial community structure and function: The effect of silvicultural burning and topographic variability in northern Alberta. Soil Biol. Biochem. 2009, 41, 770–777. [Google Scholar] [CrossRef]
- Shen, Z.T.; Zhang, Y.Y.; Jin, F.; McMillan, O.; Al-Tabbaa, A. Qualitative and quantitative characterisation of adsorption mechanisms of lead on four biochars. Sci. Total Environ. 2017, 609, 1401–1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Tang, L.; Hu, Y.; Geng, Y.; Meng, L.; Li, W.; Wang, Z.; Li, Z.; Huo, Z. Investigating the pathways of enhanced Pb immobilization by chlorine-loaded biochar. J. Clean. Prod. 2022, 344, 131097. [Google Scholar] [CrossRef]
- Srivastava, S.; Kausalya, M.T.; Archana, G.; Rupela, O.P.; Naresh-Kumar, G. Efficacy of organic acid secreting bacteria in solubilization of rock phosphate in acidic alfisols. In First International Meeting on Microbial Phosphate Solubilization; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar] [CrossRef]
- Vreulink, J.M.; Esterhuyse, A.; Jacobs, K.; Botha, A. Soil properties that impact yeast and actinomycete numbers in sandy low nutrient soils. Can. J. Microbiol. 2007, 53, 1369–1374. [Google Scholar] [CrossRef]
- Yuan, J.H.; Xu, R.K.; Wang, N.; Li, J.Y. Amendment of Acid Soils with Crop Residues and Biochars. Pedosphere 2011, 21, 302–308. [Google Scholar] [CrossRef]
- Sheng, Y.Q.; Zhu, L.Z. Biochar alters microbial community and carbon sequestration potential across different soil pH. Sci. Total Environ. 2018, 622, 1391–1399. [Google Scholar] [CrossRef]
- Jones, B.E.H.; Haynes, R.J.; Phillips, I.R. Effect of amendment of bauxite processing sand with organic materials on its chemical, physical and microbial properties. J. Environ. Manag. 2010, 91, 2281–2288. [Google Scholar] [CrossRef]
- Brockhoff, S.R.; Christians, N.E.; Killorn, R.J.; Horton, R.; Davis, D.D. Physical and Mineral-Nutrition Properties of Sand-Based Turfgrass Root Zones Amended with Biochar. Agron. J. 2010, 102, 1627–1631. [Google Scholar] [CrossRef]
- Jaafar, N.M.; Clode, P.L.; Abbott, L.K. Biochar-Soil Interactions in Four Agricultural Soils. Pedosphere 2015, 25, 729–736. [Google Scholar] [CrossRef]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic values of greenwaste biochar as a soil amendment. Aust. J. Soil Res. 2007, 45, 629–634. [Google Scholar] [CrossRef]
- Beck, D.A.; Johnson, G.R.; Spolek, G.A. Amending greenroof soil with biochar to affect runoff water quantity and quality. Environ. Pollut. 2011, 159, 2111–2118. [Google Scholar] [CrossRef] [PubMed]
- Farrell, M.; Kuhn, T.K.; Macdonald, L.M.; Maddern, T.M.; Murphy, D.V.; Hall, P.A.; Singh, B.P.; Baumann, K.; Krull, E.S.; Baldock, J.A. Microbial utilisation of biochar-derived carbon. Sci. Total Environ. 2013, 465, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Keiblinger, K.M.; Hall, E.K.; Wanek, W.; Szukics, U.; Hammerle, I.; Ellersdorfer, G.; Bock, S.; Strauss, J.; Sterflinger, K.; Richter, A.; et al. The effect of resource quantity and resource stoichiometry on microbial carbon-use-efficiency. FEMS Microbiol. Ecol. 2010, 73, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.L.; Patti, A.F.; Rose, M.T.; Schefe, C.R.; Wilkinson, K.; Cavagnaro, T.R. Functional stoichiometry of soil microbial communities after amendment with stabilised organic matter. Soil Biol. Biochem. 2014, 76, 170–178. [Google Scholar] [CrossRef] [Green Version]
- Chintala, R.; Schumacher, T.E.; Kumar, S.; Malo, D.D.; Rice, J.A.; Bleakley, B.; Chilom, G.; Clay, D.E.; Julson, J.L.; Papiernik, S.K.; et al. Molecular characterization of biochars and their influence on microbiological properties of soil. J. Hazard. Mater. 2014, 279, 244–256. [Google Scholar] [CrossRef]
- Cheng, S.; Chen, T.; Xu, W.B.; Huang, J.; Jiang, S.J.; Yan, B. Application Research of Biochar for the Remediation of Soil Heavy Metals Contamination: A Review. Molecules 2020, 25, 3167. [Google Scholar] [CrossRef]
- Seshadri, B.; Bolan, N.S.; Choppala, G.; Kunhikrishnan, A.; Sanderson, P.; Wang, H.; Currie, L.D.; Tsang, D.C.W.; Ok, Y.S.; Kim, G. Potential value of phosphate compounds in enhancing immobilization and reducing bioavailability of mixed heavy metal contaminants in shooting range soil. Chemosphere 2017, 184, 197–206. [Google Scholar] [CrossRef]
- Ayati, M.; Madsen, H.E.L. Solubility product of the cadmium phosphate Cd5H2(PO4)4·4H2O at 37 °C. J. Chem. Eng. Data 2001, 46, 113–116. [Google Scholar] [CrossRef]
- Chen, D.; Lan, Z.; Hu, S.; Bai, Y. Effects of nitrogen enrichment on belowground communities in grassland: Relative role of soil nitrogen availability vs. soil acidification. Soil Biol. Biochem. 2015, 89, 99–108. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).