Rejected Sago Starch as a Coating Material to Mitigate Urea-Nitrogen Emission
Abstract
1. Introduction
2. Materials and Methods
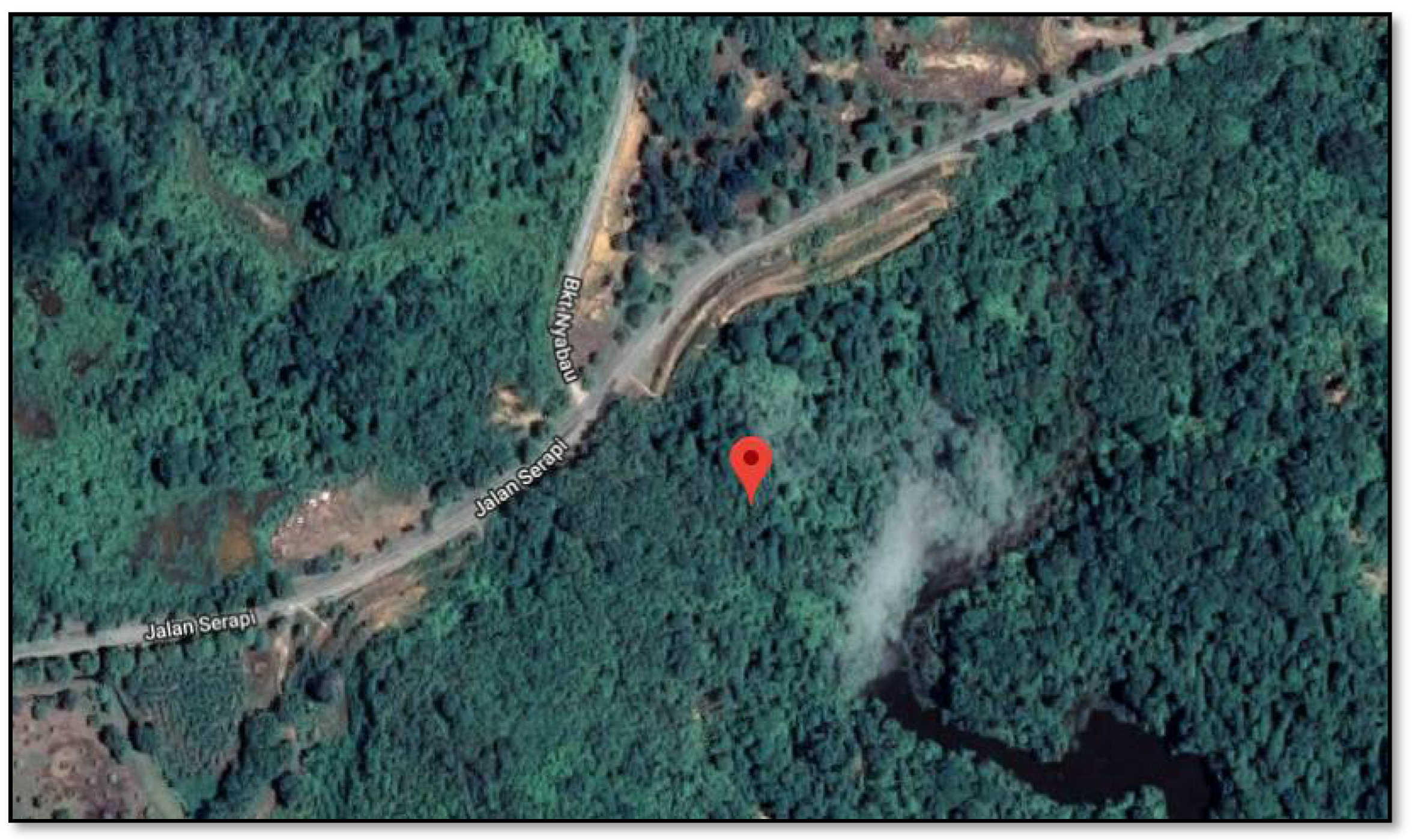
2.1. Soil Sampling Site, Preparation, and Initial Physico-Chemical Characterization
2.2. Rejected Sago Starch Characterization
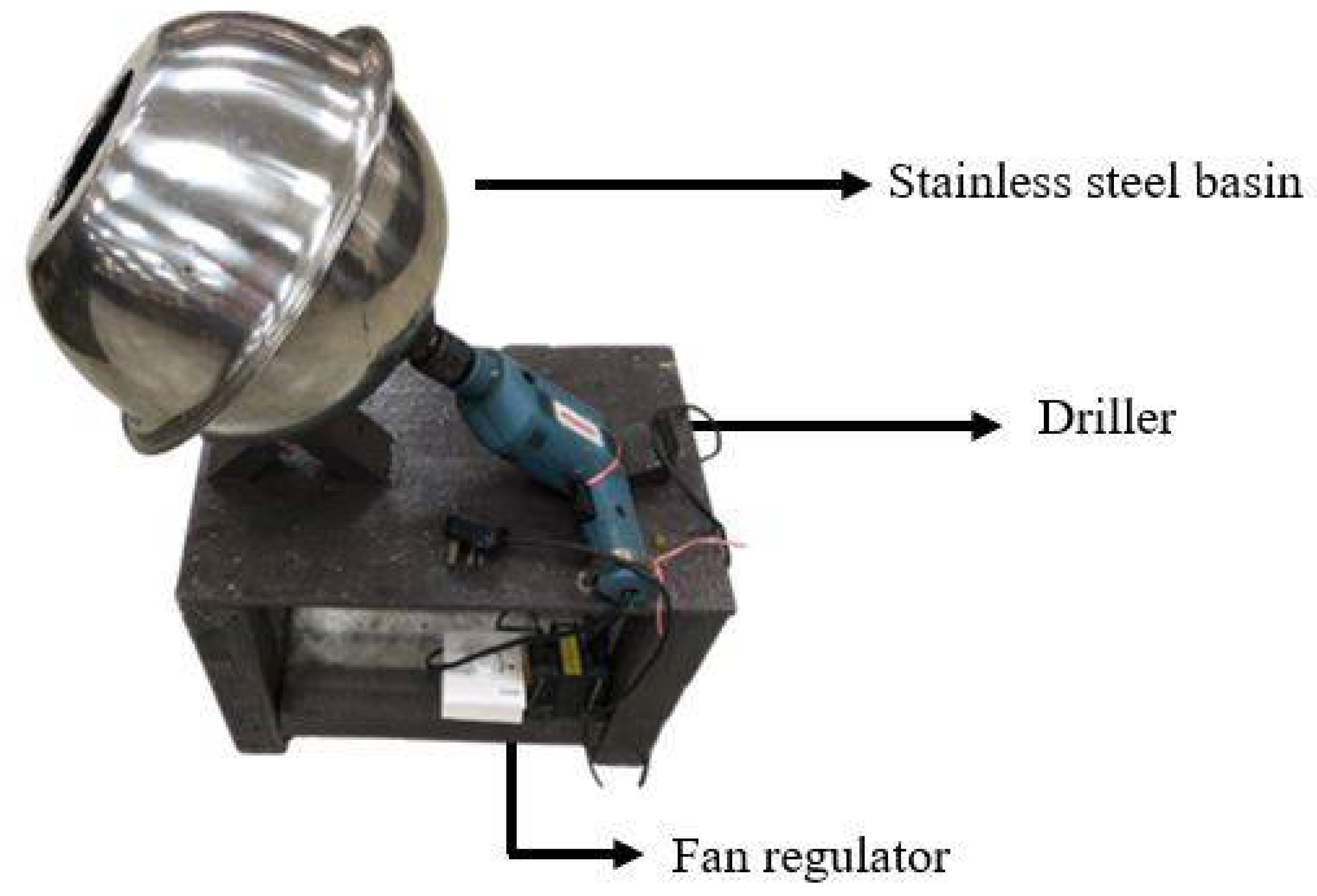
2.3. Preparation of Rejected Sago Starch-Coated Urea
2.4. Characterization of Uncoated Urea and Rejected Sago Starch-Coated Urea
2.4.1. Nitrogen Content
2.4.2. Thickness of Coating
2.4.3. Urea Dissolution Rate
2.5. Ammonia Volatilization Study
- S: soil alone
- U: soil + 5 g of uncoated urea
- CU1: soil + 5 g of urea coated with RSS (2%)
- CU2: soil + 5 g of urea coated with RSS (3%)
- CU3: soil + 5 g of urea coated with RSS (4%)
- CU4: soil + 5 g of urea coated with RSS (5%)
- CU5: soil + 5 g of urea coated with RSS (6%)
2.6. Experimental Design and Statistical Analysis
3. Results
3.1. Selected Physical and Chemical Properties of Soil
3.2. Selected Physical and Chemical Properties of Rejected Sago Starch
3.3. Characterization of Uncoated and Coated Urea
3.3.1. Nitrogen Content of Uncoated Urea and Urea Coated with Rejected Sago Starch
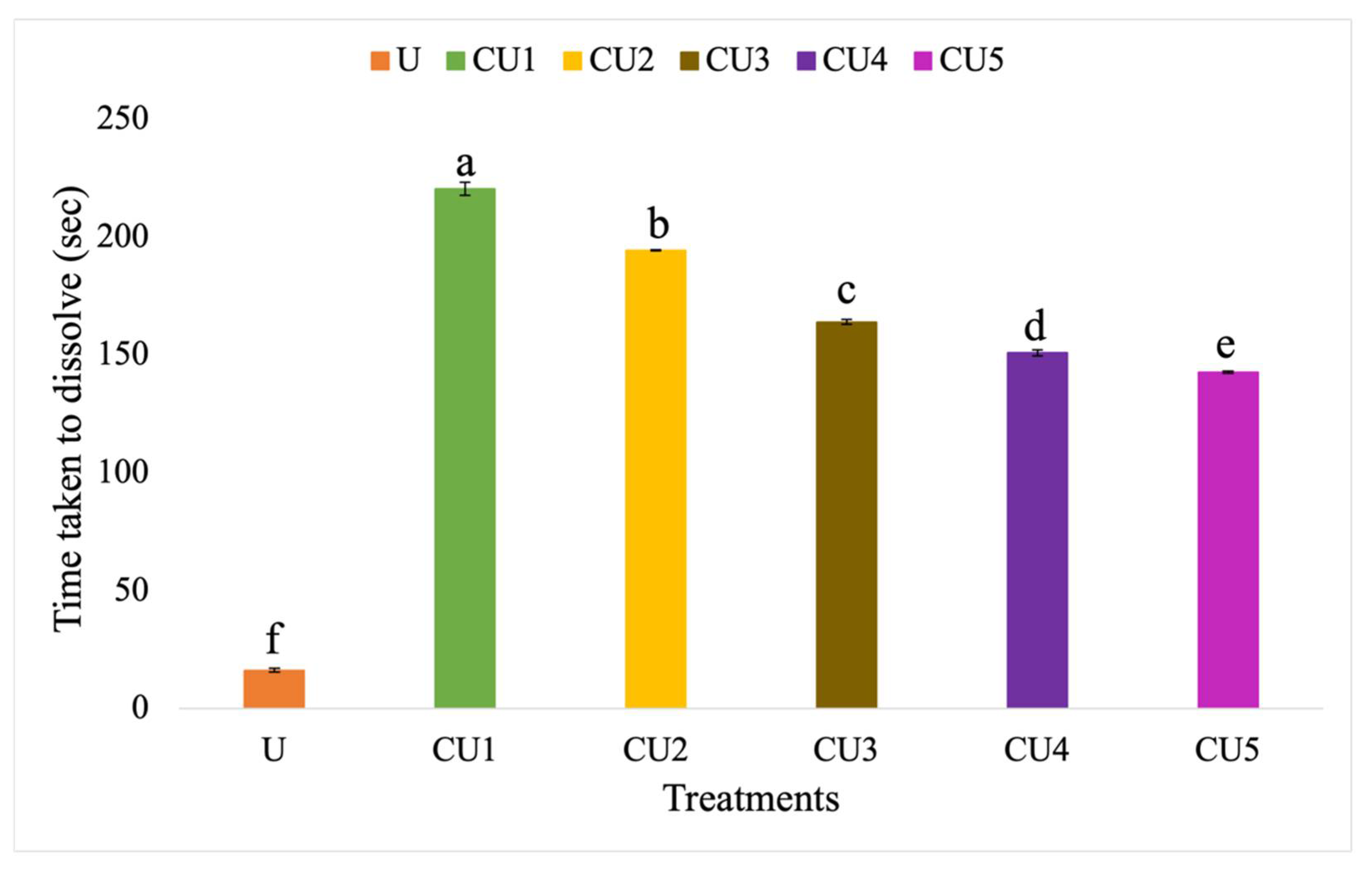
3.3.2. Dissolution Rate of Uncoated Urea and Rejected Sago Starch-Coated Urea
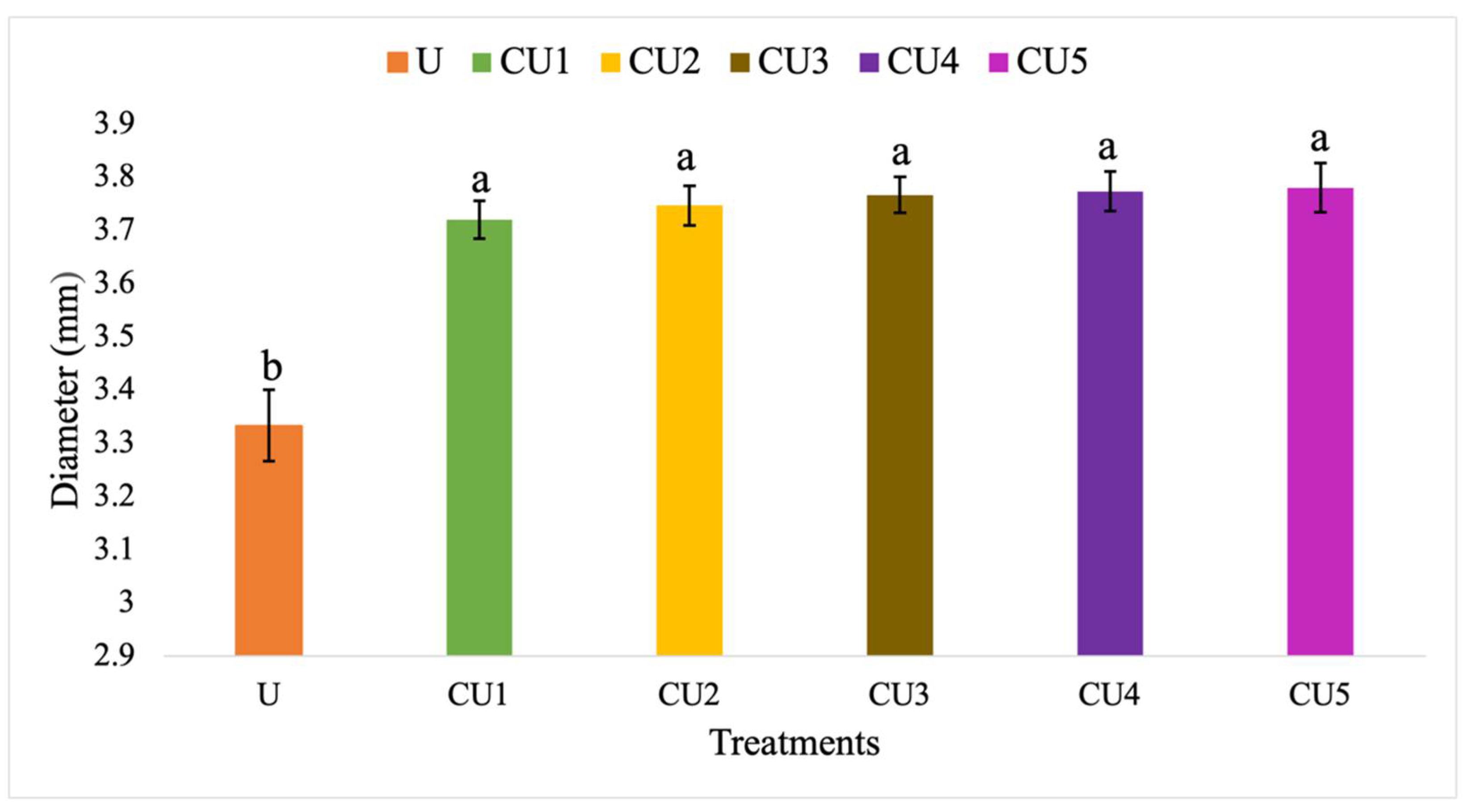
3.3.3. Granule Diameters of Uncoated Urea and Rejected Sago Starch-Coated Urea
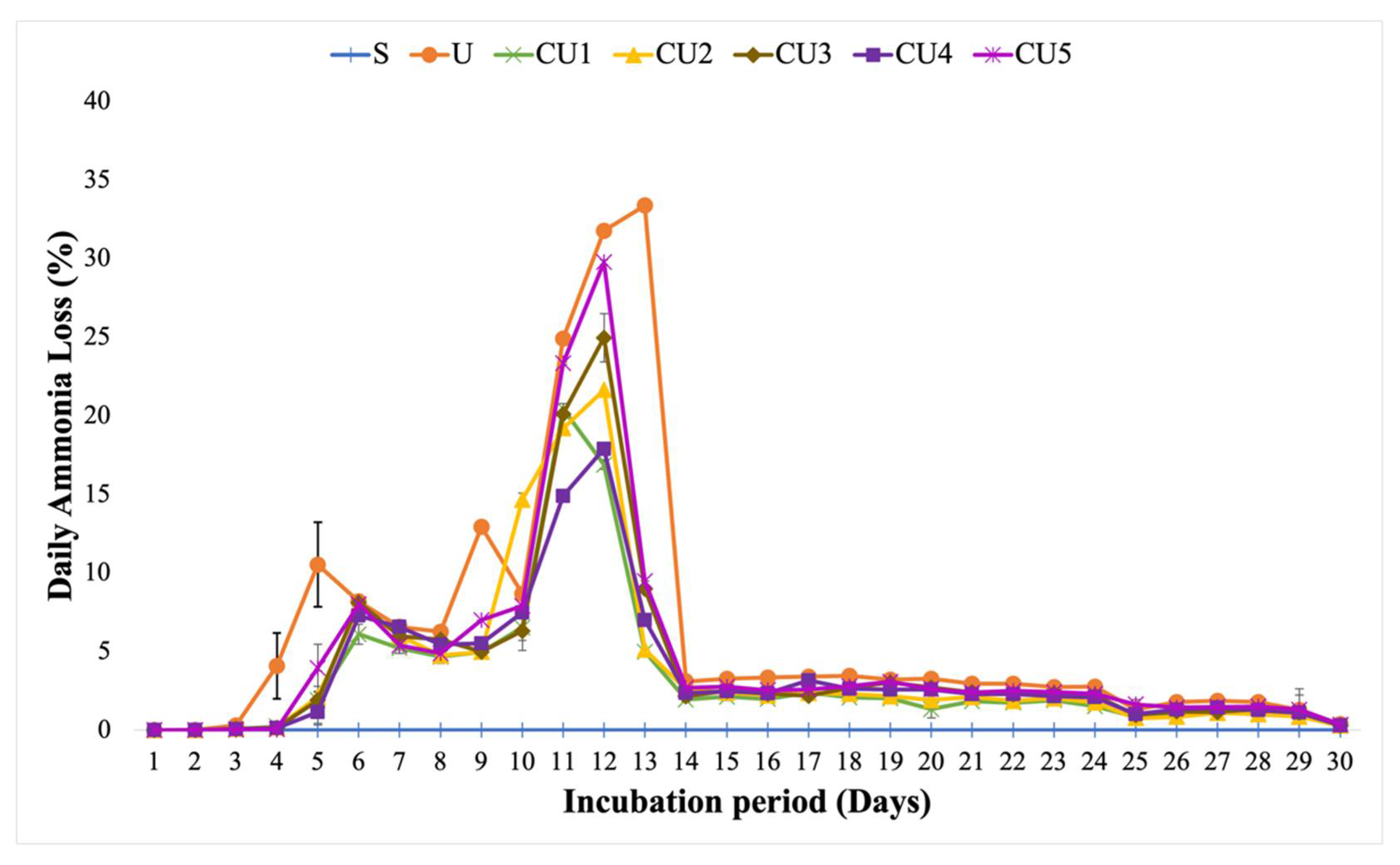
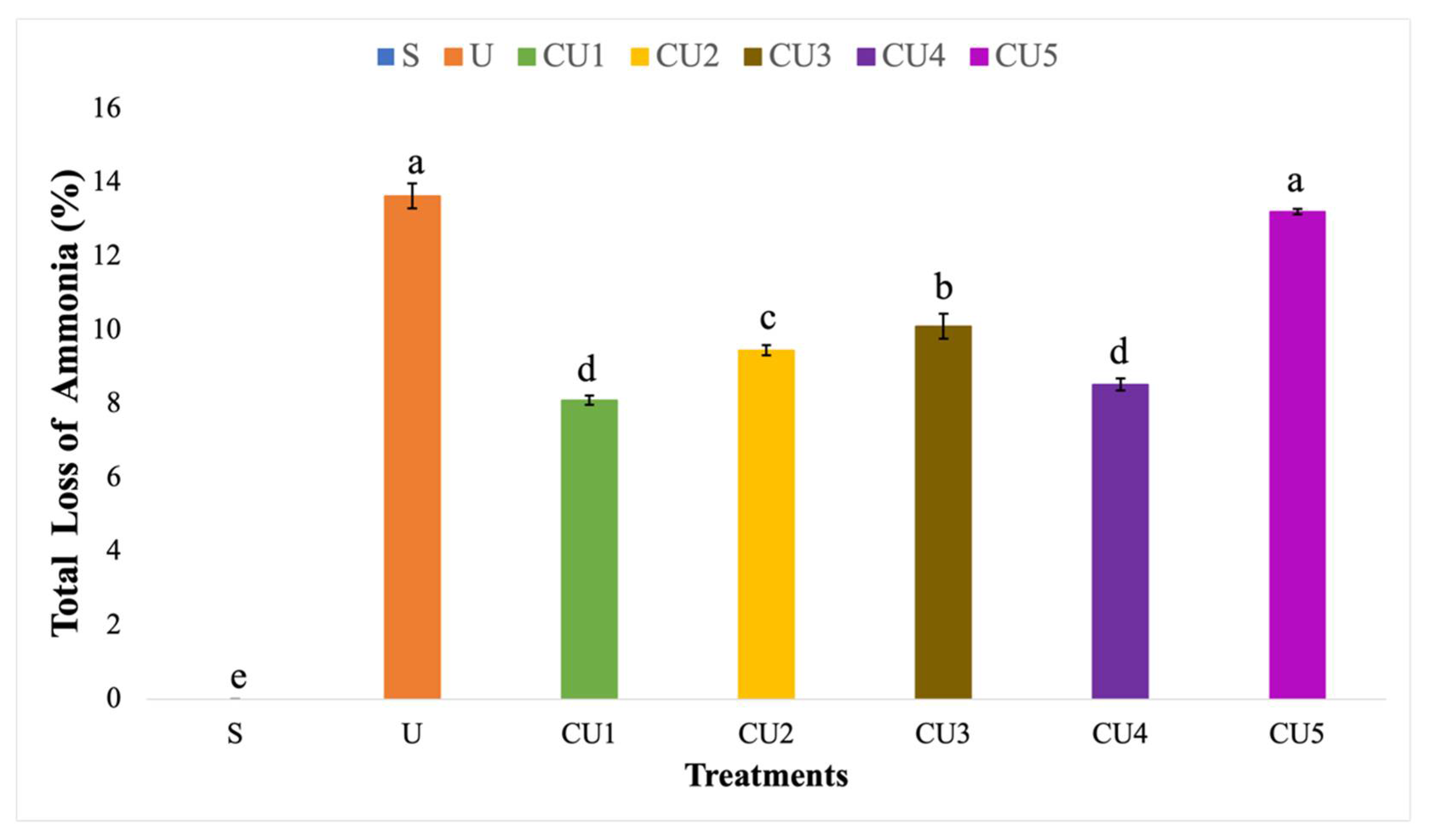
3.4. Ammonia Volatilization from Uncoated Urea and Rejected Sago Starch-Coated Urea
3.5. Soil pH, Exchangeable Ammonium, and Available Nitrate of Uncoated and Coated Urea
4. Discussion
4.1. Selected Physical and Chemical Properties of the Bekenu Series Soil
4.2. Selected Physical and Chemical Properties of Rejected Sago Starch
4.3. Characterization of Uncoated and Coated Urea
4.3.1. Nitrogen Content of Urea Coated with Rejected Sago Starch
4.3.2. Dissolution Rate of Uncoated Urea and Rejected Sago Starch-Coated Urea
4.3.3. Diameter of Uncoated Urea and Rejected Sago Starch-Coated Urea
4.4. Comparison of Ammonia Loss from Uncoated Urea and Rejected Sago Starch-Coated Urea
4.5. Soil pH, Exchangeable Ammonium, and Available Nitrate of Uncoated and Coated Urea
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rose, T.J.; Wood, R.H.; Rose, M.T.; Zwieten, L.V. A re-evaluation of the agronomic effectiveness of the nitrification inhibitors DCD and DMPP and the urease inhibitor NBPT. Agric. Ecosyst. Environ. 2018, 252, 69–73. [Google Scholar] [CrossRef]
- Bashir, M.T.; Ali, S.; Ghauri, M.; Adris, A.; Harun, R. Impact of excessive nitrogen fertilizers on the environment and associated mitigation strategies. Asian J. Microbiol. Biotechnol. Environ. Sci. 2013, 15, 213–221. [Google Scholar]
- Zavaschi, E.; Faria, L.D.; Vitti, G.C.; Nascimento, C.A.; Moura, T.A.; Vale, D.W.; Kamogawa, M.Y. Ammonia volatilization and yield components after application of polymer-coated urea to maize. Rev. Bras. Ciência Solo 2014, 38, 1200–1206. [Google Scholar] [CrossRef]
- Sanz-Cobena, A.; Misselbrook, T.; Camp, V.; Vallejo, A. Effect of water addition and the Urease Inhibitor NBPT on the abatement of ammonia emission from surface applied urea. Atmos. Environ. 2011, 45, 1517–1524. [Google Scholar] [CrossRef]
- Peng, Y.; Tian, Y.; Yin, B. Effects of NBPT Urease inhibitor on ammonia Volatilization in paddy fields with wheat straw application. Chin. J. Eco-Agric. 2012, 20, 19–23. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, J.J.; Liu, S.; Zhang, Z.; Dodla, S.K.; Myers, G. Application effects of coated urea and urease and Nitrification inhibitors on ammonia and greenhouse gas emissions from a subtropical cotton field of the Mississippi Delta region. Sci. Total Environ. 2015, 533, 329–338. [Google Scholar] [CrossRef]
- Zuki, M.M.M.; Md. Jaafar, N.; Sakimin, S.Z.; Yusop, M.K. N-(n-Butyl) thiophosphoric TRIAMIDE (NBPT)-Coated Urea (NCU) improved Maize growth and nitrogen use Efficiency (NUE) in highly Weathered Tropical Soil. Sustainability 2020, 12, 8780. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Zhang, M.; Li, Y.; Fan, X.-H.; Geng, Y.-Q. Improving the quality of polymer-coated urea with recycled plastic, proper additives, and large tablets. J. Agric. Food. Chem. 2012, 60, 11229–11237. [Google Scholar] [CrossRef]
- Farmaha, B.S.; Sims, A.L. Yield and protein response of wheat cultivars to polymer-coated urea and urea. Agron. J. 2013, 105, 229–236. [Google Scholar] [CrossRef]
- Cantarella, H.; Trivelin, P.C.; Contin, T.L.; Dias, F.L.; Rossetto, R.; Marcelino, R.; Quaggio, J.A. Ammonia volatilisation from urease inhibitor-treated urea applied to sugarcane trash blankets. Sci. Agricol. 2008, 65, 397–401. [Google Scholar] [CrossRef]
- Bhatia, A.; Sasmal, S.; Jain, N.; Pathak, H.; Kumar, R.; Singh, A. Mitigating nitrous oxide emission from soil under conventional and no-tillage in wheat using nitrification inhibitors. Agric. Ecosyst. Environ. 2010, 136, 247–253. [Google Scholar] [CrossRef]
- Li, Y.; Hu, M.; Tenuta, M.; Ma, Z.; Gui, D.; Li, X.; Gao, X.C. Agronomic evaluation of polymer-coated urea and urease and nitrification inhibitors for cotton production under drip-fertigation in a dry climate. Sci. Rep. 2020, 10, 1472. [Google Scholar] [CrossRef] [PubMed]
- Gioacchini, P.; Ramieri, N.A.; Montecchio, D.; Marzadori, C.; Ciavatta, C. Dynamics of mineral nitrogen in soils treated with slow-release fertilizers. Commun. Soil Sci. Plant Anal. 2006, 37, 1–12. [Google Scholar] [CrossRef]
- Kavoosi, M. Effects of zeolite application on rice yield, nitrogen recovery, and nitrogen use efficiency. Commun. Soil Sci. Plant Anal. 2007, 38, 69–76. [Google Scholar] [CrossRef]
- Kottegoda, N.; Sandaruwan, C.; Priyadarshana, G.; Siriwardhana, A.; Rathnayake, U.A.; Berugoda Arachchige, D.M.; Kumarasinghe, A.R.; Dahanayake, D.; Karunaratne, V.; Amaratunga, G.A.J. Urea-hydroxyapatite nanohybrids for slow release of nitrogen. ACS Nano 2017, 11, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.; Mailapalli, D.R. Zeolite coated urea fertilizer using different binders: Fabrication, material properties and nitrogen release studies. Environ. Technol. Inno. 2019, 16, 100452. [Google Scholar] [CrossRef]
- Elhassani, C.E.; Essamlali, Y.; Aqlil, M.; Nzenguet, A.M.; Ganetri, I.; Zahouily, M. Urea-impregnated HAP encapsulated by lignocellulosic biomass-extruded composites: A novel slow-release fertilizer. Environ. Technol. Inno. 2019, 15, 100403. [Google Scholar] [CrossRef]
- Beig, B.; Niazi, M.B.; Jahan, Z.; Pervaiz, E.; Abbas Shah, G.; Ul Haq, M.; Zia, M. Slow-release urea prills developed using organic and inorganic blends in fluidized bed coater and their effect on spinach productivity. Sustainability 2020, 12, 5944. [Google Scholar] [CrossRef]
- Junejo, N.; Yusof, M.K.; Dharejo, K.A.; Abdu, A.; Abdul-Hamid, H. A field evaluation of coated urea with biodegradable materials and selected urease inhibitors. Afr. J. Biotechnol. 2011, 10, 19729–19736. [Google Scholar]
- Dimkpa, C.O.; Andrews, J.; Fugice, J.; Singh, U.; Bindraban, P.S.; Elmer, W.H.; White, J.C. Facile coating of urea with low-dose ZnO nanoparticles promotes wheat performance and enhances Zn uptake under drought stress. Front. Plant Sci. 2020, 11, 168. [Google Scholar] [CrossRef]
- Himmah, N.I.; Djajakirana, G.; Darmawan, D. Nutrient release performance of Starch Coated NPK fertilizers and their effects on Corn Growth. Sains Tanah-J. Soil Sci. Agroclimatol. 2018, 15, 104. [Google Scholar] [CrossRef]
- Jyothi, A.N.; Pillai, S.S.; Aravind, M.; Salim, S.A.; Kuzhivilayil, S.J. Cassava starch-graft-poly(acrylonitrile)-coated urea fertilizer with sustained release and water retention properties. Adv. Polym. Technol. 2018, 37, 2687–2694. [Google Scholar] [CrossRef]
- Zafar, N.; Niazi, M.B.K.; Sher, F.; Khalid, U.; Jahan, Z.; Shah, G.A.; Zia, M. Starch and polyvinyl alcohol encapsulated biodegradable nanocomposites for environment friendly slow release of urea fertilizer. Chem. Eng. J. Adv. 2021, 7, 100123. [Google Scholar] [CrossRef]
- Savitri, E.; Purwanto, E.; Kodrat, A.N.; Yonathan, E. Controlled release fertilizer based on starch chitosan encapsulation. In Proceedings of the IOP Conference Series:Materials Science and Engineering, Bali, Indonesia, 22–23 August 2019; Volume 703. [Google Scholar]
- Amin, N.M.; Sabli, N.; Izhar, S.; Yoshida, H. Sago wastes and its applications. Pertanika J. Sci. Technol. 2019, 27, 1841–1862. [Google Scholar]
- Flores, L.N. Urban lakes: Ecosystems at risk, worthy of the best care. In Proceedings of the Materials of the 12th World Lake Conference, Taal 2007, Jaipur, India, 28 October–2 November 2007; pp. 1333–1337. [Google Scholar]
- Tie, A.P.; Karim, A.A.; Manan, D.M. Physicochemical properties of starch IN Sago PALMS (Metroxylon sagu) at different growth stages. Starch–Stärke 2008, 60, 408–416. [Google Scholar] [CrossRef]
- Tan, K.H. Soil Sampling, Preparation, and Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Bremner, J.M.; Keeney, D. Steam distillation methods for determination of ammonium, nitrate and nitrite. Anal. Chim. Acta. 1965, 32, 485–495. [Google Scholar] [CrossRef]
- Keeney, D.; Nelson, D. Nitrogen-inorganic forms. Agron. Monogr. Methods Soil Anal. 2015, 9, 643–698. [Google Scholar]
- Murphy, J.; Riley, R.I. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Adebowale, K.O.; Adeniyi Afolabi, T.; Lawal, O.S. Isolation, chemical modification and Physicochemical characterisation of Bambarra Groundnut (Voandzeia Subterranean) starch and flour. Food Chem. 2002, 78, 305–311. [Google Scholar] [CrossRef]
- Naz, M.Y.; Sulaiman, S.A. Testing of starch-based carbohydrate polymer coatings for enhanced urea performance. J. Coat. Technol. Res. 2014, 11, 747–756. [Google Scholar] [CrossRef]
- Siva, K.B.; Aminuddin, H.; Husni, M.H.A.; Manas, A.R. Ammonia volatilization from urea as affected by tropical-based palm oil mill effluent (Pome) and peat. Commun. Soil Sci. Plant Anal. 1999, 30, 785–804. [Google Scholar] [CrossRef]
- Ahmed, O.H.; Aminuddin, H.; Husni, A. Effect of urea, humic acid, and phosphate interactions in fertilizer microsites on ammonia volatilization, soil ammonium and nitrate contents. Int. J. Agric. Res. 2006, 1, 25–31. [Google Scholar] [CrossRef]
- Muda Agricultural Development Authority, “Rice Check,”. 2014. Available online: http://www.mada.gov.my/semakan-tanaman-padi (accessed on 8 August 2021).
- Paramananthan, S. Soil of Malaysia: Their Characteristics and Identification, Malaysia; Academy of Sciences Malaysia: Kuala Lumpur, Malaysia, 2000; Volume 1, pp. 11–125. ISBN 9839445065.
- Palanivell, P.; Ahmed, O.H.; Latifah, O.; Abdul Majid, N.M. Adsorption and desorption of nitrogen, phosphorus, potassium, and soil buffering capacity following application of chicken litter biochar to an acid soil. Appl. Sci. 2019, 10, 295. [Google Scholar] [CrossRef]
- Karim, A.A.; Tie, A.P.-L.; Manan, D.M.A.; Zaidul, I.S.M. Starch from the Sago (Metroxylon sagu) Palm Treeproperties, prospects, and challenges as a new INDUSTRIAL source for food and other uses. Compr. Rev. Food Sci. Food Saf. 2008, 7, 215–228. [Google Scholar] [CrossRef]
- Kusumayanti, H.; Handayani, N.A.; Santosa, H. Swelling power and water solubility of cassava and sweet potatoes flour. Procedia Environ. Sci. 2015, 23, 164–167. [Google Scholar] [CrossRef]
- Chan, H.T.; Bhat, R.; Karim, A.A. Physicochemical and functional properties of ozone oxidized starch. J. Agric. Food Chem. 2009, 57, 5965–5970. [Google Scholar] [CrossRef]
- Egharevba, O.H. Chemical Properties of starch and its application in the food industry. Chem. Prop. Starch 2020, 1–26. [Google Scholar] [CrossRef]
- Uthumporn, U.; Wahidah, N.; Karim, A.A. Physicochemical properties of starch from Sago (Metroxylon sagu) palm grown in mineral soil at different growth stages. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Ningbo, China, 27–29 May 2014; Volume 62, p. 012026. [Google Scholar]
- Moritsuka, N.; Matsuoka, K. An overview of the effects of heat treatments on the quality of organic wastes as a nitrogen fertilizer. Nitrogen Agric. 2018, 53. [Google Scholar] [CrossRef][Green Version]
- Azeem, B.; Kushaari, K.Z.; Man, Z. Effect of COATING thickness on Release characteristics of controlled Release urea produced in fluidized bed using waterborne starch biopolymer as coating material. Procedia Eng. 2016, 148, 282–289. [Google Scholar] [CrossRef]
- Babar, S.K.; Yusop, M.K.; Ahmed Babar, S.; Ali Khooharo, A. Consequences of cu and Zn coated urea to minimize ammonia volatilization. J. Teknol. 2016, 78, 6–12. [Google Scholar]
- Jadon, P.; Selladurai, R.; Yadav, S.S.; Coumar, M.V.; Dotaniya, M.L.; Singh, A.K.; Bhadouriya, J.; Kundu, S. Volatilization and leaching losses of nitrogen from different coated urea fertilizers. J. Soil Sci. Plant Nutr. 2018, 18, 1036–1047. [Google Scholar] [CrossRef]
- Rahman, A.N.S.; Yunus, R.; Ishak, C.F.; Hanif Khan, S. Laboratory evaluation on ammonia volatilization from coated urea fertilizers. Commun. Soil Sci. Plant Anal. 2018, 49, 717–724. [Google Scholar] [CrossRef]
- Kaneko, F.H.; Ferreira, J.P.; Leal, A.J.; Buzetti, S.; Reis, A.R.; Arf, O. Ammonia volatilization in response to coated and conventional urea in maize crop field. Biosci. J. 2019, 35, 713–722. [Google Scholar] [CrossRef]
- Faria, L.D.A.; Karp, F.H.S.; Machado, M.C.; Abdalla, A.L. Ammonia volatilization losses from urea coated with copper, boron, and selenium. Semin. Ciências Agrárias 2020, 41, 1415. [Google Scholar] [CrossRef]
- Latifah, O.; Ahmed, O.H.; Boyie Jalloh, M.; Abdul Majid, N.M. Rice husk compost production and use in mitigating ammonia volatilization from urea. Sustainability 2021, 13, 1832. [Google Scholar]
- Ahmed, O.H.; Aminuddin, H.; Husni, A. Ammonia volatilization and ammonium accumulation from urea mixed with zeolite and triple superphosphate. Acta Agric. Scand. Sect. B Soil Plant Sci. 2008, 58, 182–186. [Google Scholar]
- Latifah, O.; Ahmed, O.H.; Nik Muhamad, A.M.N. Minimizing ammonia volatilization in waterlogged soils through mixing of urea with zeolite and sago waste water. Intl. J. Phys. Sci. 2010, 5, 2193–2197. [Google Scholar]
- Osorio, A.A.; Herrera-Ruiz, G.; Pineda-Gómez, P.; Cornejo-Villegas, M.D.; Martínez-Bustos, F.; Gaytán, M.; Rodriguez García, M.E. Analysis of the Apparent viscosity of starch in aqueous suspension within agitation and temperature by using Rapid Visco Analyzer System. Mech. Eng. Res. 2011, 1, 110. [Google Scholar]
- Reddy, D.K.; Bhotmange, M.G. Viscosity of starch: A comparative study of Indian rice (Oryza Sativa L.) varieties. Int. Rev. Appl. Eng. Res. 2014, 4, 397–402. [Google Scholar]
- Latifah, O.; Ahmed, O.H.; Muhamad, A.M.N. Reducing ammonia loss from urea and improving soil exchangeable ammonium and available nitrate in non-waterlogged soils through mixing zeolite and sago (Metroxylon sagu) waste water. Int. J. Phys. Sci. 2011, 6, 866–870. [Google Scholar]
- Matin, N.H.; Jalali, M. The effect of waterlogging on electrochemical properties and soluble nutrients in paddy soils. Paddy Water Environ. 2017, 15, 443–455. [Google Scholar] [CrossRef]
- Lövblad, G.; Tarrason, L.; Tørseth, K. Base cations. EMEP Assessment Part I: European Perspective. European Monitoring and Evaluation Programme. 2004, 4. Available online: https://emep.int/publ/reports/2004/assessment/Part1_001-010_00a-Title.pdf (accessed on 8 August 2021).



| Coated Urea | Composition | ||
|---|---|---|---|
| Urea (g) | Starch Slurry (mL) | RSS (g) | |
| CU1(2%) | 200 | 0.4 g in 20 mL distilled water | 50 |
| CU2(3%) | 200 | 0.6 g in 20 mL distilled water | 50 |
| CU3(4%) | 200 | 0.8 g in 20 mL distilled water | 50 |
| CU4(5%) | 200 | 1.0 g in 20 mL distilled water | 50 |
| CU5(6%) | 200 | 1.2 g in 20 mL distilled water | 50 |
| Property | Current Study | Range * (0–36 cm) |
|---|---|---|
| Colour | Dark yellowish brown | Yellowish brown |
| pH in water | 4.67 ± 0.012 | 4.6–4.9 |
| pH in 1M KCl | 3.83 ± 0.010 | 3.8–4.0 |
| EC (µS cm−1) | 52.00 ± 1.155 | NA |
| Total organic carbon (%) | 1.44 ± 0.330 | 0.57–2.51 |
| Organic matter (%) | 2.48 ± 0.563 | NA |
| Total N (%) | 0.09 ± 0.009 | 0.04–0.17 |
| Exchangeable NH4+ (mg kg−1) | 6.30 ± 0.701 | NA |
| Available NO3− (mg kg−1) | 4.67 ± 0.234 | NA |
| Available P (mg kg−1) | 1.51 ± 0.180 | NA |
| ---------------------------------------------- (cmol (+) kg−1) ---------------------------------------- | ||
| Cation exchange capacity | 11.67 ± 0.21 | 3.86–8.46 |
| Exchangeable K+ | 0.16 ± 0.007 | 0.05–0.19 |
| Exchangeable Ca2+ | 0.03 ± 0.004 | NA |
| Exchangeable Mg2+ | 0.03 ± 0.005 | NA |
| Exchangeable Na+ | 0.22 ± 0.013 | NA |
| Exchangeable Fe2+ | 0.19 ± 0.001 | NA |
| Exchangeable Cu2+ | 0.02 ± 0.016 | NA |
| Exchangeable Zn2+ | 0.17 ± 0.011 | NA |
| Exchangeable Mn2+ | 0.02 ± 0.001 | NA |
| Sand (%) | 66 | 72–76 |
| Silt (%) | 22 | 8–9 |
| Clay (%) | 16 | 16–19 |
| Texture (USDA) | Sandy loam | Sandy clay loam |
| Property | Rejected Sago Starch |
|---|---|
| pHwater | 3.43 ± 0.050 |
| pH1M KCl | 5.10 ± 0.009 |
| EC (µS cm−1) | 1459.00 ± 58.000 |
| Total organic carbon (%) | 43.05 ± 2.666 |
| Organic matter (%) | 74.23 ± 4.596 |
| Exchangeable NH4+ (ppm) | 3.74 ± 0.234 |
| Available NO3− (ppm) | 2.34 ± 0.467 |
| Available P (mg kg−1) | 0.87 ± 0.065 |
| Exchangeable K+ (cmol (+) kg−1) | 170.27 ± 68.311 |
| Exchangeable Ca2+ (cmol (+) kg−1) | 0.78 ± 0.020 |
| Exchangeable Mg2+ (cmol (+) kg−1) | 20.13 ± 0.811 |
| Exchangeable Fe2+ (cmol (+) kg−1) | 0.41 ± 0.064 |
| Exchangeable Na+ (cmol (+) kg−1) | 0.12 ± 0.062 |
| Swelling power (g/g) | 10.0 ± 0.153 |
| Solubility (%) | 0.56 ± 0.006 |
| Samples | Nitrogen (%) |
|---|---|
| U | 46 ± 0.12 |
| CU1 | 45 ± 0.99 |
| CU2 | 44 ± 0.40 |
| CU3 | 44 ± 0.57 |
| CU4 | 43 ± 1.46 |
| CU5 | 42 ± 0.53 |
| Treatments | Exchangeable NH4+(mg kg−1) | Available NO3− (mg kg−1) |
|---|---|---|
| S | 8.640 d | 7.005 c |
| U | 81.025 b | 17.279 b |
| CU1 | 88.730 a | 31.289 a |
| CU2 | 82.893 b | 20.081 b |
| CU3 | 85.298 ab | 18.213 b |
| CU4 | 64.446 c | 25.685 b |
| CU5 | 61.644 c | 25.919 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kavitha, R.; Latifah, O.; Ahmed, O.H.; Primus, W.C.; Susilawati, K. Rejected Sago Starch as a Coating Material to Mitigate Urea-Nitrogen Emission. Agronomy 2022, 12, 941. https://doi.org/10.3390/agronomy12040941
Kavitha R, Latifah O, Ahmed OH, Primus WC, Susilawati K. Rejected Sago Starch as a Coating Material to Mitigate Urea-Nitrogen Emission. Agronomy. 2022; 12(4):941. https://doi.org/10.3390/agronomy12040941
Chicago/Turabian StyleKavitha, Rajan, Omar Latifah, Osumanu Haruna Ahmed, Walter Charles Primus, and Kasim Susilawati. 2022. "Rejected Sago Starch as a Coating Material to Mitigate Urea-Nitrogen Emission" Agronomy 12, no. 4: 941. https://doi.org/10.3390/agronomy12040941
APA StyleKavitha, R., Latifah, O., Ahmed, O. H., Primus, W. C., & Susilawati, K. (2022). Rejected Sago Starch as a Coating Material to Mitigate Urea-Nitrogen Emission. Agronomy, 12(4), 941. https://doi.org/10.3390/agronomy12040941






