Potential Use of Quartzipisamment under Agroforestry and Silvopastoral System for Large-Scale Production in Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Site Description
2.2. Experimental Design and Treatments
2.3. Field Trial and Management
- Planting a cover mix with Urochloa sp. + Cajanus cajan at a density of 15 m-1 seeds for soil decompaction and biomass production;
- Mowing of the area using equipment called “sega pasto” from Casale, which preserves the stem of the grass to ensure the quality of regrowth, after two years and opening of the strips for soil preparation using the straw rake, machinery that separates the biomass, allowing to prepare the soil for planting in windrows;
- Preparation of the planting windrows (0.20 m deep, in strips 1.2 m wide and 5.0 m long), with a rotary hoe and fertilization only in the rows, with basaltic rock powder (2 Mg ha−1 in SILVP and 3 Mg ha−1 in AGF), reactive rock phosphate (0.7 Mg ha−1), cattle manure (2 Mg ha−1 in SILVP and 5 Mg ha−1 in AGS) and biospray (3 L ha−1 in SILVP and 5 L ha−1 in AGF, after planting the seedlings). For every 1000 L of biospray produced, 500 L of biofertilizer, 40 kg of copper sulfate, 84 kg of zinc sulfate, 11.4 kg of manganese sulfate, and 4 L of liquid sulfur of trade name Sulfor M were used;
- Windrowing of the mowed material on the strips prepared for planting, using the same machinery (straw rake/windrower). Plant residues formed a thick covering layer, which controlled the growth of grasses in the ridges, in addition to providing organic matter for the soil;
- Introduction of the species of interest. Planting of seedlings and/or seeds according to the productive focus of each system, including species with the potential to supply biomass.
2.4. Soil Samplings and Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Descriptive Statistics
3.2. Principal Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poffenbarger, H.; Artz, G.; Dahlke, G.; Edwards, W.; Hanna, M.; Russell, J.; Sellers, H.; Liebman, M. An economic analysis of integrated crop-livestock systems in Iowa, USA. Agric. Syst. 2017, 157, 51–69. [Google Scholar] [CrossRef]
- Kleinman, P.J.A.; Spiegal, S.; Rigby, J.R.; Goslee, S.C.; Baker, J.M.; Bestelmeyer, B.T.; Boughton, R.K.; Bryant, R.B.; Cavigelli, M.A.; Derner, J.D.; et al. Advancing the Sustainability of US Agriculture through Long Term Research. J. Environ. Qual. 2018, 47, 1412–1425. [Google Scholar] [CrossRef] [PubMed]
- Hertel, T.W.; Baldos, U.L.C. Attaining food and environmental security in an era of globalization. Glob. Environ. Change 2016, 41, 195–205. [Google Scholar] [CrossRef]
- Guillot, E.; Hinsinger, P.; Dufour, L.; Roy, J.; Bertrand, I. With or without trees: Resistance and resilience of soil microbial communities to drought and heat stress in a Mediterranean agroforestry system. Soil Biol. Biochem. 2019, 129, 122–135. [Google Scholar] [CrossRef]
- Buller, L.S.; Bergier, I.; Ortega, E.; Moraes, A.; Bayma-Silva, G.; Zanetti, M.R. Soil improvement and mitigation of greenhouse gas emissions for integrated crop–livestock systems: Case study assessment in the Pantanal savanna highland, Brazil. Agríc. Syst. 2015, 137, 206–219. [Google Scholar] [CrossRef]
- Polanía-Hincapié, K.L.; Olaya-Montes, A.; Cherubin, M.R.; Herrera-Valencia, W.; Ortiz-Morea, F.A.; Silva-Olaya, A.M. Soil physical quality responses to silvopastoral implementation in Colombian Amazon. Geoderma 2021, 386, 114900. [Google Scholar] [CrossRef]
- Rosa-Schleich, J.; Loos, J.; Mußhoff, O.; Tscharntke, T. Ecological-economic trade-offs of Diversified Farming Systems—A review. Ecol. Econ. 2019, 160, 251–263. [Google Scholar] [CrossRef]
- Liebig, M.A.; Ryschawy, J.; Kronberg, S.L.; Archer, D.W.; Scholljegerdes, E.J.; Hendrickson, J.R.; Tanaka, D.L. Integrated crop-livestock system effects on soil N, P, and pH in a semiarid region. Geoderma 2017, 289, 178–184. [Google Scholar] [CrossRef]
- Kim, D.-G.; Miko, U.F.; Kirschbaum, M.U.F.; Beedy, T.L. Carbon sequestration and net emissions of CH4 and N2O under agroforestry: Synthesizing available data and suggestions for future studies. Agric. Ecosyst. Environ. 2016, 226, 65–78. [Google Scholar] [CrossRef]
- Monroe, P.E.M.; Gama-Rodrigues, E.F.; Gama-Rodrigues, A.C.; Marques, J.R.B. Soil carbon stocks and origin under different cacao agroforestry systems in Southern Bahia, Brazil. Agric. Ecosyst. Environ. 2016, 221, 99–108. [Google Scholar] [CrossRef]
- Gil, J.; Siebold, M.; Berger, T. Adoption and development of integrated crop–livestock–forestry systems in Mato Grosso, Brazil. Agric. Ecosyst. Environ. 2015, 199, 394–406. [Google Scholar] [CrossRef]
- Cherubin, M.R.; Chavarro-Bermeo, J.P.; Silva-Olaya, A.M. Sistemas agroflorestais melhoram a qualidade física do solo no noroeste da Amazônia colombiana. Agrofor. Syst. 2019, 93, 1741–1753. [Google Scholar] [CrossRef]
- Freitas, I.C.; Santos, F.C.V.; Custódio Filho, R.O.; Correchel, V. Carbono no solo, acúmulo e qualidade da serapilheira em sistemas de produção familiar. Floresta 2016, 46, 31–38. [Google Scholar] [CrossRef]
- Schembergue, A.; Cunha, D.A.; Matos, S.; Pires, C.M.V.; Faria, R.M. Sistemas Agroflorestais como Estratégia de Adaptação aos Desafios das Mudanças Climáticas no Brasil. Rev. Econ. Sociol. Rural 2017, 55, 9–30. [Google Scholar] [CrossRef]
- Costa, C.R.G.; Fraga, V.S.; Lambais, G.R.; Soares, K.O.; Suddarth, S.R.P.; Medeiros, S.S. Chemical and Physical Quality of the Entisol in a Natural Regeneration Area in the Semiarid Region of Paraiba. J. Exp. Agric. Int. 2019, 35, 48324. [Google Scholar] [CrossRef]
- Sampaio, T.F.; Guerrini, I.A.; Backes, C.; Heliodoro, J.C.A.; Ronchi, H.S.; Tanganelli, K.M.; Carvalho, N.C.; Oliveira, F.C. Lodo de esgoto na recuperação de áreas degradadas: Efeito nas características físicas do solo. Rev. Bras. Cienc. Solo 2012, 36, 1637–1645. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalvez, J.L.M.; Sparovek, G. Koppens climate classification map for Brazil. Meteorol. Zeitschrift 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Cepagri—Centro de Pesquisas Meteorológicas e Climáticas aplicadas a Agricultura. Available online: https://www.cpa.unicamp.br/outras-informacoes (accessed on 17 October 2016).
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.; Araujo Filho, J.C.; Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa: Brasília, Brazil, 2018; p. 358. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2014.
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Kemper, W.D.; Chepil, W.S. Size distribution of aggregates. In Methods of Soil Analysis; Black, C.A., Ed.; American Society Agronomy: Madison, WI, USA, 1965; pp. 499–510. [Google Scholar]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solos, 3rd ed.; Embrapa: Brasília, Brazil, 2017. [Google Scholar]
- Lindsay, W.L.; Norwell, W.A. Development of a DTPA soil test for zinc, iron, manganese and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis: Chemical Methods, Part 3; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Eds.; American Society of America: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Ellert, B.H.; Bettany, J.R. Calculation of organic matter and nutrients stored in soils under contrasting management regimes. Can. J. Soil Sci. 1995, 75, 529–538. [Google Scholar] [CrossRef]
- Holthusen, D.; Brandt, A.A.; Reichert, J.M.; Horn, R. Soil porosity, permeability and static and dynamic strength parameters under native forest/grassland compared to no-tillage cropping. Soil Tillage Res. 2018, 177, 113–124. [Google Scholar] [CrossRef]
- Tavares, R.L.M.; Siqueira, D.S.; Panosso, A.R.; Castioni, G.A.F.; Souza, Z.M.; La Scala, N. Soil management of sugarcane fields affecting CO2 fluxes. Sci. Agric. 2016, 73, 543–551. [Google Scholar] [CrossRef]
- Smiley, G.L.; Kroschel, J. Temporal change in carbon stocks of cocoa-gliricidia agroforests in Central Sulawesi, Indonesia. Agrofor. Syst. 2008, 73, 219–231. [Google Scholar] [CrossRef]
- Santiago, W.R.; Vasconcelos, S.S.; Kato, O.R.; Bispo, C.J.C.; Rangel-Vaconcelos, L.G.T.; Castellani, D.C. Nitrogênio mineral e microbiano do solo em sistemas agroflorestais com palma de óleo na Amazônia oriental. Acta Amazon. 2013, 43, 395–406. [Google Scholar] [CrossRef][Green Version]
- Islam, M.; Dey, A.; Rahman, M. Effect of Tree Diversity on Soil Organic Carbon Content in the Homegarden Agroforestry System of North-Eastern Bangladesh. Small-Scale For. 2015, 14, 91–101. [Google Scholar] [CrossRef]
- Rodriguez, L.; Suárez, J.S.; Pulleman, M.; Guaca, L.; Rico, A.; Romero, M.; Quintero, M.; Lavelle, P. Agroforestry systems in the Colombian Amazon improve the provision of soil ecosystem services. Appl. Soil Ecol. 2021, 164, 103933. [Google Scholar] [CrossRef]
- Cardinael, R.; Chevallier, T.; Cambouad, A.; Béral, C.; Barthès, B.G.; Dupraz, C.; Durand, C.; Kouakoua, E.; Chenu, C. Increased soil organic carbon stocks under agroforestry: A survey of six different sites in France. Agric. Ecosyst. Environ. 2017, 236, 243–255. [Google Scholar] [CrossRef]
- Dollinger, J.; Jose, S. Agroforestry for soil health. Agrofor. Syst. 2018, 92, 213–219. [Google Scholar] [CrossRef]
- Arévalo-Gardini, E.; Canto, M.; Alegre, J.; Loli, O.; Julca, A.; Baligar, V. Changes in Soil Physical and Chemical Properties in Long Term Improved Natural and Traditional Agroforestry Management Systems of Cacao Genotypes in Peruvian Amazon. Agrofor. Syst. 2018, 92, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Zhang, J.; Meng, M.; Chen, H.Y.H.; Guo, X.; Liu, S.; Ye, L. Impactos das mudanças na vegetação na condutividade hidráulica saturada do solo em florestas subtropicais. Sci. Rep. 2019, 9, 8372. [Google Scholar] [CrossRef] [PubMed]
- Reichert, J.M.; Amado, T.J.C.; Reinert, D.J.; Rodrigues, M.F.; Suzuki, E.A.S. Land use effects on subtropical, sandy soil under sandyzation/desertification processes. Agric. Ecosyst. Environ. 2016, 233, 370–380. [Google Scholar] [CrossRef]
- Kiehl, E.J. Manual de Edafologia: Relações Solo-Planta; Ceres: São Paulo, Brazil, 1979. [Google Scholar]
- Rosalem, L.M.P.; Anache, J.A.A. Wendland, E. Determining Forest litter interception in an area of the Cerrado sensu stricto. Braz. J. Water Res. 2018, 23, 1–11. [Google Scholar]
- Smith, J.L.; Doran, J.W. Measurement and use of pH and electrical conductivity for soil quality analysis. In Methods for Assessing Soil Quality; Doran, J.W., Jones, A.J., Eds.; Special Publication No. 49; Soil Science Society of America: Madison, WI, USA, 1996; pp. 169–185. [Google Scholar]
- Hair, J.F.; Black, W.C.; Babin, B.J.; Anderson, R.E.; Tatham, R.L. Análise Multivariada de Dados, 15th ed.; Bookman: Porto Alegre, Brazil, 2005. [Google Scholar]
- Franzluebbers, A.J.; Chappell, J.C.; Shi, W.; Cubbage, F.W. Greenhouse gas emissions in an agroforestry system of the southeastern USA. Nutr. Cycl. Agroecosyst. 2016, 108, 85–100. [Google Scholar] [CrossRef]
- Lima, S.S.; Leite, L.F.C.; Oliveira, F.C.; Costa, D.B. Atributos químicos e estoques de carbono e nitrogênio em Argissolo Vermelho-Amarelo sob sistemas agroflorestais e agricultura de corte e queima no norte do Piauí. Rev. Árvore 2011, 35, 51–60. [Google Scholar] [CrossRef]
- Couto, W.R.; Anjos, L.H.C.; Pereira, M.G.; Guareschi, R.F.; Assunção, S.A.; Wadt, P.G.S. Carbono, Nitrogênio, Abundância Natural de Δ13C e Δ15N do Solo sob Sistemas Agroflorestais. Floresta Ambiente 2017, 24, e00117614. [Google Scholar] [CrossRef][Green Version]
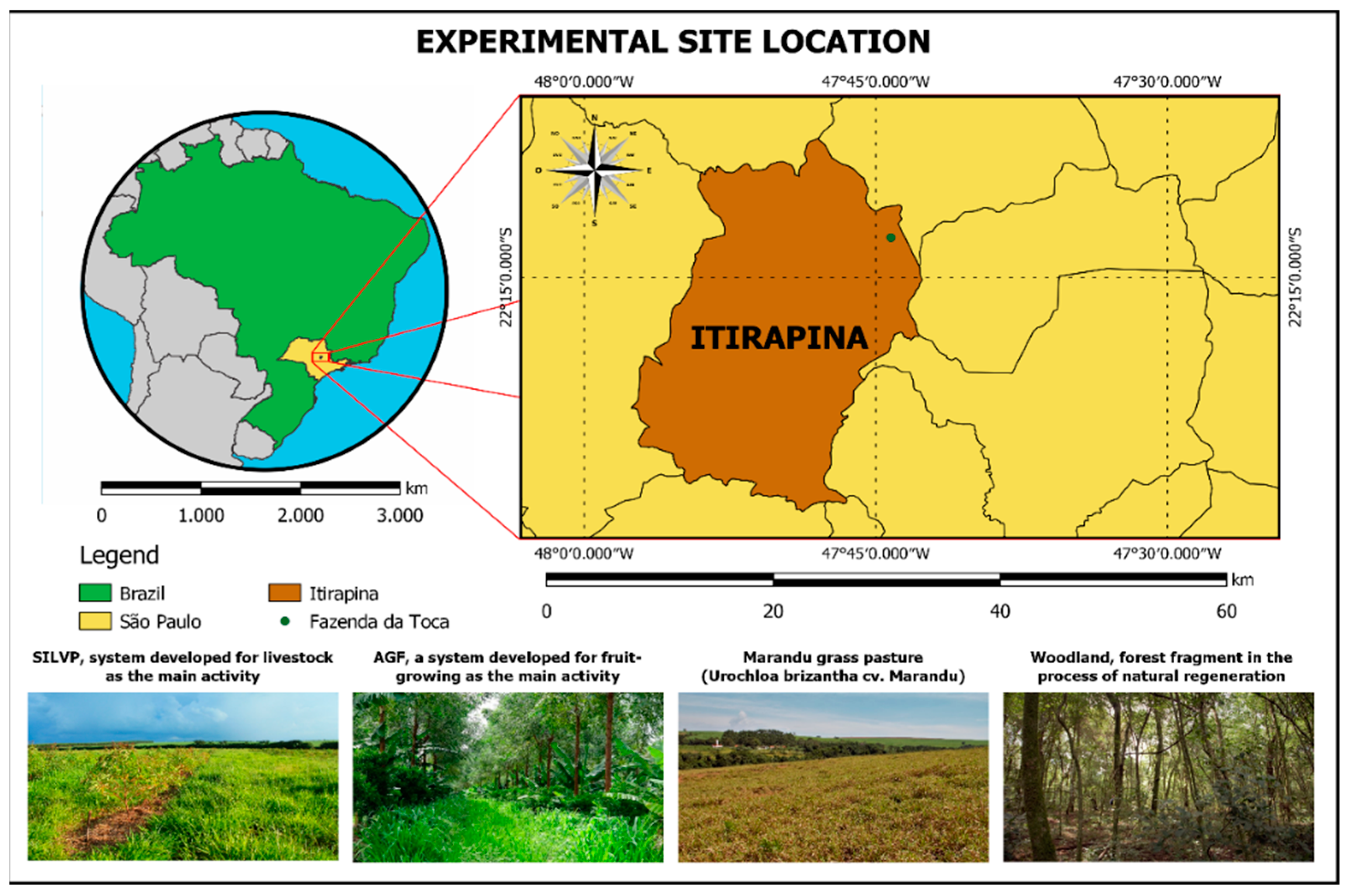
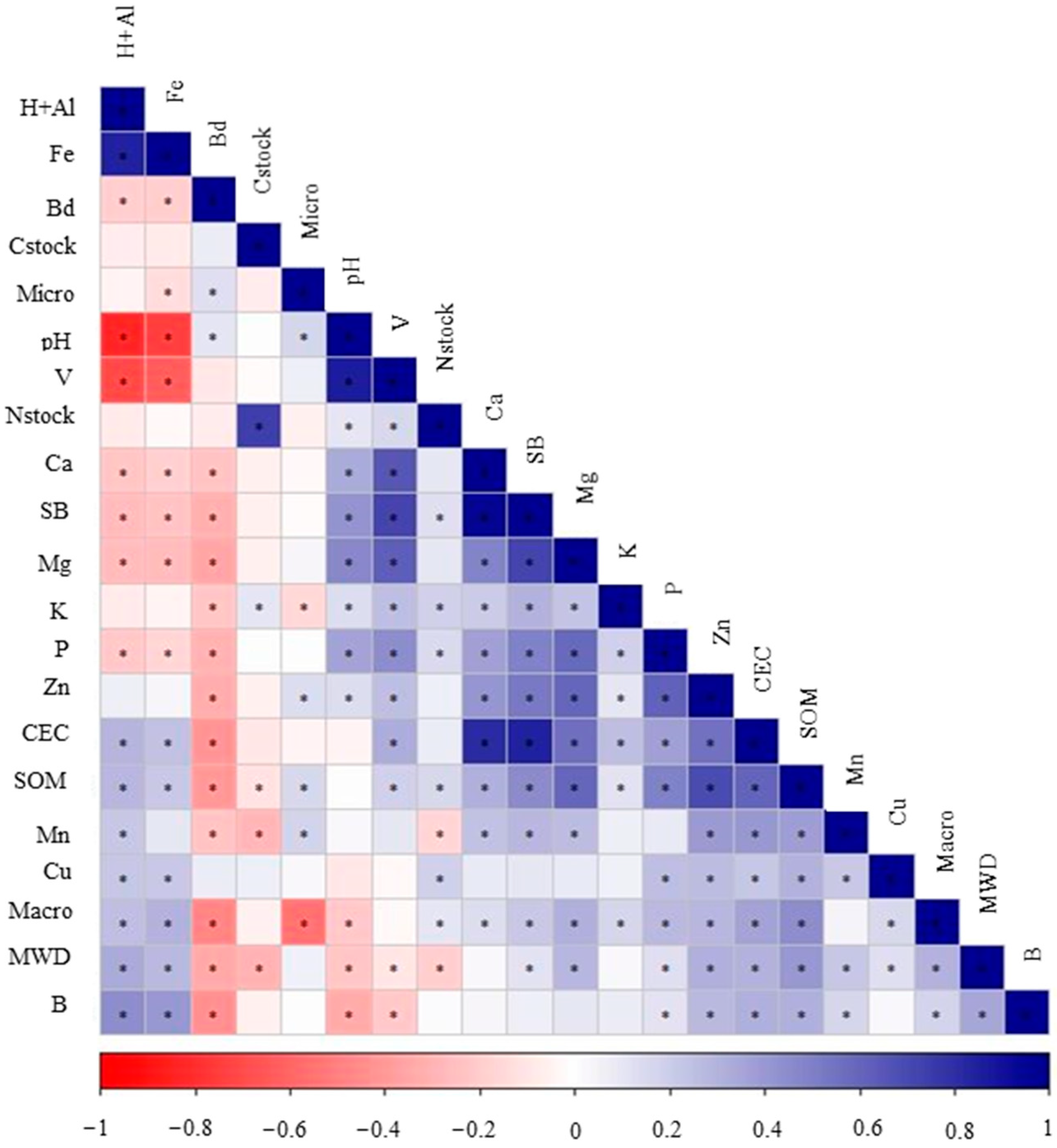

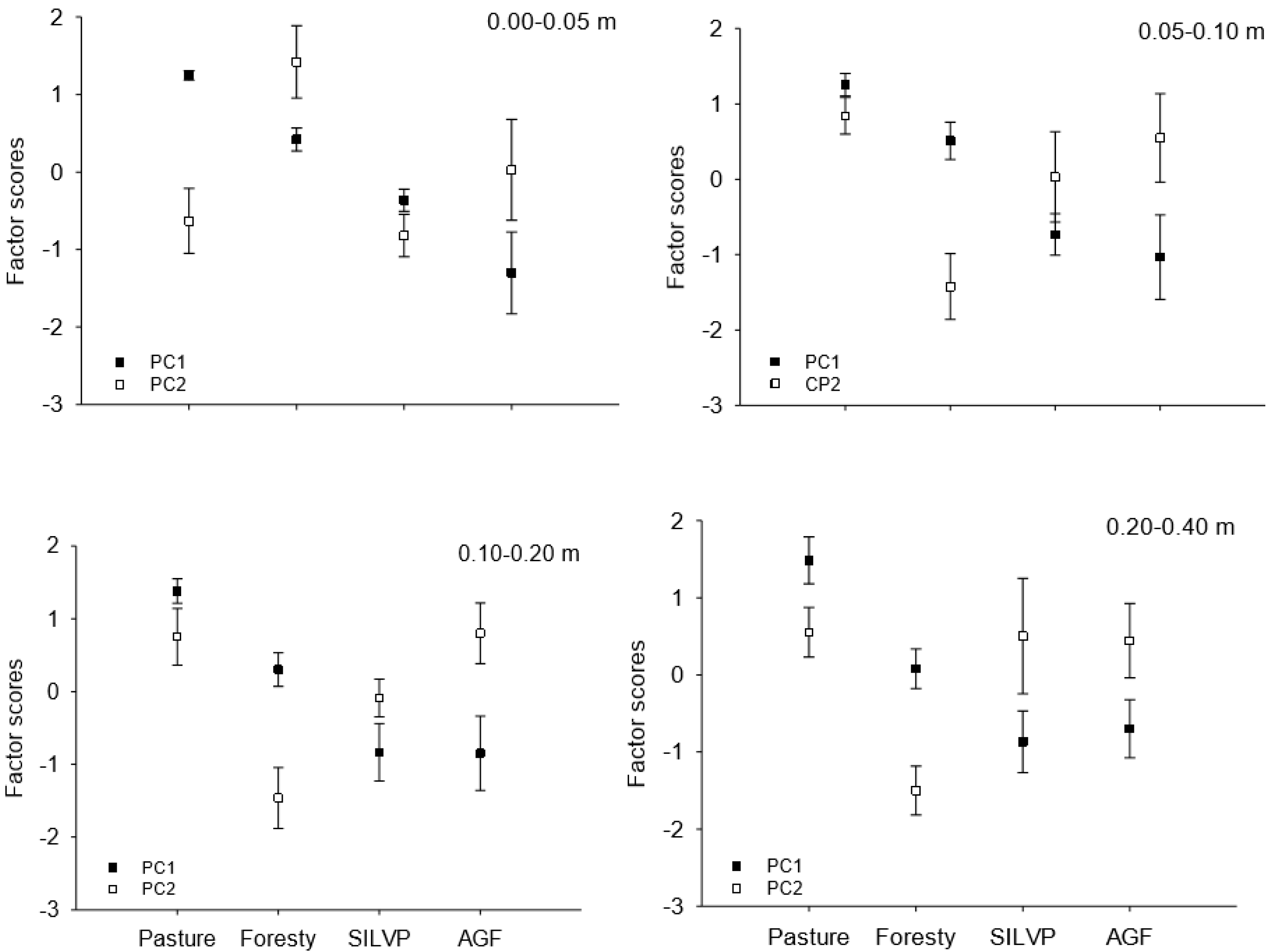
| Soil Depth | Sand | Silt | Clay | P | Ca2+ | Mg2+ | K+ | Al3+ |
|---|---|---|---|---|---|---|---|---|
| m | ------g kg−1------ | mg dm−3 | ---------cmolc dm−3 ---------- | |||||
| 0.00–0.25 | 920 | 50 | 30 | 0.001 | 1.30 | 0.76 | 0.09 | 0.01 |
| 0.25–0.50 | 911 | 19 | 70 | 0.001 | 0.46 | 0.39 | 0.06 | 0.27 |
| 0.50–1.00 | 890 | 40 | 70 | 0.001 | 0.11 | 0.14 | 0.02 | 0.46 |
| Manejo | Bd | SOM | MWD | Macro | Micro | Cstock | Nstock | pH | P | K | Ca | Mg | CEC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mg m−3 | g dm−3 | mm | -----m3 m−3----- | -----Mg ha−1----- | - | mg dm−3 | ---------------mmolc dm−3------------ | ||||||
| 0.00–0.05 m | |||||||||||||
| Foresty | 1.28 b | 22.33 a | 1.91 a | 15.96 b | 27.48 a | 5.48 b | 0.40 b | 3.51 b | 4.29 b | 0.72 b | 3.66 d | 1.35 b | 66.89 b |
| Pasture | 1.56 a | 11.25 c | 1.33 b | 5.03 c | 31.92 a | 2.50 c | 0.14 c | 5.62 a | 11.72 b | 0.47 b | 12.16 c | 5.48 b | 32.19 c |
| SILVP | 1.27 b | 19.91 b | 1.61 ab | 24.19 a | 19.77 b | 6.80 b | 0.63 b | 5.48 a | 38.06 a | 1.49 a | 21.83 b | 16.68 b | 57.24 b |
| AGF | 1.08 c | 34.00 b | 1.81 ab | 25.82 a | 26.61 a | 9.99 a | 1.19 a | 5.39 a | 41.77 a | 1.59 a | 53.51 a | 24.60 a | 94.76 a |
| 0.05–0.10 m | |||||||||||||
| Foresty | 1.44 ab | 16.16 b | 1.81 a | 16.50 a | 23.54 bc | 5.01 a | 0.30 b | 3.62 b | 3.50 c | 0.39 c | 1.47 c | 1.00 c | 41.00 ab |
| Pasture | 1.58 a | 10.25 b | 1.25 b | 6.69 b | 30.17 a | 2.27 b | 0.10 c | 5.54 a | 12.25 b | 0.38 c | 10.75 b | 5.50 b | 31.36 c |
| SILVP | 1.34 b | 17.26 a | 1.49 ab | 21.55 a | 21.32 c | 5.82 a | 0.52 b | 5.36 a | 25.50 a | 1.50 a | 19.08 a | 10.65 a | 48.51 ab |
| AGF | 1.31 b | 20.33 a | 1.63 ab | 16.52 a | 27.45 ab | 6.97 a | 0.76 a | 5.45 a | 33.00 a | 0.90 b | 25.08 a | 13.08 a | 55.53 a |
| 0.10–0.20 m | |||||||||||||
| Foresty | 1.49 ab | 13.08 ab | 1.50 a | 15.49 a | 25.37 ab | 7.95 ab | 0.57 b | 3.72 c | 3.75 c | 0,37 b | 1.46 c | 1.00 b | 44.14 a |
| Pasture | 1.61 a | 7,61 b | 0.98 a | 7.19 b | 27.72 a | 3.77 c | 0.04 c | 5.55 a | 7.37 bc | 0.30 b | 8.83 b | 3.08 b | 24.03 b |
| SILVP | 1.43 b | 14.83 a | 1.30 a | 20.14 a | 21.27 b | 10.41 a | 0.95 a | 5.11 b | 14.75 b | 0,95 a | 17.60 a | 5.12 b | 42.95 a |
| AGF | 1.35 b | 14.50 a | 0.99 a | 15.61 a | 26.45 ab | 10.01 ab | 1.06 a | 5.25 ab | 24.40 a | 0,75 a | 21.95 a | 13.71 a | 48.10 a |
| 0.20–0.40 m | |||||||||||||
| Foresty | 1.45 a | 11.08 a | 1.20 a | 16.52 a | 23.95 a | 13.11 a | 0.56 b | 3.48 c | 2.50 b | 0.28 bc | 8.16 bc | 1.00 b | 40.86 a |
| Pasture | 1.61 a | 7,36 a | 0,71 ab | 7.91 b | 27.05 a | 7.00 b | 0.08 c | 5.50 a | 3.58 b | 0.24 c | 7.37 c | 2.86 ab | 23.02 b |
| SILVP | 1.59 a | 12.10 a | 0,81 ab | 12.44 ab | 24.15 a | 15.00 a | 1.23 a | 5.03 b | 17,58 a | 0,79 a | 16.63 a | 5.87 a | 39,09 a |
| AGF | 1.56 a | 11.25 a | 0,62 b | 14.43 a | 25.76 a | 15.36 a | 1.26 a | 5.10 ab | 16.60 a | 0,61 ab | 14.25 ab | 7.10 a | 37.38 a |
| CV (%) | 7.56 | 24.83 | 26.57 | 25.39 | 15.42 | 19.97 | 24.93 | 5.55 | 31.02 | 29.07 | 27.48 | 39.54 | 18.85 |
| 0.00–0.05 m | 0.05–0.10 m | |||||||
|---|---|---|---|---|---|---|---|---|
| Variance—Var (%) | PC1 | PC2 | PC1 | PC2 | ||||
| Total | 58.29 | 17.05 | 52.60 | 21.94 | ||||
| accumulative | 58.29 | 75.34 | 52.60 | 74.54 | ||||
| Soil atributtes | Var | Corr | Var | Corr | Var | Corr | Var | Corr |
| Bd | 9.27 | 0.80 | 5.28 | −0.32 | 10.00 | 0.79 | 2.10 | 0.23 |
| Macro | 11.24 | −0.88 | 0.15 | −0.05 | 8.36 | −0.72 | 8.93 | −0.48 |
| Micro | 3.23 | 0.47 | 5.46 | 0.33 | 2.31 | 0.38 | 16.47 | 0.65 |
| MWD | 1.74 | −0.34 | 21.44 | 0.66 | 1.54 | −0.31 | 14.39 | −0.61 |
| SOM | 10.34 | −0.85 | 3.31 | 0.26 | 9.99 | −0.79 | 0.46 | −0.11 |
| pH | 0.46 | −0.17 | 37.07 | −0.87 | 1.08 | −0.26 | 27.98 | 0.85 |
| P | 8.72 | −0.78 | 15.39 | −0.56 | 9.79 | −0.78 | 11.07 | 0.54 |
| K | 11.61 | −0.90 | 2.95 | −0.24 | 8.69 | −0.74 | 0.54 | 0.12 |
| Ca | 10.00 | −0.83 | 2.06 | −0.20 | 8.65 | −0.73 | 15.22 | 0.63 |
| CEC | 10.37 | −0.85 | 5.36 | 0.33 | 12.15 | −0.87 | 0.26 | 0.08 |
| Cstock | 11.25 | −0.88 | 1.30 | 0.16 | 13.21 | −0.91 | 2.49 | −0.25 |
| Nstock | 11.73 | −0.90 | 0.17 | 0.05 | 14.16 | −0.94 | 0.028 | 0.02 |
| 0.10–0.20 m | 0.20–0.40 m | |||||||
| Variance—Var (%) | PC1 | PC2 | PC1 | PC2 | ||||
| Total | 48.23 | 19.97 | 39.35 | 26.86 | ||||
| accumulative | 48.23 | 68.2 | 39.35 | 66.21 | ||||
| Soil atributtes | Var | Corr | Var | Corr | Var | Corr | Var | Corr |
| Bd | 6.73 | 0.62 | 0.06 | −0.03 | 0.007 | 0.06 | 15.61 | 0.70 |
| Macro | 10.08 | −0.76 | 7.11 | −0.41 | 4.89 | −0.48 | 14.52 | −0.68 |
| Micro | 1.69 | 0.31 | 6.14 | 0.38 | 0.29 | 0.11 | 3.08 | 0.31 |
| MWD | 1.30 | −0.27 | 14.81 | −0.59 | 0.19 | −0.09 | 20.76 | −0.81 |
| SOM | 11.54 | −0.81 | 1.38 | −0.18 | 13.84 | −0.80 | 0.91 | −0.17 |
| pH | 0.017 | −0.03 | 33.57 | 0.89 | 0.51 | 0.15 | 22.23 | 0.84 |
| P | 8.74 | −0.71 | 13.15 | 0.56 | 11.59 | −0.73 | 9.39 | 0.55 |
| K | 12.55 | −0.85 | 1.18 | 0.16 | 10.59 | −0.70 | 5.65 | 0.42 |
| Ca | 8.27 | −0.69 | 18.42 | 0.66 | 9.91 | −0.68 | 3.02 | 0.31 |
| CEC | 10.42 | −0.77 | 3.65 | −0.29 | 12.72 | −0.77 | 3.13 | −0.31 |
| Cstock | 13.33 | −0.87 | 0.47 | −0.10 | 18.13 | −0.92 | 0.36 | −0.10 |
| Nstock | 15.28 | −0.94 | 0.013 | 0.01 | 17.21 | −0.90 | 1.28 | 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marçal, M.F.M.; de Souza, Z.M.; Tavares, R.L.M.; Farhate, C.V.V.; Júnior, R.E.M.; de Souza Lima, E.; Lovera, L.H. Potential Use of Quartzipisamment under Agroforestry and Silvopastoral System for Large-Scale Production in Brazil. Agronomy 2022, 12, 905. https://doi.org/10.3390/agronomy12040905
Marçal MFM, de Souza ZM, Tavares RLM, Farhate CVV, Júnior REM, de Souza Lima E, Lovera LH. Potential Use of Quartzipisamment under Agroforestry and Silvopastoral System for Large-Scale Production in Brazil. Agronomy. 2022; 12(4):905. https://doi.org/10.3390/agronomy12040905
Chicago/Turabian StyleMarçal, Maria Fernanda Magioni, Zigomar Menezes de Souza, Rose Luiza Moraes Tavares, Camila Vieira Viana Farhate, Raul Evaristo Monteiro Júnior, Elizeu de Souza Lima, and Lenon Henrique Lovera. 2022. "Potential Use of Quartzipisamment under Agroforestry and Silvopastoral System for Large-Scale Production in Brazil" Agronomy 12, no. 4: 905. https://doi.org/10.3390/agronomy12040905
APA StyleMarçal, M. F. M., de Souza, Z. M., Tavares, R. L. M., Farhate, C. V. V., Júnior, R. E. M., de Souza Lima, E., & Lovera, L. H. (2022). Potential Use of Quartzipisamment under Agroforestry and Silvopastoral System for Large-Scale Production in Brazil. Agronomy, 12(4), 905. https://doi.org/10.3390/agronomy12040905






