Abstract
Increasing salinization threatens the normal growth of halophytes in saline habitats, especially at the seedling stage. Soil beneficial microorganisms have the potential to promote salt tolerance of halophytes, but less attention has been paid to the various responses between different halophytes with microbial inoculations. Here we performed pot experiments to assess the responses of Nitraria tangutorum Bobr. and Elaeagnus angustifolia Linn. to arbuscular mycorrhizal fungi (AMF) and plant growth-promoting rhizobacteria (PGPR) inoculation in saline soil conditions. The results showed that neither a single AMF inoculation nor a single PGPR inoculation promoted the growth of N. tangutorum. In contrast, co-inoculation not only promoted biomass accumulation but also promoted the absorption of P, K+, and Ca2+ in the roots, and the accumulation of N, Na+, K+, and Ca2+ in the leaves. Co-inoculation also increased the K+/Na+ ratio in the roots as well as the Ca2+/Na+ ratio in both roots and leaves of N. tangutorum. Na+ is an important inorganic osmolyte, essential for both efficient osmoregulation and biomass accumulation in N. tangutorum. PGPR inoculation alone could not promote the growth of E. angustifolia. AMF inoculation, solely or combined with PGPR, was beneficial to the absorption of K+ and Ca2+ in the roots, the accumulation of N and K+ in the leaves, the maintenance of the K+/Na+ ratio and Ca2+/Na+ ratio in the leaves, the selective transportation of K+ and Ca2+ from roots to leaves, and the accumulation of proline and glycine betaine in the leaves of E. angustifolia. Increased nutrient absorption, ion homeostasis, and K+ and Ca2+ selective transportation in AMF-inoculated E. angustifolia helped reduce the toxic effects of Na+ and the damage caused by osmotic stress in saline soil conditions. “Plant-microbe specificity” leads to the different responses of N. tangutorum and E. angustifolia seedlings to AMF and PGPR inoculation in saline soil conditions. The different salt tolerance strategies for osmoregulation, nutrient acquisition, ion homeostasis, and ion transportation determine the differential responses in N. tangutorum and E. angustifolia to AMF and PGPR inoculations under saline soil conditions.
1. Introduction
Soil salinization is one of the main manifestations of land degradation and leads to reduced soil productive potential and impaired plant growth. Consequently, salinization presents a global challenge for eco-environmental protection in most arid and semi-arid regions [1]. China is amongst the countries that are most seriously affected by salinization. Saline soil is found mainly in the arid and semi-arid areas of northwestern China [2] and is closely related to the spatial distribution of Chinese halophytes [3]. Minqin oasis is a fragile oasis that suffers from extremely severe salinization and drought. However, increasing water supply from the upper reaches of the Shiyang River is raising the water table in the lower reaches and increasing the soluble salt content of the surface soil. These changes aggravate salinization, degrade the native habitats of halophytes, and ultimately threaten the survival and development of halophytes in the Minqin Oasis. Therefore, it is urgent to help increase the salt-tolerance of native halophytes and thus strengthen their ecological benefits, to ensure sustainable ecological improvement and environmental protection.
Researchers have adopted many tactics to remediate saline soils and counteract the adverse effects of salinity in plants. These tactics include physical remediation, chemical remediation, phytoremediation, and bioremediation [4]. Recently, bioremediation has drawn considerable attention due to its cost-effective, eco-friendly, and readily available traits, which benefit the alleviation of salt stress on plants and the improvement of salt-affected soil. The utilization of plant growth-promoting microorganisms, such as arbuscular mycorrhizal fungi (AMF) and plant growth-promoting rhizobacteria (PGPR), has been proven useful and promising in developing strategies to facilitate plant growth and remediate saline soil [5,6,7,8,9]. AMF are obligate biotrophs, which participate in symbiosis with the root system of nearly 80% of terrestrial plants and provide soil resources to their hosts in exchange for photosynthate [10]. PGPR are beneficial bacteria colonizing roots and improving the growth performance of host plants [11]. Both AMF and PGPR have the potential to not only stimulate plant growth but also enhance plant tolerance under salt stress conditions [12,13,14].
Previous studies on AMF and PGPR inoculation under salt stress conditions mainly concentrated on glycophytic crops, and these are closely related to global food security [15,16,17,18]. However, global climate change threatens the growth and productivity of glycophytic crops throughout the marginal agricultural ecosystems that are affected by salinity. Under climate change, glycophytic crops become unable to support the human need for food, nutrient, protein, and calories [19]. The cultivation of endemic halophytes is a potential method to alleviate or solve the insecurity of food, nutrient, protein, and calories [19]. Some halophytes have already been cultivated as cash crops or utilized in functional food, thereby benefitting sustainable agriculture and securing farmers’ incomes [19,20]. In comparison to glycophytic crops, little is known about how halophytes respond to AMF and PGPR inoculation in saline soil conditions. In addition, although some studies focused on the various responses of different halophytes to AMF and PGPR inoculation, less attention has been paid to the exploration of possible reasons for the different responses in terms of the salt-tolerant strategies in halophytes [21,22,23,24,25].
High salinity in soil initially causes osmotic stress and toxic ionic stress in plant cells [26]. Both stresses, alone or in combination, inhibit the absorption of water and nutrients, consequently leading to physiological drought, ion imbalance, and nutrient deficiency [27]. Salt stress also adversely affects the number and structure of beneficial microorganisms living within the vicinity of the roots, again hindering plant growth and development [28]. Our previous meta-analyses have proved that some AMF and PGPR strains can help to regulate osmotic balance by increasing both inorganic ions absorption and compatible solutes accumulation in host plants, and also help to protect host plants from salt toxicity by increasing nutrients absorption, maintaining ionic homeostasis, and minimizing toxic ion acquisition [27,29]. However, plant growth responses to AMF and PGPR inoculation range from positive to neutral and even negative [8,10,30,31]. The inoculation efficiency induced by soil microorganisms is highly controlled by both introduced microbial strains and host plants [32]. Salt tolerance strategies vary greatly among different halophytes and also depend on different mechanisms involving osmoregulation, nutrient uptake, and ion transportation [26,33]. Whether the effectiveness of salt tolerance induced by microbial inoculation is dependent on the salt tolerance strategies of halophytic species, including the control of osmolytes accumulation, nutrient acquisition, and ion transportation, is an open question needing further study.
Nitraria tangutorum Bobr. and Elaeagnus angustifolia Linn. are the dominant halophytes that are widespread in the saline area in arid and semi-arid regions of northwestern China [34]. Both also play important roles in the restoration of saline land and the maintenance of ecological balance in the saline desert ecosystem of the Minqin Oasis [35,36,37]. Although both halophytic species could reduce the damage induced by osmotic stress and ionic stress in saline soils via various strategies, the inhibition of seed germination and seedling growth occurs when salinity concentration exceeds their respective salt-tolerant thresholds in the Minqin Oasis. Therefore, the elevation of salt tolerance in both halophytic species and the protection of halophytic seedling establishment become more and more important in arid and semi-arid areas under the background of climate change worldwide. Beneficial microorganism inoculation may be an effective way to achieve this goal. Here, a pot experiment was carried out to analyze the influence of single and co-inoculation of AMF and PGPR on N. tangutorum and E. angustifolia in saline soil conditions. The objectives of the present study were (1) to evaluate the effects of AMF and PGPR on the biomass accumulation, succulence, nutrient acquisition, ion distributions, and osmolytes accumulation in both N. tangutorum and E. angustifolia; and (2) to compare the differences in salt tolerance strategies between N. tangutorum and E. angustifolia after inoculation with AMF and PGPR in saline soil conditions.
2. Materials and Methods
2.1. Study Site Description
The study area is the typical area with long-term salt accumulation in the lower reaches of the Shiyang River [2]. A pot experiment was conducted at the Drylands Salinization Research Station affiliated with the Northwest Institute of Eco-Environment and Resources, Chinese Academy of Sciences, China (39°02′38″ N, 103°36′36″ E), which is located on the northern margin of the Minqin Oasis. During the experiment, the daily precipitation, photosynthetically active radiation, vapor pressure deficit, and temperature were 0.75 mm, 476.48 μmol·m−2 s−1, 1.74 kPa, and 21.81 °C, respectively (Figure 1).
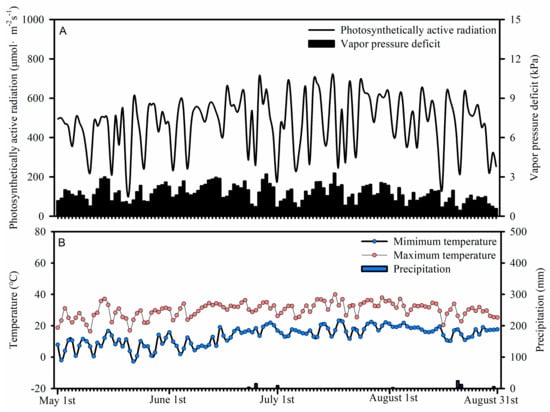
Figure 1.
Daily photosynthetically active radiation (A), vapor pressure deficit (A), temperature (B), and precipitation (B) in Drylands Salinization Research Station during the experiment in 2018.
2.2. Soil Sampling
Prior to planting, the soil was collected from an irrigated agricultural field affected by saline-alkalinity in the Drylands Salinization Research Station. The soil samples were air-dried for several days, passed through a 1 cm sieve to remove root-stone residue, and mixed to maximize homogeneity. The soil was loamy and classified as Orthic Halosols in the Chinese taxonomic system [36]. The soil studied here was not sterilized, and the chemical properties were determined as follows (Table 1), which are severely salinized soils [38].

Table 1.
Soil characterization in the experiment.
2.3. Plant Materials
The plants used in this experiment were N. tangutorum and E. angustifolia. N. tangutorum belongs to the Nitraria genus in the Zygophyllaceae family. E. angustifolia belongs to the Elaeagnus genus in the Elaeagnaceae family. N. tangutorum and E. angustifolia are dominant halophytes in the Minqin Oasis; both of them are best known for excellent tolerance to salt and form the primary ecological barrier for that area [36,37]. However, both could not grow normally when salinity concentration exceeds 300 mM NaCl [39,40]. An increasingly serious salinization problem in the Huqu sub-region of Minqin Oasis still has a negative impact on the normal growth of N. tangutorum and E. angustifolia at the seedling stage. Healthy and mature seeds of the two halophytes were collected in the Drylands Salinization Research Station in the autumn of 2017. Seeds were surface disinfected with commercial NaOCl (5%) for 10 min, then thoroughly rinsed with deionized water to remove all traces of NaOCl completely and leave the seeds dry. Five dry seeds were sown in each plastic pot (31 cm height × 26 cm top diameter × 21 cm bottom diameter) under open natural conditions. Thinning was performed after 15 days of emergence, and only one seedling was left in each pot. Seedlings had almost uniform heights across the pots. No fertilizer was applied during the experiment. Seedlings were irrigated with water from the Shiyang River in the late afternoon of every day to maintain the optimum moisture level and exclude the influences of hydrological factors.
2.4. Microbial Inoculum and Experimental Design
The AMF inoculum, Glomus mosseae, was commercially obtained from the Institute of Plant Nutrition and Resources, Beijing Academy of Agriculture and Forestry Sciences, China. Glomus mosseae was isolated from the rhizosphere soil of Populus euphratica in the Xinjiang Autonomous Region, China. The required PGPR, Bacillus amyloliquefaciens FZB42 strain used in this experiment, was obtained from the Northwest Institute of Eco-environment and Resource, Chinese Academy of Sciences, China [41]. The AMF inoculum and PGPR inoculum were multiplied following the methods in the previous study [36]. The experiment was arranged in a randomized design containing four treatments with ten replications: (1) control (without microbe inoculation), (2) AMF inoculation treatment, (3) PGPR inoculation treatment, and (4) co-inoculation treatment (AMF combined with PGPR). For mycorrhizal treatment, the soil in each pot was mixed with 100 g AMF inoculum that contained growth substrate, spores (density of approximately 75 per 10 g dry substrate), mycelium and infected root fragments. For non-mycorrhizal treatment, the soil in each pot was mixed with 100 g sterilized AM inoculum. For both PGPR inoculation treatment and co-inoculation treatment, seeds were soaked in PGPR inoculum at 108 CFU/mL for 30 min, and seedlings were extra-irrigated with 40 mL PGPR inoculum at 108 CFU/mL after thinning. Seeds were sown on 11 May 2018, and seedlings were harvested on 29 August 2018. Three randomly selected pots of each treatment were sampled for the following measurements at the end of the pot experiment.
2.5. Parameters Measurement
2.5.1. Measurement of Plant Biomass, AMF Colonization Rate, and Succulence Degree
Plant fresh weight was determined at the final harvest. The seedlings were carefully removed from the pots 110 days after sowing and divided into leaves, stems, and roots. Plant dry weight was measured after the plant samples were dried at 80 °C in an oven to constant weight [42]. Mycorrhizal colonization was measured using the slide method [43]. Fresh roots were cleared and cut into approximately 1 cm length segments, then stained root segments and stored in water. After random selection, the stained root samples were mounted on slides in groups of 15, then 2 to 3 drops of lactic acid were added to the slide, coverslips were placed, and the root segments were viewed under a stereomicroscope. Mycorrhizal colonization rate was calculated as the proportion of the number of root segments with vesicle, arbuscules, or hyphae in total observed root segments [44]. The succulence degree was calculated as sample fresh weight divided by dry weight [45].
2.5.2. Measurement of Mineral Nutrients Concentration
Leaves and roots samples are dried in an oven at 80 °C to constant weight. Dry samples that were crushed through a 40-mesh sieve were used for chemical analyses. The concentrations of nitrogen (N) and phosphorus (P) were determined using a 0.3 g powdered sample through the Kjeldahl nitrogen determination method and molybdenum antimony colorimetric method, respectively. The atomic absorption spectrometry method was applied to determine the concentrations of potassium ion (K+) and sodium ion (Na+) using 0.3 g powdered samples as well as the concentrations of calcium ion (Ca2+) using 0.5 g powdered samples. The K+, Ca2+, and Na+ concentrations in the samples of leaves and roots were then used to calculate the K+/Na+ ratio and Ca2+/Na+ ratio.
2.5.3. Measurement of Proline, Soluble Sugar, and Glycine Betaine Concentration
Proline, soluble sugar, and glycine betaine concentrations were determined from leaf samples with respective reagent kits according to the manufacturer’s instructions (Cominbio, Suzhou, China). Fresh leaf tissue stored in liquid nitrogen was used to measure the proline and soluble sugar concentrations via the ninhydrin colorimetry and anthrone colorimetry methods, respectively. Each 0.1 g leaf sample that was ground into a powder with liquid nitrogen was homogenized using 1 mL sodium phosphate (Na2HPO4/NaH2PO4) buffer to extract proline [46]. The homogenates were shaken and extracted in boiling water at a temperature of 90 °C for 10 min. After cooling, the homogenates were centrifuged at 10,000× g at a temperature of 25 °C for 10 min. Proline concentration was measured and calculated at 520 nm with a spectrophotometer. For the soluble sugar measurement, each 0.1 g leaf sample was homogenized with 1 mL distilled water and extracted in a boiling water bath at a temperature of 95 °C for 10 min. After cooling, the homogenates were centrifuged at a rotating speed of 8000× g at a temperature of 25 °C for 10 min to collect the supernatants [46]. Soluble sugar concentration was measured and calculated at 620 nm with a spectrophotometer. A 0.2 g powdered dry sample, crushed through a 40-mesh sieve, was collected to measure the glycine betaine concentration [47]. Leaf powdered samples were shaken with 1 mL 80% methanol at a temperature of 60 °C for 30 min. Then the supernatants were produced at a rotating speed of 10,000× g at a temperature of 25 °C for 10 min. Finally, the concentration of glycine betaine was measured and collected at 525 nm with a spectrophotometer.
2.6. Calculation of the Selective Transportation Coefficient
To compare the capacities of selective transportation of K+, Ca2+, and Na+ from roots to shoots, the selective transportation coefficient (STC) was calculated using the following equation [48]:
where X+ is K+ or Ca2+ concentration.
2.7. Statistical Analysis
Variance analysis and comparison among treatment mean with Tukey’s multiple range test, were carried out using SPSS 21 Software at p ≤ 0.05 (SPSS for Windows, Version 21.0, Chicago, IL, USA). All figures were drawn by Sigma Plot version 10.0 (Systat Software Inc., Point Richmond, CA, USA).
3. Results
3.1. Effects of AMF and PGPR Inoculation on Biomass Accumulation
Leaf, stem, root, and total dry weight of N. tangutorum seedlings were significantly increased in co-inoculation treatment compared with the control, while the inoculation of either AMF or PGPR alone showed no effect on the total dry weight of N. tangutorum seedlings (Figure 2A–D). The leaf, stem, root, and total dry weight of E. angustifolia seedlings were significantly promoted in AMF inoculation treatment and co-inoculation treatment, while only PGPR inoculation showed a non-significant effect on the biomass accumulation of E. angustifolia seedlings compared with the control (Figure 2E–H).
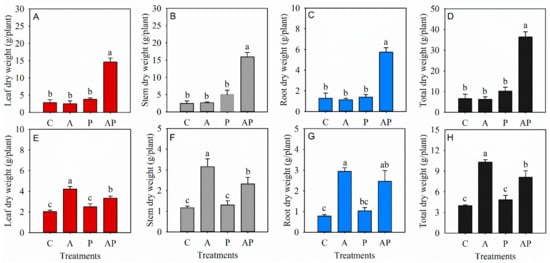
Figure 2.
Effects of AMF and PGPR inoculation on the leaf dry weights (A,E), stem dry weights (B,F), root dry weights (C,G), and total dry weights (D,H) in Nitraria tangutorum Bobr. (first row) and Elaeagnus angustifolia Linn. (second row). Each value represents the mean of three replicates. Each bar represents the standard deviation of three replicates. Values are significantly different at the 0.05 level (Tukey’s test) if followed by different letters above the bars. C: control, A: AMF inoculation treatment, P: PGPR inoculation treatment, AP: co-inoculation treatment.
3.2. Effects of AMF and PGPR Inoculation on Succulence Degree and AMF Colonization
The succulence degree in the root was significantly lower than that of the leaf but higher than that of the stem in N. tangutorum seedlings. Decreasing succulence degree (compared with the control) was only observed in the stem of N. tangutorum seedlings with co-inoculation (Figure 3A). The root succulence degree was significantly higher than that of both leaf and stem in E. angustifolia seedlings, and no statistical difference was recorded in succulence degree between leaves and stems. The presence of AMF, solely or combined with PGPR, statistically decreased the succulence degree of leaves and roots in E. angustifolia seedlings (Figure 3C). AMF colonization rates in N. tangutorum seedlings (range from 6.55% to 40.54%) were considerably lower than those in E. angustifolia seedlings (range from 60.15% to 71.77%) in the presence of AMF (Figure 3B,D).
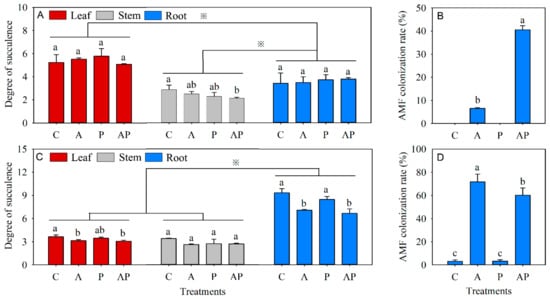
Figure 3.
Effects of AMF and PGPR inoculation on the succulence degree (A,C) and AMF colonization rates (B,D) in Nitraria tangutorum Bobr. (first row) and Elaeagnus angustifolia Linn. (second row). Asterisk indicates significant difference between leaves, stems, and roots at the 0.05 level (Tukey’s test). Each value represents the mean of three replicates. Each bar represents the standard deviation of three replicates. Values are significantly different at the 0.05 level (Tukey’s test) if followed by different letters above the bars. C: control, A: AMF inoculation treatment, P: PGPR inoculation treatment, AP: co-inoculation treatment.
3.3. Effects of AMF and PGPR Inoculation on N and P Concentrations
The inoculation of either AMF or PGPR alone showed no effect on the N and P concentration of the leaves, while co-inoculation statistically increased the N concentration of the leaves and the P concentration of the roots in N. tangutorum seedlings by 31.22% and 15.38%, respectively (Figure 4A,B). The N concentration of roots and the P concentration in leaves did not differ significantly between the control and the co-inoculation treatments in N. tangutorum seedlings (Figure 4A,B). AMF inoculation and co-inoculation significantly increased N concentration in both leaves and roots when compared with the control in E. angustifolia seedlings (Figure 4C). AMF, solely or combined with PGPR, statistically increased the P concentration in leaves while markedly decreasing the P concentration in roots of E. angustifolia seedlings (Figure 4D). However, the PGPR inoculation alone showed no effect on the N concentration of the leaves as well as the P concentration of the leaves and roots of E. angustifolia seedlings (Figure 4C,D).
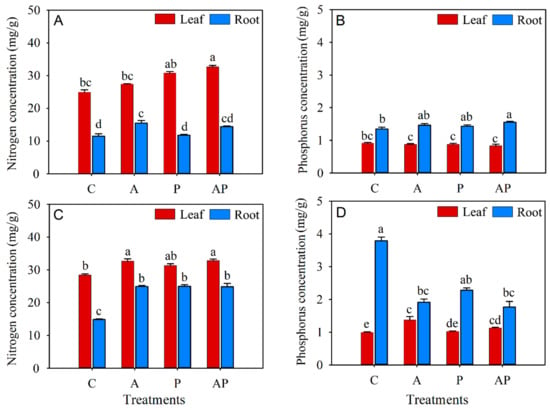
Figure 4.
Effects of AMF and PGPR inoculation on the N (A,C) and P (B,D) concentrations in Nitraria tangutorum Bobr. (first row) and Elaeagnus angustifolia Linn. (second row). Each value represents the mean of three replicates. Each bar represents the standard deviation of three replicates. Values are significantly different at the 0.05 level (Tukey’ test) if followed by different letters above the bars. C: control, A: AMF inoculation treatment, P: PGPR inoculation treatment, AP: co-inoculation treatment.
3.4. Effects of AMF and PGPR Inoculation on K+, Ca2+, and Na+ Concentrations
The K+ concentration in leaves significantly increased in PGPR inoculation and co-inoculation by 10.55% and 47%, respectively, but K+ concentration in roots only statistical increased in the N. tangutorum seedlings with co-inoculation (Figure 5A). The three microbial inoculation treatments statistically increased Ca2+ concentration in the leaves when compared with the control, but only co-inoculation markedly increased the Ca2+ concentration in the roots of N. tangutorum seedlings (Figure 5B). Compared to the control, co-inoculation significantly increased Na+ concentration in leaves while statistically decreasing Na+ concentration in the roots of N. tangutorum seedlings (Figure 5C). The three microbial inoculations significantly increased the K+ concentration in both leaves and roots of E. angustifolia seedlings when compared to the control (Figure 5D). The Ca2+ concentration in leaves statistical decreased in the E. angustifolia seedlings with PGPR inoculation and co-inoculation, while the Ca2+ concentration in roots significantly increased in three microbial inoculations when compared with the control (Figure 5E). Na+ concentration markedly decreased in the leaves of AMF inoculation treatment and significantly increased in the roots of co-inoculation treatment when compared to the control in E. angustifolia seedlings (Figure 5F).
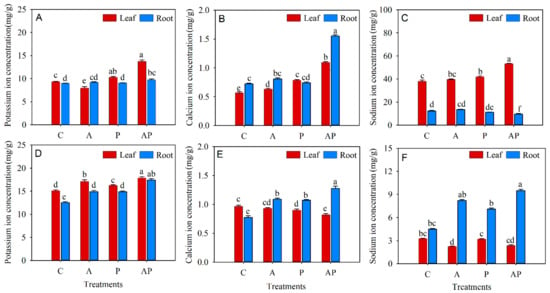
Figure 5.
Effects of AMF and PGPR inoculation on the K+ (A,D), Ca2+ (B,E), and Na+ (C,F) concentrations in Nitraria tangutorum Bobr. (first row) and Elaeagnus angustifolia Linn. (second row). Each value represents the mean of three replicates. Each bar represents the standard deviation of three replicates. Values are significantly different at the 0.05 level (Tukey’s test) if followed by different letters above the bars. C: control, A: AMF inoculation treatment, P: PGPR inoculation treatment, AP: co-inoculation treatment.
3.5. Effects of AMF and PGPR Inoculation on K+/Na+ Ratio, Ca2+/Na+ Ratio and STC
Compared to the control, there was no change in the K+/Na+ ratio of N. tangutorum seedlings inoculated with AMF or PGPR alone, while the K+/Na+ ratio in the roots of N. tangutorum seedlings with co-inoculation significantly increased by 37.42%. STCK,Na decreased significantly in three microbial treatments, by 13.33% to 30.77%, compared to the control (Figure 6A). The Ca2+/Na+ ratio in roots significantly increased in N. tangutorum seedlings with co-inoculation by 170.65%, while the STCCa,Na in the co-inoculation treatment was significantly decreased in N. tangutorum seedlings by 92.31% (Figure 6B). The presence of AMF, solely or combined with PGPR, significantly increased the K+/Na+ ratio in leaves and STCK,Na when compared with the control, while the K+/Na+ ratio in roots decreased markedly only in the E. angustifolia seedlings with AMF inoculation (Figure 6C). Both AMF inoculation and co-inoculation statistically decreased the Ca2+/Na+ ratio in roots but significantly increased STCCa,Na in E. angustifolia seedlings compared to the control. Only AMF inoculation significantly increased the Ca2+/Na+ ratio in the leaves of E. angustifolia seedlings (Figure 6D).
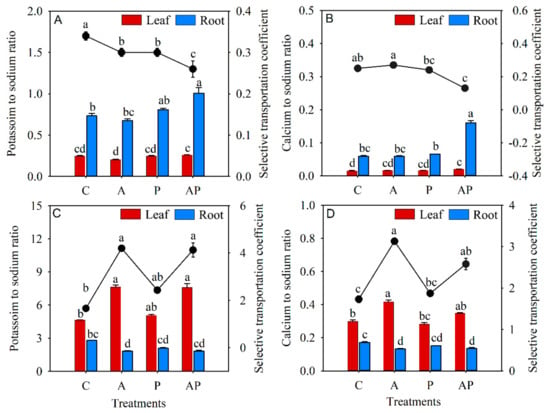
Figure 6.
Effects of microbial inoculation on the K+/Na+ ratio (A,C), Ca2+/Na+ ratio (B,D), STCK,Na (A,C), and STCCa,Na (B,D) in Nitraria tangutorum Bor. (first row) and Elaeagnus angustifolia Linn. (second row). Black dots represent the values of STC. Each value represents the mean of three replicates. Each bar represents the standard deviation of three replicates. Values are significantly different at the 0.05 level (Tukey’s test) if followed by different letters above the bars. C: control, A: AMF inoculation treatment, P: PGPR inoculation treatment, AP: co-inoculation treatment.
3.6. Effects of AMF and PGPR Inoculation on Compatible Osmolyte Concentrations
Compared to the control, PGPR inoculation and co-inoculation had no influence on the concentrations of proline, soluble sugar, and glycine betaine in N. tangutorum seedlings, while AMF inoculation decreased the concentrations of proline, soluble sugar, and glycine betaine in N. tangutorum seedlings by 21.28%, 20.88%, and 11.16%%, respectively (Figure 7A–C). Compared to the control, AMF inoculation, PGPR inoculation, and co-inoculation significantly increased the proline concentration in E. angustifolia seedlings by 121.24%, 84.15%, and 106.34%, respectively (Figure 7D). AMF inoculation and PGPR inoculation statistical increased the soluble sugar concentration in the leaves of E. angustifolia seedlings by 19.93% and 6.95%, respectively (Figure 7E). Compared to the control, AMF inoculation and co-inoculation increased the glycine betaine concentration of E. angustifolia seedlings by 18.6% and 11.04%, respectively (Figure 7F).
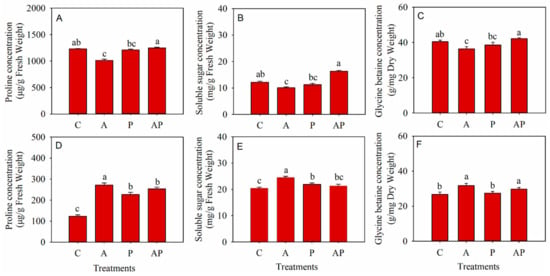
Figure 7.
Effects of microbial inoculation on the proline (A,D), soluble sugar (B,E), and glycine betaine (C,F) concentration in the leaves of Nitraria tangutorum Bobr. (first row) and Elaeagnus angustifolia Linn. (second row). Each value represents the mean of three replicates. Each bar represents the standard deviation of three replicates. Values are significantly different at the 0.05 level (Tukey’s test) if followed by different letters above the bars. C: control, A: AMF inoculation treatment, P: PGPR inoculation treatment, AP: co-inoculation treatment.
4. Discussion
Salinity disturbs the normal metabolic activity and ultimately inhibits the growth of seedlings [49]. Utilization of microbial inoculation may be an effective way to ensure the easier establishment of halophytic seedlings in soils with high salinity. N. tangutorum and E. angustifolia developed special strategies to adapt to saline soils, respectively, but high salinity is not conducive to seed germination and seedling establishment of two species [50,51]. Distinctive salt tolerance strategies of N. tangutorum and E. angustifolia may lead to different inoculation effects. N. tangutorum and E. angustifolia inoculated with AMF and PGPR may employ different tactics to accumulate biomass, avoid Na+ toxicity, absorb nutrients, maintain ion homeostasis, and conduct osmoregulation to adapt to saline soil environments. Therefore, we compared the tactics of the above physiological, metabolic processes to uncover the underlying mechanisms that determine the different inoculation effects in the N. tangutorum and E. angustifolia inoculated with AMF and PGPR in saline soil conditions.
4.1. “Plant-Microbe Specificity” Leads to Different Biomass Accumulation Responses between N. tangutorum and E. angustifolia Seedlings Inoculated with AMF and PGPR
Biomass accumulation, as the most intuitive response of plants to salt stress, is often used to quantify salt tolerance in plants [36]. More biomass accumulation means faster plant growth and stronger salt tolerance, and vice versa [12]. Our results showed that the combination of AMF and PGPR had the most beneficial effect on biomass accumulation in N. tangutorum seedlings, while AMF plays an important role in promoting biomass accumulation of E. angustifolia seedlings under severely salinized soil conditions (Figure 2). “Plant-microbe specificity” may be used to explain the different responses of N. tangutorum and E. angustifolia seedlings to AMF and PGPR inoculation. Specialization in plant-microbe symbiosis exists widely in nature [52], and the symbiotic interaction between plants and microbes could cause harmful, neutral, and beneficial effects on the host plants [53]. In this study, the existence of “functional specificity” in the combination of E. angustifolia and AMF (whether or not associated with PGPR) benefits biomass accumulation. In contrast, PGPR may serve as mycorrhizal facilitator bacteria to improve the AMF colonization in the roots and promote the growth of the N. tangutorums seedlings [54]. Therefore, the formation of mycorrhizal symbionts in co-inoculation treatments is beneficial to the growth of N. tangutorum, the formation of mycorrhizal symbionts with high colonization rate in both AMF and co-inoculation treatments are beneficial to the growth of E. angustifolia in saline soil conditions. The exertion of the mycorrhizal effect requires long periods to develop [55]. The effect induced by mycorrhizal symbiosis deserves further study in the long-term experimental observation.
Plants must cope with various challenges and concurrently support microbial symbioses under abiotic stress conditions [10,56]. In our studies, the mycorrhizal colonization in N. tangutorum seedlings in both AMF inoculation treatment and co-inoculation treatment is lower than that in E. angustifolia seedlings (Figure 3). This could be explained based on the innately evolutionary traits of the two halophytic species that were used in this experiment. N. tangutorum belongs to the Nitraria genus in the Zygophyllaceae family, which rarely forms mycorrhizas [55], but AMF can easily form mycorrhizas with E. angustifolia [40]. There is the inability of the introduced AMF strain, previously proved effective in E. angustifolia [36], to consistently form a symbiosis with N. tangutorum may have due, in large part, to the variations in AMF colonization between two halophytic species. Meanwhile, high biomass accumulation corresponded to high mycorrhizal colonization in both N. tangutorum and E. angustifolia seedlings (Figure 2 and Figure 3). This result highlights the fact that mycorrhizal symbionts are well-positioned to exert strong impacts on biomass accumulation of halophytes, supporting previous findings [24].
However, although previous studies already demonstrated the plant growth-promoting ability of the introduced PGPR strain in Arabidopsis thaliana planted on Petri dishes at 100 mM NaCl [41,57], PGPR inoculation alone failed to significantly promote the growth of N. tangutorum and E. angustifolia seedlings in saline soil conditions (Figure 2). Similar results that demonstrate the inconsistent responses of host plants to benefit microorganisms in real soil conditions are frequently reported and are not surprising due to the following three possible reasons [31]. Firstly, the physical, chemical, and microbial characteristics in real soil conditions are more complex than that in controlled sterile conditions; multiple soil factors are harmful to the performance of the introduced PGPR strain. Secondly, biological interaction between the introduced PGPR strain and the indigenous soil microflora may affect the ability of the bacterial inoculant to promote halophyte growth. Thirdly, a high degree of “plant-microbe specificity” may exist, which influences the microbial inoculation efficiency and further leads to different responses in biomass accumulation between different plant species. A complex array of multiple factors affect the effectiveness induced by introduced microorganisms [31,58]. Verification of the above hypothesis would need more research in future studies to reveal interactions between native microbial communities and the introduced AMF and PGPR strains, as well as the effects of experimental soil and irrigation water on microbial inoculation efficiency.
4.2. Distinctive Strategies of Na+ Acquisition and Distribution in N. tangutorum and E. angustifolia Affect the Salt Tolerance Strategies and Succulence Degree after Microbial Inoculations
Na+ is a key nutrient element and an essential physiological osmolyte for the osmotic adjustment and biomass production in succulent halophytes [59,60]. The capacity to regulate the acquisition, distribution, and compartmentalization of Na+ determines the salt tolerance of plants under salt stress conditions [61]. Previous studies have suggested that the leaves of N. tangutorum develop abundant parenchyma tissues and are able to compartmentalize Na+ from cells into vacuoles via vacuolar Na+/H+ antiporter, which would consequently lower the Na+ toxicity in cytosolic [39,60]. Our study showed that the Na+ concentration in leaves is higher than that in roots in N. tangutorum seedlings (Figure 5C) and that N. tangutorum in the co-inoculation treatment had the highest TFNa+ among all treatments (Table S1). This result suggests that the transportation of Na+ from roots to leaves is an essential strategy to enhance salt tolerance in N. tangutorum seedlings with co-inoculation. E. angustifolia has the scalable capacity to store Na+ and Cl- in roots, constrain Na+ transportation from stems to leaves, and maintain low Na+ concentration in leaves [62]. Both the compensatory growth and salt storage in roots, as well as the constrained transportation of salt from stems to leaves, play key roles in the salt tolerance of E. angustifolia [62]. In this study, Na+ concentration in roots was higher than that in leaves in E. angustifolia seedlings (Figure 5F), and the TFNa+ of E. angustifolia seedlings with microbial inoculations was statistically lower than that of the control (Table S1). This result supports previous findings suggesting that: (1) the Na+ storage in roots might be an important strategy that augments the salt tolerance of the E. angustifolia seedlings with or without microbial inoculation [62]. (2) the restriction of Na+ transportation from roots to leaves is a key microbial strategy enhancing the salt tolerance of E. angustifolia seedlings [24].
Different halophytic species show varying levels of succulence degree [63]. Increasing succulence degree in the leaf, stem, and root is an adaptive strategy to improve the halophyte’s salt tolerance [36,59]. This study revealed that with or without microbial inoculation, the succulence degree in the leaves is higher than that in the roots and stems of N. tangutorum seedlings, while the succulence degree in the roots is higher than that in the leaves and stems of E. angustifolia seedlings (Figure 3A,C). This difference indicates that microbial inoculation cannot change the pattern of succulence degree between the roots, stems, and leaves in N. tangutorum and E. angustifolia seedlings under saline soil conditions. Na+ may be a beneficial element for succulent halophytes, which are inclined to assimilate a large amount of Na+ without toxicity symptoms [39,60]. Na+ concentration was positively correlated with succulence degree in both N. tangutorum and E. angustifolia seedlings (Figure S1). This result suggests that both the acquisition and distribution of Na+ affect the succulence degree of N. tangutorum and E. angustifolia seedlings in saline soil conditions. Therefore, whether inoculated with AMF and PGPR or not, N. tangutorum seedlings may rely on both the transportation of Na+ from roots to leaves and the enhancement of succulence in leaves to reduce the damage induced by soil salinity. E. angustifolia seedlings may rely on both the restriction of Na+ transportation from roots to leaves and the enhanced succulence degree in roots to increase salt tolerance in saline soil conditions.
4.3. Mechanisms of Nutrient Acquisition and Ion Homeostasis in Organ-Level Are Different between N. tangutorum and E. angustifolia Seedlings with Microbial Inoculation
N is an essential nutrient for plant growth, but Na+ is detrimental to N acquisition by plants and thus induces plant N deficiency in the soil with high salinity [64,65]. Previous studies showed that the N concentration in leaves was significantly higher than that in roots in both N. tangutorum [66] and E. angustifolia [67]. In this study, the N concentration in the leaves was higher than that in the roots in both N. tangutorum and E. angustifolia seedlings, whether inoculated with AMF and PGPR or not (Figure 3A,C). This result indicates that: (1) N. tangutorum and E. angustifolia preferred to distribute N to leaves under saline soil conditions. (2) AMF and PGPR inoculation, singly or dually, did not change the N distribution pattern between leaves and roots in N. tangutorum and E. angustifolia seedlings. Meanwhile, co-inoculation significantly increased the N concentration in the leaves of N. tangutorum seedlings, and AMF inoculation and co-inoculation markedly increased the N concentration in E. angustifolia seedlings (Figure 4A,C). This indicates that AMF combined with PGPR could enhance the acquisition and utilization of N in the two halophytic species. The seedlings in the above treatments also have formed arbuscular mycorrhizal symbiosis and had relatively higher mycorrhizal colonization rates when compared with the control (Figure 3). Thus, we hypothesize that arbuscular mycorrhizal symbiosis formed in the roots of both N. tangutorum and E. angustifolia plays a pivotal role in N acquisition because arbuscular mycorrhizal symbiosis can effectively uptake the mineral nitrogen, some amino acids, and other complex organic nitrogen in soil [68], and further transfer N from fungus to plants [69].
P has poor solubility and fluidity in saline soil because the competition between Na+ and H2 reduces the activity of , thus reducing the mobility and availability of P in soil [64,70,71]. In this study, the P concentration in the leaves was lower than that in the roots in both N. tangutorum and E. angustifolia seedlings to a different extent, whether inoculated with AMF and PGPR or not (Figure 4B,D). This result indicates that N. tangutorum and E. angustifolia preferred to distribute P to roots in saline soil conditions; AMF and PGPR inoculation did not radically change the P partitioning between the leaves and roots in N. tangutorum and E. angustifolia. Furthermore, co-inoculation was conducive to increasing P concentration in the roots of N. tangutorum seedlings, while AMF inoculation, solely or combined with PGPR, increased the P concentration in the leaves and decreased its concentration in the roots of E. angustifolia seedlings. These results indicate that microbial inoculation and halophytic species co-regulated P acquisition, allocation, and accumulation, which might be caused by the salt-tolerant mechanisms and the P utilization strategies in both N. tangutorum and E. angustifolia with microbial inoculations. A previous study documented that the increased P concentration is helpful in promoting Na+ compartmentalization in vacuoles, thereby attenuating the adverse effects of Na+ on intracellular metabolic pathways and consequently enhancing salt tolerance of plants in salt stress conditions [64]. We suppose that the increased P concentration helps to reduce the damage induced by Na+ in the roots of N. tangutorum seedlings with co-inoculation and in the leaves of E. angustifolia seedlings with AMF inoculation.
Increasing Na+ in soil reduces K+ and Ca2+ availability and impairs K+ and Ca2+ acquisition by plants, leading to nutritional deficiency and intracellular ion imbalance in plants under saline soil conditions [24,26]. Our previous meta-analyses demonstrated that the growth-promoting effect of microbial inoculation on plants under salt stress partially benefits from the improvement of nutrient acquisition and the re-establishment of ion homeostasis [27,29]. In this study, co-inoculation increased the K+, Ca2+, and Na+ concentration of the leaves, enhanced the K+/Na+ ratio and Ca2+/Na+ ratio of the roots and reduced the STCK,Na and STCCa,Na in N. tangutorum seedlings (Figure 5 and Figure 6). The Na+ concentration is positively related to K+ and Ca2+ concentrations in the leaves but negatively related to the K+ and Ca2+ concentrations in the roots of N. tangutorum seedlings (Figure S2). However, AMF inoculation promoted the K+, Ca2+, and Na+ concentrations of the roots, enhanced the K+/Na+ ratio and Ca2+/Na+ ratio of the leaves, and increased the STCK,Na and STCCa,Na in E. angustifolia seedlings (Figure 5 and Figure 6). The Na+ concentration is negatively related to the K+ and Ca2+ concentrations in the leaves but positively related to the K+ and Ca2+ concentrations in the roots of E. angustifolia seedlings (Figure S3). These results indicate that AMF and PGPR have different effects on the accumulation and distribution of K+, Ca2+, and Na+ in N. tangutorum and E. angustifolia seedlings at the organ-level, demonstrating that the increases in both K+ and Ca2+ concentrations were not always accompanied by a decrease in Na+ concentration [26]. For N. tangutorum, co-inoculation not only alleviates nutrient deficiency by increasing K+ and Ca2+ accumulations in leaves but also reduces salt toxicity by maintaining ion balance in roots. For E. angustifolia, AMF inoculation promoted the Na+ accumulation in roots, preferentially promoted the transportation of K+ and Ca2+ from roots to leaves, and restricted the Na+ transportation to leaves, thus achieving ion homeostasis in leaves and alleviating the nutrient deficiency under saline soil conditions.
4.4. Inorganic Ions and Organic Solutes Play Different Roles in the Osmoregulation of N. tangutorum and E. angustifolia Seedlings
Halophytes can perform osmoregulation by absorbing inorganic ions (such as K+, Ca2+, and Na+) from the environment and synthesizing organic solutes (such as proline, soluble sugar, and glycine betaine) in vivo [72]. The usage of inorganic ions to maintain osmoregulation is more energy-efficient than the synthesis of organic solutes [60,61,73]. Previous studies demonstrated that N. tangutorum maintains osmotic homeostasis by increasing Na+ more than K+ accumulation [60] and accumulating high amounts of metabolically compatible compounds [74]. E. angustifolia accumulates organic compatible solutes to re-establish cytoplastic homeostasis under salt-induced osmotic stress [62]. In this study, co-inoculation increased K+, Ca2+, and Na+ concentrations to different extents but showed no effect on the proline, soluble, and glycine betaine concentrations in the leaves of N. tangutorum seedlings (Figure 5 and Figure 7). This result demonstrates that N. tangutorum, with co-inoculation, could acquire more inorganic ions to alleviate physiological drought and maintain osmoregulation in an energy-efficient manner. Different from N. tangutorum, K+ and organic solutes (such as proline, soluble sugar, and glycine betaine) increased in E. angustifolia seedlings with AMF inoculation (Figure 5D and Figure 7), which indicates that E. angustifolia inoculated with AMF could utilize both K+ and soluble osmolytes to maintain osmoregulation. Thus, the relative contribution of inorganic ions and organic solutes to osmoregulation varies between N. tangutorum and E. angustifolia under saline soil conditions.
The quantitative increase in inorganic ions and organic solutes does not always directly imply improved salt tolerance and promotion of biomass accumulation [26,75]. Stepwise multiple regression analysis showed that the biomass accumulation was positively correlated with Na+ and glycine betaine in the leaves of N. tangutorum seedlings, whilst the biomass accumulation was negatively correlated with Na+ in the leaves of E. angustifolia seedlings (Table S2). It is reasonable to make two assumptions, as follows. First, Na+, an important inorganic osmoticum, is beneficial to maintain osmotic equilibrium, increase water content within the cells, and promote the plant growth of N. tangutorum seedlings [39]. Therefore, increasing Na+ more than K+ is beneficial to improving the tolerance to water deficit in N. tangutorum seedlings under salt-induced osmotic stress [60]. Second, increasing K+ and soluble osmolytes concentrations are conducive to maintaining osmotic balance in the leaves, restricting Na+ accumulation, and finally promoting biomass accumulation of E. angustifolia seedlings. However, it is risky to rule out the potential contribution of organic osmolytes to the salt tolerance of N. tangutorum seedlings. Roots are the “first line of defense” of a plant to combat salt stress in soil [64], and overall, roots accumulate more compatible osmolytes than that in shoots [76]. Soil salinity may induce the accumulation of osmolytes in roots to aid the plant in maintaining osmoregulation and enhance water absorption for N. tangutorum [77]. Thus, the accumulation of compatible osmolytes at the organ-level needs to be further studied before elucidating the role of compatible osmolytes in the osmoregulation of N. tangutorum.
5. Conclusions
N. tangutorum and E. angustifolia inoculated with AMF and PGPR employed different tactics to adapt saline soil environment. The “plant-microbe specificity” induced the different growth and salt tolerance physiological responses to AMF and PGPR inoculations between N. tangutorum and E. angustifolia seedlings in saline soil conditions. The combination of AMF and PGPR is a key factor promoting the growth of N. tangutorum seedlings. Co-inoculation alleviates nutrient deficiency by increasing the absorption of P, K+, and Ca2+ in the roots, as well as the accumulation of N, K+, and Ca2+ in the leaves of N. tangutorum seedlings. Co-inoculation also reduces salt toxicity by optimizing the distribution of K+, Na+, and Ca2+, and alleviates physiological drought by accumulating inorganic ions in leaves to enhance energy-efficient osmoregulation in N. tangutorum seedlings. AMF is the key microorganism promoting the growth of E. angustifolia seedlings in saline soil conditions. AMF inoculation enhances N accumulation to alleviate N deficiency in the leaves of E. angustifolia seedlings; it also restricts Na+ transportation, and preferentially promotes the transportation of K+ and Ca2+ from roots to leaves to reduce ion toxicity and re-establish ion homeostasis in E. angustifolia seedlings. AMF inoculation also promotes the synthesis of proline, soluble sugar, and glycine betaine to alleviate osmotic stress. The different salt tolerance strategies with regard to osmoregulation, nutrient absorption, ion homeostasis, and ion transportation determine the microbial mechanisms underlying the improved salt tolerance in N. tangutorum and E. angustifolia inoculated with AMF and PGPR in saline soil conditions. These findings are helpful for the utilization of halophytes to better restore and reconstruct the ecosystem influenced by salinization.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy12040913/s1, Table S1: Effects of microbial inoculation on the acquisition and transportation of Na+ and P in Nitraria tangutorum Bobr. and Elaeagnus angustifolia Linn. seedlings. Table S2: Stepwise multiple regression equation between total biomass and osmolytes indices. Figure S1: Relationships between Na+ and succulence degree in Nitraria tangutorum Bobr. and Elaeagnus angustifolia Linn. seedlings under saline soil conditions. Figure S2: Correlation coefficients among different nutrient parameters in the leaf and root of Nitraria tangutorum Bobr. seedlings. Figure S3: Correlation coefficients among different nutrient parameters in the leaf and root of Elaeagnus angustifolia Linn. seedlings.
Author Contributions
Conceptualization, J.P., X.X., C.H. and F.P.; methodology, J.P. and C.H.; software, J.P.; formal analysis, J.P.; investigation, J.P. and C.H.; writing—original draft preparation, J.P. and F.P.; writing—review and editing, J.P., X.X., F.P. and Q.Y.; visualization, J.P.; funding acquisition, J.L., S.M. and T.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Key Research and Development Program of China (No. 2017YFE0119100), the National Natural Science Foundation of China (No. 42107513), and Key Research and Development Program of Gansu (No. 21YF5FA151).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We would like to thank Wenjuan Zhang for helping us to measure the plant parameters together and thank Pinglin Guo for his valuable suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Saidi, S.; Cherif-Silini, H.; Bouket, A.C.; Silini, A.; Eshelli, M.; Luotakova, L.; Alenezi, F.N.; Belbahri, L. Improvement of Medicago sativa crops productivity by the co-inoculation of Sinorhizobium meliloti–Actinobacteria under salt stress. Curr. Microbiol. 2021, 78, 1344–1357. [Google Scholar] [CrossRef] [PubMed]
- Qian, T.; Tsunekawa, A.; Peng, F.; Masunaga, T.; Wang, T.; Li, R. Derivation of salt content in salinized soil from hyperspectral reflectance data: A case study at Minqin Oasis, Northwest China. J. Arid Land 2019, 11, 111–122. [Google Scholar] [CrossRef]
- Zhao, K.; Song, J.; Feng, G.; Zhao, M.; Liu, J. Species, types, distribution, and economic potential of halophytes in China. Plant Soil 2011, 342, 495–509. [Google Scholar] [CrossRef]
- Nouri, H.; Borujeni, S.C.; Nirola, R.; Hassanli, A.; Beecham, S.; Alaghmand, S.; Saint, C.; Mulcahy, D. Application of green remediation on soil salinity treatment: A review on halophytoremediation. Proc. Saf. Env. Prot. 2017, 107, 94–107. [Google Scholar] [CrossRef]
- Rabie, G.H.; Aboul-Nasr, M.B.; Al-Humiany, A. Increased salinity tolerance of cowpea plants by dual inoculation of an arbuscular mycorrhizal fungus Glomus clarum and a nitrogen-fixer Azospirillum brasilense. Mycobiology 2005, 33, 51–60. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qurashi, A.W.; Sabri, A.N. Bacterial exopolysaccharide and biofilm formation stimulate chickpea growth and soil aggregation under salt stress. Braz. J. Microbiol. 2012, 43, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, X.; Li, G.; Qin, P. Interactions between arbuscular mycorrhizal fungi and phosphate-solubilizing fungus (Mortierella sp.) and their effects on Kostelelzkya virginica growth and enzyme activities of rhizosphere and bulk soils at different salinities. Biol. Fert. Soils 2011, 47, 543–554. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, H.; Liang, Y.; Cao, Y. Full-length transcriptome analysis of asparagus roots reveals the molecular mechanism of salt tolerance induced by arbuscular mycorrhizal fungi. Environ. Exp. Bot. 2021, 185, 104402. [Google Scholar] [CrossRef]
- Santos, S.S.; Rask, K.A.; Vestergård, M.; Johansen, J.L.; Priemé, A.; Frøslev, T.G.; González, A.M.M.; He, H.; Ekelund, F. Specialized microbiomes facilitate natural rhizosphere microbiome interactions counteracting high salinity stress in plants. Environ. Exp. Bot. 2021, 186, 104430. [Google Scholar] [CrossRef]
- Klironomos, J.N. Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 2003, 84, 2292–2301. [Google Scholar] [CrossRef]
- Kumar, A.; Verma, J.P. The role of microbes to improve crop productivity and soil health. In Ecological Wisdom Inspired Restoration Engineering; Achal, V., Mukherjee, A., Eds.; Springer: Singapore, 2019; pp. 249–265. [Google Scholar]
- Ilangumaran, G.; Smith, D.L. Plant growth promoting rhizobacteria in amelioration of salinity stress: A systems biology perspective. Front. Plant Sci. 2017, 8, 1768. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, M.; Boughattas, S.; Hu, S.; Oh, S.H.; Sa, S.H. A meta-analysis of arbuscular mycorrhizal effects on plants grown under salt stress. Mycorrhiza 2014, 24, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Porcel, R.; Aroca, R.; Ruiz-Lozano, J.M. Salinity stress alleviation using arbuscular mycorrhizal fungi. A review. Agron. Sustain. Dev. 2012, 32, 181–200. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, C.; Lu, T.; Zheng, Y. Cooperation between arbuscular mycorrhizal fungi and earthworms promotes the physiological adaptation of maize under a high salt stress. Plant Soil 2018, 423, 125–140. [Google Scholar] [CrossRef]
- Zhu, X.; Song, F.; Liu, S.; Liu, F. Role of arbuscular mycorrhiza in alleviating salinity stress in wheat (Triticum aestivum L.) grown under ambient and elevated CO2. J. Agron. Crop Sci. 2016, 202, 486–496. [Google Scholar] [CrossRef]
- Liu, J.; Hu, J.; Cheng, Z.; Li, M.; Liu, Z.; Wang, J.; Lin, X. Can phosphorus (P)-releasing bacteria and earthworm (Eisenia fetida L.) co-enhance soil P mobilization and mycorrhizal P uptake by maize (Zea mays L.)? J. Soil Sediments 2020, 21, 842–852. [Google Scholar] [CrossRef]
- Barnawal, D.; Bharti, N.; Pandey, S.S.; Pandey, A.; Chanotiya, C.S.; Kalra, A. Plant growth-promoting rhizobacteria enhance wheat salt and drought stress tolerance by altering endogenous phytohormone levels and TaCTR1/TaDREB2 expression. Physiol. Plant. 2017, 161, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Atzori, G. The Potential of Edible Halophytes as New Crops in Saline Agriculture. In Future of Sustainable Agriculture in Saline Environments; Negacz, K., Vellinga, P., Barrett-Lennard, E., Choukr-Allah, R., Elzenga, T., Eds.; CSC Press: St. Paul, MN, USA, 2021; pp. 443–460. [Google Scholar]
- Koyro, H.W.; Lieth, H.; Gul, B.; Ansari, R.; Huchzermeyer, B.; Abideen, Z.; Hussain, T.; Ajhman Khan, M. Importance of the Diversity within the Halophytes to Agriculture and Land Management in Arid and Semiarid Countries. In Sabkha Ecosystems: Volume IV: Cash Crop Halophyte and Biodiversity Conservation; Khan, M.A., Böer, B., Öztürk, M., et al., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 175–198. [Google Scholar]
- Qin, S.; Zhang, Y.; Yuan, B.; Xu, P.Y.; Xing, K.; Wang, J.; Jiang, J.H. Isolation of ACC deaminase-producing habitat-adapted symbiotic bacteria associated with halophyte Limonium sinense (Girard) Kuntze and evaluating their plant growth-promoting activity under salt stress. Plant Soil 2014, 374, 753–766. [Google Scholar] [CrossRef]
- Rueda-Puente, E.O.; García-Hernández, J.L.; Preciado-Rangel, P.; Murillo-Amador, B.; Tarazón-Herrera, M.A.; Flores-Hernández, A.; Holguin-Peña, J.; Aybar, A.N.; Barrón Hoyos, J.M.; Weimers, D.; et al. Germination of Salicornia bigelovii ecotypes under stressing conditions of temperature and salinity and ameliorative effects of plant growth-promoting bacteria. J. Agron. Crop Sci. 2007, 193, 167–176. [Google Scholar] [CrossRef]
- Liu, C.; Dai, Z.; Cui, M.; Lu, W.; Sun, H. Arbuscular mycorrhizal fungi alleviate boron toxicity in Puccinellia tenuiflora under the combined stresses of salt and drought. Environ. Pollut. 2018, 240, 557–565. [Google Scholar] [CrossRef]
- Chang, W.; Sui, X.; Fan, X.X.; Jia, T.T.; Song, F.Q. Arbuscular mycorrhizal symbiosis modulates antioxidant response and ion distribution in salt-stressed Elaeagnus angustifolia seedlings. Front. Microbiol. 2018, 9, 652. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Li, H.; Paré, P.W.; Aziz, M.; Wang, S.M.; Shi, H.; Li, J.; Han, Q.Q.; Guo, S.Q.; Li, J.; et al. Induced growth promotion and higher salt tolerance in the halophyte grass Puccinellia tenuiflora by beneficial rhizobacteria. Plant Soil 2016, 407, 217–230. [Google Scholar] [CrossRef]
- Maimaiti, A.; Yunus, Q.; Iwanaga, F.; Mori, N.; Tanaka, K.; Yamanaka, N. Effects of salinity on growth, photosynthesis, inorganic and organic osmolyte accumulation in Elaeagnus oxycarpa seedlings. Acta Physiol. Plant. 2014, 36, 881–892. [Google Scholar] [CrossRef]
- Pan, J.; Peng, F.; Tedeschi, A.; Xue, X.; Wang, T.; Liao, J.; Zhang, W.; Huang, C. Do halophytes and glycophytes differ in their interactions with arbuscular mycorrhizal fungi under salt stress? A meta-analysis. Bot. Stud. 2020, 61, 13. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Abd Allah, E.F.; Alqarawi, A.A.; Al-Huqail, A.A.; Wirth, S.; Egamberdieva, D. The interaction between arbuscular mycorrhizal fungi and endophytic bacteria enhances plant growth of Acacia gerrardii under salt stress. Front. Microbiol. 2016, 7, 1089. [Google Scholar] [CrossRef]
- Pan, J.; Peng, F.; Xue, X.; You, Q.; Zhang, W.; Wang, T.; Huang, C. The growth promotion of two salt-tolerant plant groups with PGPR inoculation: A meta-analysis. Sustainability 2019, 11, 378. [Google Scholar] [CrossRef]
- Hidri, R.; Barea, J.M.; Mahmoud, O.M.B.; Abdelly, C.; Azcón, R. Impact of microbial inoculation on biomass accumulation by Sulla carnosa provenances, and in regulating nutrition, physiological and antioxidant activities of this species under non-saline and saline conditions. J. Plant Physiol. 2016, 201, 28–41. [Google Scholar] [CrossRef]
- Walley, F.L.; Germida, J.J. Plant growth-promoting rhizobacteria alter rooting patterns and arbuscular mycorrhizal fungi colonization of field-grown spring wheat. Biol. Fert. Soils 1996, 23, 113–120. [Google Scholar]
- Seckbach, J.; Grube, M. Symbiosis and Stress; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Breckle, S.W. Salinity tolerance of different halophyte types. In Genetic Aspects of Plant Mineral Nutrition; El Bassam, N., Dambroth, M., Loughman, B.C., Eds.; Springer: Dordrecht, The Netherlands, 1990; pp. 167–175. [Google Scholar]
- Liu, L.; Wang, B. Protection of halophytes and their uses for cultivation of saline-alkali soil in China. Biology 2021, 10, 353. [Google Scholar] [CrossRef]
- Du, J.; Ping, Y.; Dong, Y. Phenological response of Nitraria tangutorum to climate change in Minqin County, Gansu Province, northwest China. Int. J. Biometeorol. 2010, 54, 583–593. [Google Scholar] [CrossRef]
- Pan, J.; Huang, C.; Peng, F.; Zhang, W.; Luo, J.; Ma, S.; Xue, X. Effect of arbuscular mycorrhizal fungi (AMF) and plant growth-promoting bacteria (PGPR) inoculations on Elaeagnus angustifolia L. in saline soil. Appl. Sci. 2020, 10, 945. [Google Scholar] [CrossRef]
- Wang, X.; Ma, Q.; Jin, H.; Fan, B.; Wang, D.; Lin, H. Change in characteristics of soil carbon and nitrogen during the succession of Nitraria Tangutorum in an arid desert area. Sustainability 2019, 11, 1146. [Google Scholar] [CrossRef]
- Chen, L.; Feng, Q.; Cheng, A. Spatial distribution of soil water and salt contents and reasons of saline soils’ development in the Minqin Oasis. J. Arid Land Res. Environ. 2013, 27, 99–105. [Google Scholar]
- Liu, W.; Yuan, X.; Zhang, Y.; Xuan, Y.; Yan, Y. Effects of salt stress and exogenous Ca2+ on Na+ compartmentalization, ion pump activities of tonoplast and plasma membrane in Nitraria tangutorum Bobr. leaves. Acta Physiol. Plant. 2014, 36, 2183–2193. [Google Scholar] [CrossRef]
- Liang, B.B.; Wang, W.J.; Fan, X.X.; Kurakov, A.V.; Liu, Y.F.; Song, F.; Chang, W. Arbuscular mycorrhizal fungi can ameliorate salt stress in Elaeagnus angustifolia by improving leaf photosynthetic function and ultrastructure. Plant Biol. 2020, 23, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hao, H.; Lu, X.; Zhao, X.; Wang, Y.; Zhang, Y.; Xie, Z.; Wang, R. Transcriptome profiling of genes involved in induced systemic salt tolerance conferred by Bacillus amyloliquefaciens FZB42 in Arabidopsis thaliana. Sci. Rep. 2017, 7, 10795. [Google Scholar] [CrossRef]
- Weremijewicz, J.; Da Silveira Lobo, O.; Janos, D.P. Arbuscular common mycorrhizal networks mediate intra- and interspecific interactions of two prairie grasses. Mycorrhiza 2018, 28, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular-arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Biermann, B.; Linderman, R. Quantifying vesicular-arbuscular mycorrhizae: A proposed method towards standardization. New Phytol. 1981, 87, 63–67. [Google Scholar] [CrossRef]
- Han, Y.; Wang, W.; Sun, J.; Ding, M.; Zhao, R.; Deng, S.; Wang, F.; Hu, Y.; Wang, Y.; Lu, Y.; et al. Populus euphratica XTH overexpression enhances salinity tolerance by the development of leaf succulence in transgenic tobacco plants. J. Exp. Bot. 2013, 64, 4225–4238. [Google Scholar] [CrossRef]
- Yang, L.; Lai, L.; Zhou, J.; Li, Q.; Yi, S.; Sun, Q.; Zheng, Y. Changes in levels of enzymes and osmotic adjustment compounds in key species and their relevance to vegetation succession in abandoned croplands of a semiarid sandy region. Ecol. Evol. 2020, 10, 2269–2280. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Richard, J.; Chen, S.; Lv, H.; Zhou, J.; Li, C. Infection by the fungal endophyte Epichlo bromicola enhances the tolerance of wild barley (Hordeum brevisubulatum) to salt and alkali stresses. Plant Soil 2018, 428, 353–370. [Google Scholar] [CrossRef]
- Chakraborty, K.; Mondal, S.; Ray, S.; Samal, P.; Pradhan, B.; Chattopadhyay, K.; Kar, M.K.; Swain, P.; Sarkar, R.K. Tissue tolerance coupled with ionic discrimination can potentially minimize the energy cost of salinity tolerance in rice. Front. Plant Sci. 2020, 11, 265. [Google Scholar] [CrossRef] [PubMed]
- Orhan, F. Alleviation of salt stress by halotolerant and halophilic plant growth-promoting bacteria in wheat (Triticum aestivum). Braz. J. Microbiol. 2016, 47, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, Y.; Yuan, X.; Xuan, Y.; Gao, Y.; Yan, Y. Exogenous salicylic acid improves salinity tolerance of Nitraria tangutorum. Russ. J. Plant Physiol. 2016, 63, 132–142. [Google Scholar] [CrossRef]
- Zheng, X.; Lin, J.; Xin, J.; Li, L.; Qi, Y.; Chen, M. Response to NaCl stress and salinity threshold by two species of Elaeagnus from the same provenance. Crops 2017, 4, 143–149. [Google Scholar]
- Cobian, G.M.; Egan, C.P.; Amend, A.S. Plant–microbe specificity varies as a function of elevation. ISME J. 2019, 13, 2778–2788. [Google Scholar] [CrossRef]
- Drew, G.C.; Stevens, E.J.; King, K.C. Microbial evolution and transitions along the parasite–mutualist continuum. Nat. Rev. Microbiol. 2021, 19, 623–638. [Google Scholar] [CrossRef]
- Diagne, N.; Ndour, M.; Djighaly, P.I.; Ngom, D.; Ngom, M.C.N.; Ndong, G.; Svistoonoff, S.; Cherif-Silini, H. Effect of plant growth promoting rhizobacteria (PGPR) and arbuscular mycorrhizal fungi (AMF) on salt stress tolerance of Casuarina obesa (Miq.). Front. Sustain. Food Syst. 2020, 4, 601004. [Google Scholar] [CrossRef]
- Brundrett, M. Mycorrhizas in natural ecosystems. Adv. Ecol. Res. 1991, 21, 171–313. [Google Scholar]
- Porter, S.S.; Bantay, R.; Friel, C.A.; Garoutte, A.; Gdanetz, K.; Ibarreta, K.; Moore, B.M.; Shetty, P.; Siler, E.; Friesen, M.L. Beneficial microbes ameliorate abiotic and biotic sources of stress on plants. Funct. Ecol. 2020, 34, 2075–2086. [Google Scholar] [CrossRef]
- Hao, H.; Zhao, X.; Shang, Q.; Wang, Y.; Guo, Z.H.; Zhang, Y.B.; Xie, Z.K.; Wang, R.Y. Comparative digital gene expression analysis of the Arabidopsis response to volatiles emitted by Bacillus amyloliquefaciens. PLoS ONE 2016, 11, e0158621. [Google Scholar] [CrossRef] [PubMed]
- Leifheit, E.F.; Veresoglou, S.D.; Lehmann, A.; Morris, E.K.; Rillig, M.C. Multiple factors influence the role of arbuscular mycorrhizal fungi in soil aggregation-a meta-analysis. Plant Soil 2014, 374, 523–537. [Google Scholar] [CrossRef]
- Matinzadeha, Z.; Akhania, H.; Abedib, M.; Palacio, S. The elemental composition of halophytes correlates with key morphological adaptations and taxonomic groups. Plant Physiol. Biochem. 2019, 141, 259–278. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Yue, L.; Wang, S.; Zhao, W.; Bao, A. Na compound fertilizer stimulates growth and alleviates water deficit in the succulent xerophyte Nitraria tangutorum (Bobr) after breaking seed dormancy. Soil Sci. Plant Nutr. 2016, 62, 489–499. [Google Scholar] [CrossRef][Green Version]
- Auge, R.M.; Toler, H.D.; Saxton, A.M. Arbuscular mycorrhizal symbiosis and osmotic adjustment in response to NaCl stress: A meta-analysis. Front. Plant Sci. 2014, 5, 562. [Google Scholar] [PubMed]
- Liu, Z.; Zhu, J.; Yang, X.; Wu, H.; Wei, Q.; Wei, H.; Zhang, H. Growth performance, organ-level ionic relations and organic osmoregulation of Elaeagnus angustifolia in response to salt stress. PLoS ONE 2018, 13, e0191552. [Google Scholar] [CrossRef]
- Flowers, T.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Azooz, M.M.; Prasad, M.N.V. Ecophysiology and Responses of Plants under Salt Stress; Springer: New York, NY, USA; Heidelberg, Germany; Dordrecht, The Netherlands; London, UK, 2013. [Google Scholar]
- Osman, K.T. Saline and Sodic Soils. In Management of Soil Problems; Osman, K.T., Ed.; Springer International Publishing AG, Part of Springer Nature: Cham, Switzerland, 2018; pp. 255–293. [Google Scholar]
- Xing, L.; Xue, H.; Li, Q.; Gao, T. Scaling from leaf to whole plant in biomass and nitrogen content of Nitraria tangutorum seedlings. J. Beijing For. Univ. 2018, 40, 76–82. [Google Scholar]
- Wei, Q.; Wu, H.; Liu, Z.; Li, H.; Yang, X.; Zhang, H. Biological nitrogen fixation ability and nitrogen distribution of Elaeagnus angustifolia under salt stress. For. Res. 2017, 30, 985–992. [Google Scholar]
- Govindarajulu, M.; Pfeffer, P.E.; Jin, H.; Abubaker, J.; Douds, D.D.; Allen, J.W.; Bücking, H.; Lammers, P.J.; Shachar-Hill, Y. Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 2005, 435, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Pfeffer, P.E.; Douds, D.D.; Piotrowski, E.; Lammers, P.J.; Shachar-Hill, Y. The uptake, metabolism, transport and transfer of nitrogen in an arbuscular mycorrhizal symbiosis. New Phytol. 2005, 168, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Tester, M. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 2003, 91, 503–527. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Ye, C.; Zhang, J.; Koziol, L.; Bever, J.D.; Li, X. Asymmetric facilitation induced by inoculation with arbuscular mycorrhizal fungi leads to overyielding in maize/faba bean intercropping. J. Plant Interact. 2019, 14, 10–20. [Google Scholar] [CrossRef]
- Ashraf, M. Some important physiological selection criteria for salt tolerance in plants. Flora 2004, 199, 361–376. [Google Scholar] [CrossRef]
- Himabindu, Y.; Chakradhar, T.; Reddy, M.C.; Kanygin, A.; Redding, K.E.; Chandrasekhar, T. Salt-tolerant genes from halophytes are potential key players of salt tolerance in glycophytes. Environ. Exp. Bot. 2016, 124, 39–63. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, X.; Shi, R.; Fan, Q.; An, L. Salinity-induced Physiological Modification in the Callus from Halophyte Nitraria tangutorum Bobr. J. Plant Growth Regul. 2010, 29, 465–476. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004, 166, 3–16. [Google Scholar] [CrossRef]
- Borde, M.; Dudhane, M.; Kulkarni, M. Role of Arbuscular Mycorrhizal Fungi (AMF) in Salinity Tolerance and Growth Response in Plants under Salt Stress Conditions. In Mycorrhiza—Eco-Physiology, Secondary Metabolites. Nanomaterials; Varma, A., Prasad, R., Tuteja, N., Eds.; Springer: Cham, Switzerland, 2017; pp. 71–86. [Google Scholar]
- Wang, W.; Jiang, W.; Xie, Z. Effect of NaCl stress on physiological index of Nitraria tangutorum seedling. Acta Agrestia Sin. 2012, 20, 907–913. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).