Non-Essential Elements and Their Role in Sustainable Agriculture
Abstract
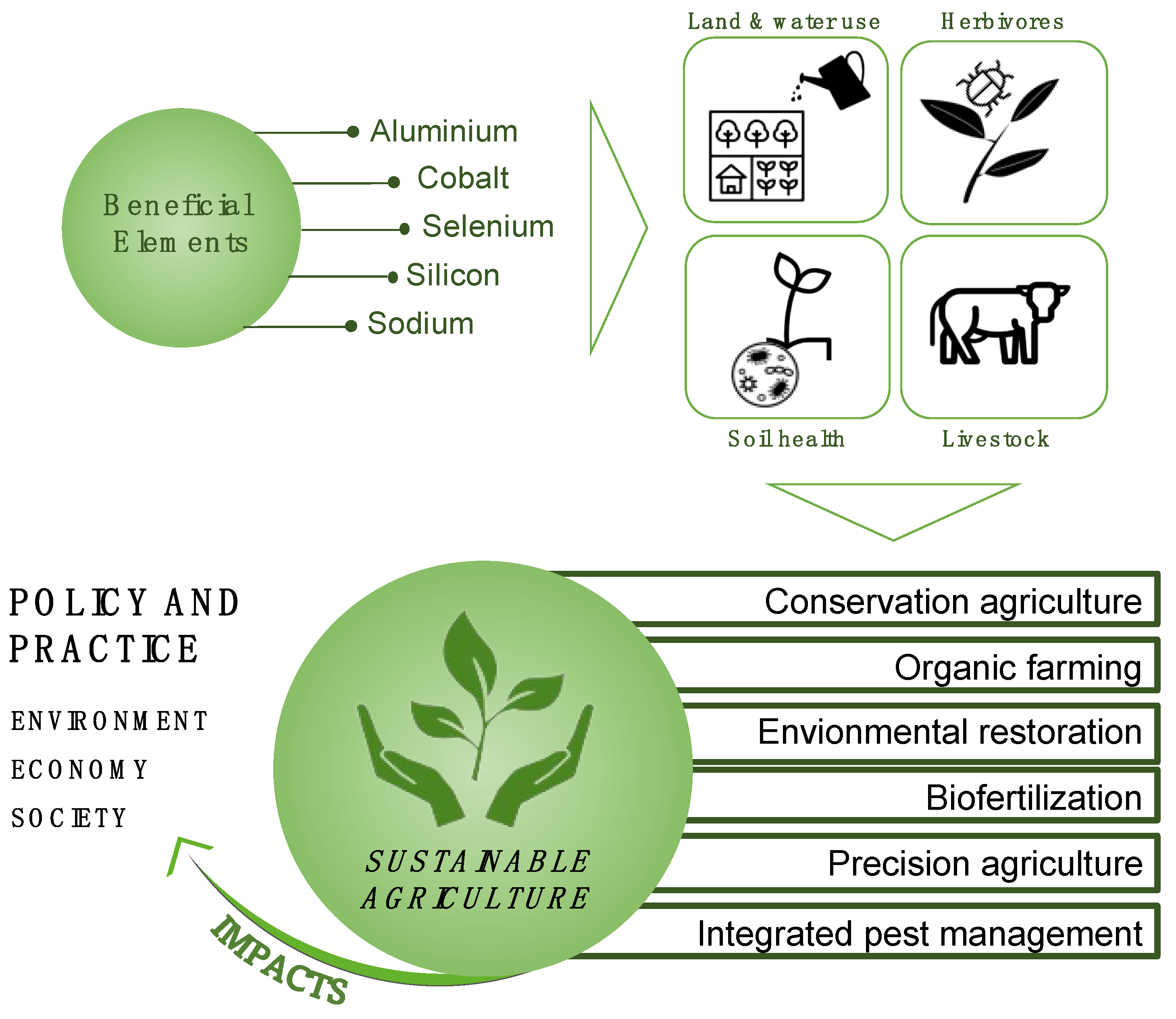
1. Introduction
2. Aluminium
2.1. Plant Growth and Productivity
2.2. Nutrient Balance
| Plant Species | Al Supply | Al Concentration Tested | Effects | Reference |
|---|---|---|---|---|
| Brachiaria ruziziensis | AlCl3 | 15 ppm | ↑N, P and K and ↓ Ca in L; ↑ K and ↓ Ca in St | [46] |
| Camellia sinensis | Al2(SO4)3 | 43 ppm, low P 345 ppm, high P | Low P: ↑ K in L, St and R and Mn in L; ↓Ca in L; ↑ DW High P: ↑ K and Mn in L, St and R and N in L; ↓Mg in L, St and R; ↑ DW | [37] |
| AlCl3 | 200 μM | ↑ Ca and Fe and ↓ P, Mn, Mg and B in L; ↓ catechin and epicatechin | [50] | |
| Coffea arabica | AlCl3 | 2.7 ppm | ↑ K and Ca in R; ↑ root growth; ↑ phospholipase C activity | [28] |
| Hordeum vulgare | Al2(SO4)3 | 100 μM | ↑ NO3− and PO4 in R; ↓P, Ca, and NH4+ in R | [49] |
| 600 µM | ↑ Zn in Sh and R and P in R; ↓ P in Sh and K, Ca, Mg, Fe and Mn in Sh and R | [51] | ||
| Musa spp. | Al2(SO4)3 | 78.5 μM | ↓ Ca, K, P, Mg, NO3_N and NH4_N; ↓ Water uptake | [48] |
| Oryza sativa | AlCl3 | 3 ppm | ↑ N and P in L, P and K in St and P in R; ↑ DW | [46] |
| Phaseolus vulgaris | AlCl3 | 10 ppm | ↓ Ca in R, St and L, and ↑ R length, DW and St length in cv. ‘Hilds Maxi’; ↓ Ca in R, L area, Sh DW in cv. ‘Seleccion F-15′; ↑ Ca in St and ↓ Ca in L, R and St length, L area, R and Sh DW in cv. ‘Eagle’ | [52] |
| Prunus persica | NS | 1000 µM | ↑ N in St ↓ P, K, Ca, Mg, in R, St and L; ↓ Fe, Mo, in R and L; ↓ Mn in L; ↓ total growth, lateral Sh number and length, L number and area | [53] |
| Secale cereale | AlK(SO4)2 | 10 ppm | ↑ P and ↓ K, Ca, Mg and Mn in L; ↑ K and ↓ Ca and Mn in R; ↓ Chl. b and carotenoid | [54] |
| × Triticosecale | AlK(SO4)2 | 10 ppm | ↑ Mn in R; ↓K in L and R; ↓ Chl. a and Chl. b; ↑ carotenoid | [54] |
| Triticum aestivum | AlK(SO4)2 | 5 ppm | ↑ K and ↓ P and Mn in L; ↑ Ca and ↓ Mn in R; ↑ Chl. a | [54] |
| Vaccinium macrocarpon | AlCl3 | 3 ppm | ↑ N, P and K in L, St and R; ↑ DW | [46] |
2.3. Environmental Stress-Mitigation
2.4. Aluminium Potential in Plant Protection
2.5. Enhancing Postharvest Performance
3. Cobalt
3.1. Cobalt Uptake and Stimulatory Effects on Plant Growth
3.2. Cobalt in Plant Nutrition
3.3. Cobalt and Increased Shelf-Life
4. Selenium
4.1. Selenium as Green Fertilizer
4.2. Crop Biofortification
4.3. Phytoremediation for Sustainable Fertilization
4.4. Interactions with Other Trace Elements
4.5. Improving Water-Use Efficiency (WUE)
5. Silicon
5.1. Quality and Yield Improvement
5.2. Pest and Pathogen Resistance
5.3. Tolerance to Drought and Salinity
5.4. Alleviation of Heavy-Metal Toxicity
6. Sodium
6.1. Promotion of Plant Growth and Sensorial Quality
6.2. Potassium Nutrition
6.3. Promoting Water Use Efficiency (WUE)
7. Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soares, J.C.; Santos, C.S.; Carvalho, S.M.P.; Pintado, M.M.; Vasconcelos, M.W. Preserving the nutritional quality of crop plants under a changing climate: Importance and strategies. Plant Soil 2019, 443, 1–26. [Google Scholar] [CrossRef]
- Matthiesen, R.L.; Ahmad, A.A.; Robertson, A.E. Temperature Affects Aggressiveness and Fungicide Sensitivity of Four Pythium spp. that Cause Soybean and Corn Damping Off in Iowa. Plant Dis. 2015, 100, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Matzrafi, M.; Brunharo, C.; Tehranchian, P.; Hanson, B.D.; Jasieniuk, M. Increased temperatures and elevated CO2 levels reduce the sensitivity of Conyza canadensis and Chenopodium album to glyphosate. Sci. Rep. 2019, 9, 2228. [Google Scholar] [CrossRef] [PubMed]
- Vasileiadis, V.P. Economic sustainability: Less pesticide rarely causes loss. Nat. Plants 2017, 3, 17016. [Google Scholar] [CrossRef] [PubMed]
- Scown, M.W.; Winkler, K.J.; Nicholas, K.A. Aligning research with policy and practice for sustainable agricultural land systems in Europe. Proc. Natl. Acad. Sci. USA 2019, 116, 4911. [Google Scholar] [CrossRef]
- FAO. Sustainable Options for Addressing Land and Water Problems—A Problem Tree and Case Studies. Available online: http://www.fao.org/fileadmin/templates/solaw/files/thematic_reports/TR_15_web.pdf (accessed on 14 August 2020).
- Awasthi, S.; Chauhan, R.; Srivastava, S. Chapter 2—The importance of beneficial and essential trace and ultratrace elements in plant nutrition, growth, and stress tolerance. In Plant Nutrition and Food Security in the Era of Climate Change; Kumar, V., Srivastava, A.K., Suprasanna, P., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 27–46. [Google Scholar]
- Pretty, J.; Bharucha, Z.P. Sustainable intensification in agricultural systems. Ann. Bot. 2014, 114, 1571–1596. [Google Scholar] [CrossRef]
- U.S. Congress. Food, Agriculture, Conservation, and Trade Act, Public Law. Available online: https://www.gpo.gov/fdsys/pkg/USCODE-2007-title7/pdf/USCODE-2007-title7-chap64-subchapI.pdf (accessed on 14 August 2020).
- National Research Council. Impact of Genetically Engineered Crops on Farm Sustainability in the United States; National Academies Press: Washington, DC, USA, 2010. [Google Scholar]
- Vanlauwe, B.; Hungria, M.; Kanampiu, F.; Giller, K.E. The role of legumes in the sustainable intensification of African smallholder agriculture: Lessons learnt and challenges for the future. Agric. Ecosyst. Environ. 2019, 284, 106583. [Google Scholar] [CrossRef]
- Mahanty, T.; Bhattacharjee, S.; Goswami, M.; Bhattacharyya, P.; Das, B.; Ghosh, A.; Tribedi, P. Biofertilizers: A potential approach for sustainable agriculture development. Environ. Sci. Pollut. Res. 2017, 24, 3315–3335. [Google Scholar] [CrossRef]
- Niggli, U. Sustainability of organic food production: Challenges and innovations. Proc. Nutr. Soc. 2014, 74, 83–88. [Google Scholar] [CrossRef]
- Lo Piccolo, E.; Ceccanti, C.; Guidi, L.; Landi, M. Role of beneficial elements in plants: Implications for the photosynthetic process. Photosynthetica 2021, 59, 349–360. [Google Scholar] [CrossRef]
- Rahman, M.A.; Lee, S.-H.; Ji, H.C.; Kabir, A.H.; Jones, C.S.; Lee, K.-W. Importance of Mineral Nutrition for Mitigating Aluminum Toxicity in Plants on Acidic Soils: Current Status and Opportunities. Int. J. Mol. Sci. 2018, 19, 3073. [Google Scholar] [CrossRef] [PubMed]
- Muszyńska, E.; Labudda, M. Dual Role of Metallic Trace Elements in Stress Biology—From Negative to Beneficial Impact on Plants. Int. J. Mol. Sci. 2019, 20, 3117. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y.Y.; Hu, L.; Yang, M.Y.; Zeng, R.S. Plant anti-herbivore defense priming: Concept, mechanisms and application. J. Appl. Ecol. 2018, 29, 2068–2078. [Google Scholar] [CrossRef]
- Abd-Alla, M.H.; Bagy, M.K.; El-enany, A.-W.E.-s.; Bashandy, S.R. Activation of Rhizobium tibeticum With Flavonoids Enhances Nodulation, Nitrogen Fixation, and Growth of Fenugreek (Trigonella foenum-graecum L.) Grown in Cobalt-Polluted Soil. Arch. Environ. Contam. Toxicol. 2014, 66, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Cai, M.; Hu, C.; Sun, X.; Cheng, Q.; Jia, W.; Yang, T.; Nie, M.; Zhao, X. Selenium (Se) reduces Sclerotinia stem rot disease incidence of oilseed rape by increasing plant Se concentration and shifting soil microbial community and functional profiles. Environ. Pollut. 2019, 254, 113051. [Google Scholar] [CrossRef] [PubMed]
- Dumont, B.; Groot, J.C.J.; Tichit, M. Review: Make ruminants green again—How can sustainable intensification and agroecology converge for a better future? Animal 2018, 12, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, M.W.; Pinto, A.G.E.; Ferreira, H.; Vieira, E.; Pimenta, A.; Santos, C.S. The push-, pull- and enabling—Capacities necessary for legume grain inclusion into sustainable agri-food systems and healthy diets. World Rev. Nutr. Diet. 2020, 121, 193–211. [Google Scholar]
- Abarca-Gómez, L.; Ezzati, M. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Poschenrieder, C.; Gunsé, B.; Corrales, I.; Barceló, J. A glance into aluminum toxicity and resistance in plants. Sci. Total Environ. 2008, 400, 356–368. [Google Scholar] [CrossRef]
- Barceló, J.; Poschenrieder, C. Fast root growth responses, root exudates, and internal detoxification as clues to the mechanisms of aluminium toxicity and resistance: A review. Environ. Exp. Bot. 2002, 48, 75–92. [Google Scholar] [CrossRef]
- Wood, S.; Rosen, C.; Billings, H.; Powell, M.; Sebastian, K.; Scherr, S.; Batjes, N.; Farrow, A.; Faurs, J.; Iiasa, G.; et al. Pilot Analysis of Global Ecosystems: Agroecosystems; Wood, S., Sebastian, K., Scherr, S.J., Eds.; World Resources Institute (WRI) and International Food Policy Reseach Institute (IFPRI): Washington, DC, USA, 2001. [Google Scholar]
- Pilon-Smits, E.A.H.; Quinn, C.F.; Tapken, W.; Malagoli, M.; Schiavon, M. Physiological functions of beneficial elements. Curr. Opin. Plant Biol. 2009, 12, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Kaur, N.; Siddique, K.H.M.; Nayyar, H. Beneficial elements for agricultural crops and their functional relevance in defence against stresses. Arch. Agron. Soil Sci. 2016, 62, 905–920. [Google Scholar] [CrossRef]
- Bojórquez-Quintal, D.J.E.; Sánchez-Cach, L.A.; Ku-González, Á.; de los Santos-Briones, C.; de Fátima Medina-Lara, M.; Echevarría-Machado, I.; Muñoz-Sánchez, J.A.; Teresa Hernández Sotomayor, S.M.; Estévez, M.M. Differential effects of aluminum on in vitro primary root growth, nutrient content and phospholipase C activity in coffee seedlings (Coffea arabica). J. Inorg. Biochem. 2014, 134, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Hajiboland, R.; Bastani, S.; Bahrami-Rad, S.; Poschenrieder, C. Interactions between aluminum and boron in tea (Camellia sinensis) plants. Acta Physiol. Plant. 2015, 37, 54. [Google Scholar] [CrossRef]
- Moreno-Alvarado, M.; García-Morales, S.; Trejo-Téllez, L.I.; Hidalgo-Contreras, J.V.; Gómez-Merino, F.C. Aluminum Enhances Growth and Sugar Concentration, Alters Macronutrient Status and Regulates the Expression of NAC Transcription Factors in Rice. Front. Plant Sci. 2017, 8, 73. [Google Scholar] [CrossRef]
- Pan, J.; Li, D.; Zhu, J.; Shu, Z.; Ye, X.; Xing, A.; Wen, B.; Ma, Y.; Zhu, X.; Fang, W.; et al. Aluminum relieves fluoride stress through stimulation of organic acid production in Camellia sinensis. Physiol. Mol. Biol. Plants 2020, 26, 1127–1137. [Google Scholar] [CrossRef]
- Poot-Poot, W.; Teresa Hernandez-Sotomayor, S.M. Aluminum stress and its role in the phospholipid signaling pathway in plants and possible biotechnological applications. IUBMB Life 2011, 63, 864–872. [Google Scholar] [CrossRef]
- Famoso, A.N.; Zhao, K.; Clark, R.T.; Tung, C.-W.; Wright, M.H.; Bustamante, C.; Kochian, L.V.; McCouch, S.R. Genetic Architecture of Aluminum Tolerance in Rice (Oryza sativa) Determined through Genome-Wide Association Analysis and QTL Mapping. PLoS Genet. 2011, 7, e1002221. [Google Scholar] [CrossRef]
- Bertrand, D.; Wolf, A.D. Physiologie vegetale. L’aluminium, oligo-element necessaire au mais. CR Acad. Sci 1968, 267, 2325–2326. [Google Scholar]
- Wang, L.; Fan, X.-W.; Pan, J.-L.; Huang, Z.-B.; Li, Y.-Z. Physiological characterization of maize tolerance to low dose of aluminum, highlighted by promoted leaf growth. Planta 2015, 242, 1391–1403. [Google Scholar] [CrossRef]
- Yu, H.N.; Liu, P.; Wang, Z.Y.; Chen, W.R.; Xu, G.D. The effect of aluminum treatments on the root growth and cell ultrastructure of two soybean genotypes. Crop Prot. 2011, 30, 323–328. [Google Scholar] [CrossRef]
- Konishi, S.; Miyamoto, S.; Taki, T. Stimulatory Effects of Aluminum on Tea Plants Grown under Low and High Phosphorus Supply. Soil Sci. Plant Nutr. 1985, 31, 361–368. [Google Scholar] [CrossRef]
- Fung, K.F.; Carr, H.P.; Zhang, J.; Wong, M.H. Growth and nutrient uptake of tea under different aluminium concentrations. J. Sci. Food Agric. 2008, 88, 1582–1591. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, Y.; Ding, Z.; Song, L.; Li, Y.; Ma, D.; Wang, Y.; Shen, J.; Jia, S.; Sun, H.; et al. Aluminum induced metabolic responses in two tea cultivars. Plant Physiol. Biochem. 2016, 101, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Hajiboland, R.; Barceló, J.; Poschenrieder, C.; Tolrà, R. Amelioration of iron toxicity: A mechanism for aluminum-induced growth stimulation in tea plants. J. Inorg. Biochem. 2013, 128, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Kidd, P.S.; Proctor, J. Effects of aluminium on the growth and mineral composition of Betula pendula Roth. J. Exp. Bot. 2000, 51, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Tomioka, R.; Oda, A.; Takenaka, C. Root growth enhancement by rhizospheric aluminum treatment in Quercus serrata Thunb. seedlings. J. For. Res. 2005, 10, 319–324. [Google Scholar] [CrossRef]
- Tomioka, R. Stimulation of Root Growth Induced by Aluminum in Quercus serrata Thunb. Is Related to Activity of Nitrate Reductase and Maintenance of IAA Concentration in Roots. Am. J. Plant Sci. 2012, 03, 1619–1624. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, R.F.; Shen, R.F. Coadaptation of Plants to Multiple Stresses in Acidic Soils. Soil Sci. 2014, 179, 503–513. [Google Scholar] [CrossRef]
- Kinraide, T.B. Aluminum enhancement of plant growth in acid rooting media. A case of reciprocal alleviation of toxicity by two toxic cations. Physiol. Plant. 1993, 88, 619–625. [Google Scholar] [CrossRef]
- Osaki, M.; Watanabe, T.; Tadano, T. Beneficial effect of aluminum on growth of plants adapted to low pH soils. Soil Sci. Plant Nutr. 1997, 43, 551–563. [Google Scholar] [CrossRef]
- Tomioka, R.; Takenaka, C. Enhancement of root respiration and photosynthesis in Quercus serrata Thunb. seedlings by long-term aluminum treatment. Environ. Sci. Int. J. Environ. Physiol. Toxicol. 2007, 14, 141–148. [Google Scholar]
- Rufyikiri, G.; Nootens, D.; Dufey, J.E.; Delvaux, B. Effect of aluminium on bananas (Musa spp.) cultivated in acid solutions. I. Plant growth and chemical composition. Fruits 2001, 55, 367–379. [Google Scholar] [CrossRef]
- Nichol, B.E.; Oliveira, L.A.; Glass, A.D.M.; Siddiqi, M.Y. The Effects of Aluminum on the Influx of Calcium, Potassium, Ammonium, Nitrate, and Phosphate in an Aluminum-Sensitive Cultivar of Barley (Hordeum vulgare L.). Plant Physiol. 1993, 101, 1263. [Google Scholar] [CrossRef]
- Tolrà, R.; Martos, S.; Hajiboland, R.; Poschenrieder, C. Aluminium alters mineral composition and polyphenol metabolism in leaves of tea plants (Camellia sinensis). J. Inorg. Biochem. 2020, 204, 110956. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.M. Influence of aluminium on plant growth and mineral nutrition of barley. Commun. Soil Sci. Plant Anal. 1981, 12, 121–138. [Google Scholar] [CrossRef]
- Massot, N.G.; Poschenrieder, C.; Barceló, J. Differential response of three bean (Phaseolus vulgaris) cultivars to aluminium. Acta Bot. Neerl. 1992, 41, 293–298. [Google Scholar] [CrossRef]
- Graham, C. The influence of nitrogen source and aluminum on growth and elemental composition of ‘Nemaguard’ peach seedlings. J. Plant Nutr. 2001, 24, 423–439. [Google Scholar] [CrossRef]
- Dinev, N.; Stancheva, I. Changes in nitrate reductase activity, plastid pigment content, and plant mineral composition of wheat, rye, and triticale grown in the presence of aluminum. J. Plant Nutr. 1993, 16, 2397–2409. [Google Scholar] [CrossRef]
- Mihailovic, N.; Vucinic, Z.; Hadzi-Taskovic Sukalovic, V. Ammonium Enables Aluminum-Induced Stimulation of Nitrogen Assimilation in Roots of Al-Tolerant Maize Genotypes. J. Plant Nutr. 2015, 38, 371–383. [Google Scholar] [CrossRef]
- Hajiboland, R.; Bahrami-Rad, S.; Bastani, S. Aluminum alleviates boron-deficiency induced growth impairment in tea plants. Biol. Plant. 2014, 58, 717–724. [Google Scholar] [CrossRef]
- Farrukh, M.A.; Naseem, F. Nano-Leucite for Slow Release Nitrogen Fertilizer and Green Environment. Available online: https://patents.google.com/patent/US20140190226A1/en (accessed on 27 August 2020).
- Sornhiran, N.; Tuntrachanida, J.; Malachey, P.; Thongtuk, P.; Wisawapipat, W.; Aramrak, S.; Prakongkep, N. Aluminum- and iron-engineered biochar from sugarcane filter cake as phosphorus adsorbents and fertilizers. ScienceAsia 2021, 47, 220–227. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Gianoncelli, A.; Kourousias, G.; Green, K.; McKenna, B.A. Alleviation of Al Toxicity by Si Is Associated with the Formation of Al–Si Complexes in Root Tissues of Sorghum. Front. Plant Sci. 2017, 8, 2189. [Google Scholar] [CrossRef] [PubMed]
- Thornton, F.C.; Schaedle, M.; Raynal, D.J. Tolerance of Red Oak and American and European Beech Seedlings to Aluminum. J. Environ. Qual. 1989, 18, 541–545. [Google Scholar] [CrossRef]
- Hiatt, A.J.; Amos, D.F.; Massey, H.F. Effect of Aluminum on Copper Sorption by Wheat1. Agron. J. 1963, 55, 284–287. [Google Scholar] [CrossRef]
- Yang, Z.B.; You, J.F.; Xu, M.Y.; Yang, Z.M. Interaction between aluminum toxicity and manganese toxicity in soybean (Glycine max). Plant Soil 2009, 319, 277–289. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, X.Q.; Hu, Z.M.; Shao, J.F.; Che, J.; Chen, R.F.; Dong, X.Y.; Shen, R.F. Aluminium alleviates manganese toxicity to rice by decreasing root symplastic Mn uptake and reducing availability to shoots of Mn stored in roots. Ann. Bot. 2015, 116, 237–246. [Google Scholar] [CrossRef]
- Deluca, T.H.; Shew, H.D. Inhibition of growth and reproduction of Phytophthora parasitica var. nicotianae by aluminum. Phytopathology 1988, 78, 1576. [Google Scholar]
- Yang, Y.; Liu, Y.; Huang, C.-F.; de Silva, J.; Zhao, F.-J. Aluminium alleviates fluoride toxicity in tea (Camellia sinensis). Plant Soil 2016, 402, 179–190. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, Y.; Chen, Z.; Song, N.; Li, X.; Ren, D.; Zhang, S. Evaluation of fluoride and cadmium adsorption modification of corn stalk by aluminum trichloride. Appl. Surf. Sci. 2021, 543, 148727. [Google Scholar] [CrossRef]
- Hanafiah, M.M.; Zainuddin, M.F.; Mohd Nizam, N.U.; Halim, A.A.; Rasool, A. Phytoremediation of Aluminum and Iron from Industrial Wastewater Using Ipomoea aquatica and Centella asiatica. Appl. Sci. 2020, 10, 3064. [Google Scholar] [CrossRef]
- Beach, B.G.W.; Chalandon, A.; Gallinelli, G. The control of various Phytophthora diseases in tropical crops with aluminium tris(ethyl phosphonate). In Proceedings of the 1979 British Crop Protection Conference, Brighton, UK, 19–22 November 1979; pp. 319–329. [Google Scholar]
- Guest, D.I. Modification of defense responses in tobacco and capsicum following treatment with Fosetyl-Al [Aluminium tris (o-ethyl phosphonate)]. Physiol. Plant Pathol. 1984, 25, 125–134. [Google Scholar] [CrossRef]
- Andreu, A.B.; Guevara, M.G.; Wolski, E.A.; Daleo, G.R.; Caldiz, D.O. Enhancement of natural disease resistance in potatoes by chemicals. Pest Manag. Sci. 2006, 62, 162–170. [Google Scholar] [CrossRef]
- Zhao, X.; Ren, L.; Yin, H.; Zhou, J.; Han, J.; Luo, Y. Sensitivity of Pseudoperonospora cubensis to dimethomorph, metalaxyl and fosetyl-aluminium in Shanxi of China. Crop Prot. 2013, 43, 38–44. [Google Scholar] [CrossRef]
- Davis, R.M. Control of Phytophthora Root and Foot Rot of Citrus with Systemic Fungicides Metalaxyl and Phosethyl Aluminum. Am. Phytopathol. Soc. 1981, 66, 218–220. [Google Scholar] [CrossRef]
- Farih, A.; Menge, J.A.; Tsao, P.H.; Ohr, H.D. Metalaxyl and efosite aluminium for control of Phytophthora gummosis and root rot on citrus. Plant Dis. 1981, 65, 654–657. [Google Scholar] [CrossRef]
- Goswami, A.; Roy, I.; Sengupta, S.; Debnath, N. Novel applications of solid and liquid formulations of nanoparticles against insect pests and pathogens. Thin Solid Films 2010, 519, 1252–1257. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, X.Q.; Li, X.; Ni, Y.Q.; Li, H.Y. Aluminum uptake and disease resistance in Nicotiana rustica leaves. Ecotoxicol. Environ. Saf. 2010, 73, 655–663. [Google Scholar] [CrossRef]
- Satapathy, P.; Achary, V.M.M.; Panda, B.B. Aluminum-induced abiotic stress counteracts Fusarium infection in Cajanus cajan (L.) Millsp. J. Plant Interact. 2012, 7, 121–128. [Google Scholar] [CrossRef]
- Kolaei, E.A.; Cenatus, C.; Tweddell, R.J.; Avis, T.J. Antifungal activity of aluminium-containing salts against the development of carrot cavity spot and potato dry rot. Ann. Appl. Biol. 2013, 163, 311–317. [Google Scholar] [CrossRef]
- Hamel, F.; Breton, C.; Houde, M. Isolation and characterization of wheat aluminum-regulated genes: Possible involvement of aluminum as a pathogenesis response elicitor. Planta 1998, 205, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Arasimowicz-Jelonek, M.; Floryszak-Wieczorek, J.; Drzewiecka, K.; Chmielowska-Bąk, J.; Abramowski, D.; Izbiańska, K. Aluminum induces cross-resistance of potato to Phytophthora infestans. Planta 2014, 239, 679–694. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.R.; Shew, H.D. Soils suppressive to black root rot of burley tobacco, caused by Thielaviopsis basicola. Phytopathology 1991, 81, 946–954. [Google Scholar] [CrossRef]
- Yanık, F.; Vardar, F. Toxic Effects of Aluminum Oxide (Al2O3) Nanoparticles on Root Growth and Development in Triticum aestivum. Water Air Soil Pollut. 2015, 226, 296. [Google Scholar] [CrossRef]
- Avis, T.J.; Michaud, M.; Tweddell, R.J. Role of lipid composition and lipid peroxidation in the sensitivity of fungal plant pathogens to aluminum chloride and sodium metabisulfite. Appl. Environ. Microbiol. 2007, 73, 2820–2824. [Google Scholar] [CrossRef][Green Version]
- Avis, T.J.; Rioux, D.; Simard, M.; Michaud, M.; Tweddell, R.J. Ultrastructural Alterations in Fusarium sambucinum and Heterobasidion annosum Treated with Aluminum Chloride and Sodium Metabisulfite. Phytopathology 2009, 99, 167–175. [Google Scholar] [CrossRef]
- Al-Mughrabi, K.I.; Coleman, W.K.; Vikram, A.; Poirier, R.; Jayasuriya, K.E. Effectiveness of Essential Oils and Their Combinations with Aluminum Starch Octenylsuccinate on Potato Storage Pathogens. J. Essent. Oil Bear. Plants 2013, 16, 23–31. [Google Scholar] [CrossRef]
- Hu, X.; Wei, X.; Ling, J.; Chen, J. Cobalt: An Essential Micronutrient for Plant Growth? Front. Plant Sci. 2021, 12, 768523. [Google Scholar] [CrossRef]
- Lange, B.; van der Ent, A.; Baker, A.J.M.; Echevarria, G.; Mahy, G.; Malaisse, F.; Meerts, P.; Pourret, O.; Verbruggen, N.; Faucon, M.-P. Copper and cobalt accumulation in plants: A critical assessment of the current state of knowledge. New Phytol. 2017, 213, 537–551. [Google Scholar] [CrossRef]
- Hamilton, E. Environmental variables in a holistic evaluation of land contaminated by historic mine wastes: A study of multi-element mine wastes in West Devon, England using arsenic as an element of potential concern to human health. Sci. Total Environ. 2000, 249, 171–221. [Google Scholar] [CrossRef]
- Palit, S.; Sharma, A.; Talukder, G. Effects of cobalt on plants. Bot. Rev. 1994, 60, 149–181. [Google Scholar] [CrossRef]
- Wa Lwalaba, J.L.; Zvogbo, G.; Mulembo, M.; Mundende, M.; Zhang, G. The effect of cobalt stress on growth and physiological traits and its association with cobalt accumulation in barley genotypes differing in cobalt tolerance. J. Plant Nutr. 2017, 40, 2192–2199. [Google Scholar] [CrossRef]
- Wang, Y.-M.; Yang, Q.; Xu, H.; Liu, Y.-J.; Yang, H.-L. Physiological and transcriptomic analysis provide novel insight into cobalt stress responses in willow. Sci. Rep. 2020, 10, 2308. [Google Scholar] [CrossRef]
- Korshunova, Y.O.; Eide, D.; Gregg Clark, W.; Lou Guerinot, M.; Pakrasi, H.B. The IRT1 protein from Arabidopsis thaliana is a metal transporter with a broad substrate range. Plant Mol. Biol. 1999, 40, 37–44. [Google Scholar] [CrossRef]
- Gad, N.; El-Moez, M.R.A. Broccoli growth, yield quantity and quality as affected by cobalt nutrition. Agric. Biol. J. N. Am. 2011, 2, 226–231. [Google Scholar] [CrossRef]
- Kandil, H.; Farid, I.; El-Maghraby, A. Effect of Cobalt Level and Nitrogen Source on Quantity and Quality of Soybean Plant. J. Basic. Appl. Sci. Res. 2013, 3, 185–192. [Google Scholar]
- Trejo-Tapia, G.; Jimenez-Aparicio, A.; Rodriguez-Monroy, M.; De Jesus-Sanchez, A.; Gutierrez-Lopez, G. Influence of cobalt and other microelements on the production of betalains and the growth of suspension cultures of Beta vulgaris. Plant Cell Tissue Organ Cult. 2001, 67, 19–23. [Google Scholar] [CrossRef]
- Tosh, S.; Choudhuri, M.A.; Chatterjee, S.K. Retardation of lettuce (Lactuca sativa L.) leaf senescence by cobalt ions. Plant Physiol. Biochem. 1979, 17, 1134–1136. [Google Scholar]
- Abbas, M.; Magdy, M. Maximization of drought tolerance of bean plants using cobalt supplementation A-Growth, Yield and nutritional status. Middle East J. 2018, 7, 1819–1826. [Google Scholar]
- Cheruiyot, J.; Boyd, R.; Moar, W. Exploring Lower Limits of Plant Elemental Defense by Cobalt, Copper, Nickel, and Zinc. J. Chem. Ecol. 2013, 39, 666–674. [Google Scholar] [CrossRef]
- Gad, N. Increasing the efficiency of nitrogen fertilization through cobalt application to Pea plant. Res. J. Agric. Biol. Sci. 2006, 2, 433–442. [Google Scholar]
- Gad, N.; Sękara, A.; Abdelhamid, M. The Potential Role of Cobalt and/or Organic Fertilizers in Improving the Growth, Yield, and Nutritional Composition of Moringa oleifera. Agronomy 2019, 9, 862. [Google Scholar] [CrossRef]
- Hala, K. Effect of Cobalt Fertilizer on Growth, Yield and Nutrients Status of Faba Bean (Vicia faba L.) Plants. J. Appl. Sci. Res. 2007, 3, 867–872. [Google Scholar]
- Gad, N.; Kandil, H. Maximizing the Tolerance of Wheat Plants to Soil Salinity Using Cobalt 1-Growth and Mineral Composition. J. Appl. Sci. Res. 2011, 7, 1569–1574. [Google Scholar]
- Prasetyo, I.; Mukti, N.I.; Ariyanto, T. Ethylene Adsorption Using Cobalt Oxide-Loaded Polymer-Derived Nanoporous Carbon and Its Application to Extend Shelf Life of Fruit. Molecules 2019, 24, 1507. [Google Scholar] [CrossRef]
- Schiavon, M.; Pilon-Smits, E.A.H. Selenium Biofortification and Phytoremediation Phytotechnologies: A Review. J. Environ. Qual. 2017, 46, 10–19. [Google Scholar] [CrossRef]
- Yang, H.; Yang, X.; Ning, Z.; Kwon, S.Y.; Li, M.-L.; Tack, F.M.G.; Kwon, E.E.; Rinklebe, J.; Yin, R. The beneficial and hazardous effects of selenium on the health of the soil-plant-human system: An overview. J. Hazard. Mater. 2022, 422, 126876. [Google Scholar] [CrossRef]
- Puccinelli, M.; Malorgio, F.; Pezzarossa, B. Selenium Enrichment of Horticultural Crops. Molecules 2017, 22, 933. [Google Scholar] [CrossRef]
- Hartikainen, H. Biogeochemistry of selenium and its impact on food chain quality and human health. J. Trace Elem. Med. Biol. 2005, 18, 309–318. [Google Scholar] [CrossRef]
- Hanson, B.; Garifullina, G.F.; Lindblom, S.D.; Wangeline, A.; Ackley, A.; Kramer, K.; Norton, A.P.; Lawrence, C.B.; Pilon-Smits, E.A.H. Selenium accumulation protects Brassica juncea from invertebrate herbivory and fungal infection. New Phytol. 2003, 159, 461–469. [Google Scholar] [CrossRef]
- Eurola, M.; Ekholm, P.; Ylinen, M.; Koivistoinen, P.; Varo, P. Effects of Selenium Fertilization on the Selenium Content of Selected Finnish Fruits and Vegetables. Acta Agric. Scand. 1989, 39, 345–350. [Google Scholar] [CrossRef]
- Farzaneh, G.; Béla, K.; Éva, D.-S.; Szilvia, V. Biological changes of green pea (Pisum sativum L.) by selenium enrichment. Acta Biol. Hung. Acta Biol. Hung. 2017, 68, 60–72. [Google Scholar] [CrossRef][Green Version]
- Huang, Y.; Xu, J.; Hu, Q. Effect of Selenium on Preservation Quality of Green Tea during Autumn Tea-Processing Season. J. Agric. Food Chem. 2005, 53, 7444–7447. [Google Scholar] [CrossRef] [PubMed]
- Ngigi, P.B.; Lachat, C.; Masinde, P.W.; Du Laing, G. Agronomic biofortification of maize and beans in Kenya through selenium fertilization. Environ. Geochem. Health 2019, 41, 2577–2591. [Google Scholar] [CrossRef] [PubMed]
- Puccinelli, M.; Malorgio, F.; Rosellini, I.; Pezzarossa, B. Production of selenium-biofortified microgreens from selenium-enriched seeds of basil. J. Sci. Food Agric. 2019, 99, 5601–5605. [Google Scholar] [CrossRef]
- Poblaciones, M.J.; Rodrigo, S.; Santamaría, O.; Chen, Y.; McGrath, S.P. Agronomic selenium biofortification in Triticum durum under Mediterranean conditions: From grain to cooked pasta. Food Chem. 2014, 146, 378–384. [Google Scholar] [CrossRef]
- Thavarajah, D.; Ruszkowski, J.; Vandenberg, A. High Potential for Selenium Biofortification of Lentils (Lens culinaris L.). J. Agric. Food Chem. 2008, 56, 10747–10753. [Google Scholar] [CrossRef]
- Lavu, R.V.S.; Du Laing, G.; Van De Wiele, T.; Pratti, V.L.; Willekens, K.; Vandecasteele, B.; Tack, F. Fertilizing Soil with Selenium Fertilizers: Impact on Concentration, Speciation, and Bioaccessibility of Selenium in Leek (Allium ampeloprasum). J. Agric. Food Chem. 2012, 60, 10930–10935. [Google Scholar] [CrossRef]
- Hawkesford, M.J.; Zhao, F.-J. Strategies for increasing the selenium content of wheat. J. Cereal Sci. 2007, 46, 282–292. [Google Scholar] [CrossRef]
- Dhillon, K.; Dhillon, S.K. Distribution and Management of Seleniferous Soils. Adv. Agron. 2003, 79, 119–184. [Google Scholar] [CrossRef]
- Terry, N.; Zayed, A.M.; de Souza, M.P.; Tarun, A.S. Selenium in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 401–432. [Google Scholar] [CrossRef] [PubMed]
- Bocchini, M.; D’Amato, R.; Ciancaleoni, S.; Fontanella, M.C.; Palmerini, C.A.; Beone, G.M.; Onofri, A.; Negri, V.; Marconi, G.; Albertini, E.; et al. Soil Selenium (Se) Biofortification Changes the Physiological, Biochemical and Epigenetic Responses to Water Stress in Zea mays L. by Inducing a Higher Drought Tolerance. Front. Plant Sci. 2018, 9, 389. [Google Scholar] [CrossRef] [PubMed]
- Proietti, P.; Nasini, L.; Del Buono, D.; D’Amato, R.; Tedeschini, E.; Businelli, D. Selenium protects olive (Olea europaea L.) from drought stress. Sci. Hortic. 2013, 164, 165–171. [Google Scholar] [CrossRef]
- Balal, R.; Shahid, M.; Javaid, M.; Anjum, M.; Ali, H.H.; Mattson, N.; García-Sánchez, F. Foliar treatment with Lolium perenne (Poaceae) leaf extract alleviates salinity and nickel-induced growth inhibition in pea. Braz. J. Bot. 2016, 39, 453–463. [Google Scholar] [CrossRef]
- Yao, X.; Chu, J.; Wang, G. Effects of Selenium on Wheat Seedlings Under Drought Stress. Biol. Trace Elem. Res. 2009, 130, 283–290. [Google Scholar] [CrossRef]
- Quinn, C.F.; Freeman, J.L.; Reynolds, R.J.B.; Cappa, J.J.; Fakra, S.C.; Marcus, M.A.; Lindblom, S.D.; Quinn, E.K.; Bennett, L.E.; Pilon-Smits, E.A.H. Selenium hyperaccumulation offers protection from cell disruptor herbivores. BMC Ecol. 2010, 10, 19. [Google Scholar] [CrossRef]
- El Mehdawi, A.F.; Pilon-Smits, E.A.H. Ecological aspects of plant selenium hyperaccumulation. Plant Biol. 2012, 14, 1–10. [Google Scholar] [CrossRef]
- Quinn, C.F.; Freeman, J.L.; Galeas, M.L.; Klamper, E.M.; Pilon-Smits, E.A.H. The role of selenium in protecting plants against prairie dog herbivory: Implications for the evolution of selenium hyperaccumulation. Oecologia 2008, 155, 267–275. [Google Scholar] [CrossRef]
- Andrade, F.R.; da Silva, G.N.; Guimarães, K.C.; Barreto, H.B.F.; de Souza, K.R.D.; Guilherme, L.R.G.; Faquin, V.; Reis, A.R.d. Selenium protects rice plants from water deficit stress. Ecotoxicol. Environ. Saf. 2018, 164, 562–570. [Google Scholar] [CrossRef]
- Alfthan, G.; Eurola, M.; Ekholm, P.; Venäläinen, E.R.; Root, T.; Korkalainen, K.; Hartikainen, H.; Salminen, P.; Hietaniemi, V.; Aspila, P.; et al. Effects of nationwide addition of selenium to fertilizers on foods, and animal and human health in Finland: From deficiency to optimal selenium status of the population. J. Trace Elem. Med. Biol. 2015, 31, 142–147. [Google Scholar] [CrossRef]
- González-Morales, S.; Pérez-Labrada, F.; García-Enciso, E.L.; Leija-Martínez, P.; Medrano-Macías, J.; Dávila-Rangel, I.E.; Juárez-Maldonado, A.; Rivas-Martínez, E.N.; Benavides-Mendoza, A. Selenium and Sulfur to Produce Allium Functional Crops. Molecules 2017, 22, 558. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Sharma, N.; Sharma, S.; Kapoor, P.; Kumar, A.; Ch, V.; Arora, P. Biofortified Crops Generated by Breeding, Agronomy, and Transgenic Approaches Are Improving Lives of Millions of People around the World. Front. Nutr. 2018, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Bañuelos, G.S.; Lin, Z.Q.; Liu, Y.; Yuan, L.; Yin, X.; Li, M. Biofortification and phytoremediation of selenium in China. Front.Plant Sci. 2015, 6, 136. [Google Scholar] [CrossRef] [PubMed]
- Haug, A.; Graham, R.D.; Christophersen, O.A.; Lyons, G.H. How to use the world’s scarce selenium resources efficiently to increase the selenium concentration in food. Microb. Ecol. Health Dis. 2007, 19, 209–228. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets—Iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Wu, L. Review of 15 years of research on ecotoxicology and remediation of land contaminated by agricultural drainage sediment rich in selenium. Ecotoxicol. Environ. Saf. 2004, 57, 257–269. [Google Scholar] [CrossRef]
- Lin, Z.-Q.; Terry, N. Selenium Removal by Constructed Wetlands: Quantitative Importance of Biological Volatilization in the Treatment of Selenium-Laden Agricultural Drainage Water. Environ. Sci. Technol. 2003, 37, 606–615. [Google Scholar] [CrossRef]
- Bañuelos, G.S.; Arroyo, I.; Pickering, I.J.; Yang, S.I.; Freeman, J.L. Selenium biofortification of broccoli and carrots grown in soil amended with Se-enriched hyperaccumulator Stanleya pinnata. Food Chem. 2015, 166, 603–608. [Google Scholar] [CrossRef]
- Peralta-Videa, J.R.; Lopez, M.L.; Narayan, M.; Saupe, G.; Gardea-Torresdey, J. The biochemistry of environmental heavy metal uptake by plants: Implications for the food chain. Int. J. Biochem. Cell Biol. 2009, 41, 1665–1677. [Google Scholar] [CrossRef]
- Zembala, M.; Filek, M.; Walas, S.; Mrowiec, H.; Kornaś, A.; Miszalski, Z.; Hartikainen, H. Effect of selenium on macro- and microelement distribution and physiological parameters of rape and wheat seedlings exposed to cadmium stress. Plant Soil 2010, 329, 457–468. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, S.; Huang, X.; Zhang, F.; Pang, Y.; Huang, Q.; Yi, Q. Selenium uptake, dynamic changes in selenium content and its influence on photosynthesis and chlorophyll fluorescence in rice (Oryza sativa L.). Environ. Exp. Bot. 2014, 107, 39–45. [Google Scholar] [CrossRef]
- Feng, T.; Chen, S.S.; Gao, D.Q.; Liu, G.Q.; Bai, H.X.; Li, A.; Peng, L.X.; Ren, Z.Y. Selenium improves photosynthesis and protects photosystem II in pear (Pyrus bretschneideri), grape (Vitis vinifera), and peach (Prunus persica). Photosynthetica 2015, 53, 609–612. [Google Scholar] [CrossRef]
- Alyemeni, M.N.; Ahanger, M.A.; Wijaya, L.; Alam, P.; Bhardwaj, R.; Ahmad, P. Selenium mitigates cadmium-induced oxidative stress in tomato (Solanum lycopersicum L.) plants by modulating chlorophyll fluorescence, osmolyte accumulation, and antioxidant system. Protoplasma 2018, 255, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Ismael, M.A.; Elyamine, A.M.; Moussa, M.G.; Cai, M.; Zhao, X.; Hu, C. Cadmium in plants: Uptake, toxicity, and its interactions with selenium fertilizers. Met. Integr. Biometal Sci. 2019, 11, 255–277. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Zia-ur-Rehman, M.; Rinklebe, J.; Tsang, D.; Tack, F.; Abbasi, G.; Hussain, A.; Igalavithana, A.; Lee, B.C.; et al. Effects of selenium on the uptake of toxic trace elements by crop plants: A review. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2531–2566. [Google Scholar] [CrossRef]
- Mandryk, M.; Reidsma, P.; van Ittersum, M.K. Scenarios of long-term farm structural change for application in climate change impact assessment. Landsc. Ecol. 2012, 27, 509–527. [Google Scholar] [CrossRef]
- Cartes, P.; Jara, A.A.; Pinilla, L.; Rosas, A.; Mora, M.L. Selenium improves the antioxidant ability against aluminium-induced oxidative stress in ryegrass roots. Ann. Appl. Biol. 2010, 156, 297–307. [Google Scholar] [CrossRef]
- Luyckx, M.; Hausman, J.-F.; Lutts, S.; Guerriero, G. Silicon and Plants: Current Knowledge and Technological Perspectives. Front. Plant Sci. 2017, 8, 411. [Google Scholar] [CrossRef]
- Epstein, E. The anomaly of silicon in plant biology. Proc. Natl. Acad. Sci. USA 1994, 91, 11. [Google Scholar] [CrossRef]
- Frew, A.; Weston, L.A.; Reynolds, O.L.; Gurr, G.M. The role of silicon in plant biology: A paradigm shift in research approach. Ann. Bot. 2018, 121, 1265–1273. [Google Scholar] [CrossRef]
- Ranjan, A.; Sinha, R.; Bala, M.; Pareek, A.; Singla-Pareek, S.L.; Singh, A.K. Silicon-mediated abiotic and biotic stress mitigation in plants: Underlying mechanisms and potential for stress resilient agriculture. Plant Physiol. Biochem. 2021, 163, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, J.; Kostic, L.; Bosnic, P.; Kirkby, E.A.; Nikolic, M. Interactions of Silicon with Essential and Beneficial Elements in Plants. Front. Plant Sci. 2021, 12, 1224. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Réthoré, E.; Yvin, J.-C.; Hosseini, S.A. The Regulatory Role of Silicon in Mitigating Plant Nutritional Stresses. Plants 2020, 9, 1779. [Google Scholar] [CrossRef]
- Rastogi, A.; Tripathi, D.K.; Yadav, S.; Chauhan, D.K.; Živčák, M.; Ghorbanpour, M.; El-Sheery, N.I.; Brestic, M. Application of silicon nanoparticles in agriculture. 3 Biotech 2019, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Zargar, S.M.; Mahajan, R.; Bhat, J.A.; Nazir, M.; Deshmukh, R. Role of silicon in plant stress tolerance: Opportunities to achieve a sustainable cropping system. 3 Biotech 2019, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Lavinsky, A.O.; Detmann, K.C.; Reis, J.V.; Ávila, R.T.; Sanglard, M.L.; Pereira, L.F.; Sanglard, L.M.V.P.; Rodrigues, F.A.; Araújo, W.L.; DaMatta, F.M. Silicon improves rice grain yield and photosynthesis specifically when supplied during the reproductive growth stage. J. Plant Physiol. 2016, 206, 125–132. [Google Scholar] [CrossRef]
- Marxen, A.; Klotzbücher, T.; Jahn, R.; Kaiser, K.; Nguyen, V.S.; Schmidt, A.; Schädler, M.; Vetterlein, D. Interaction between silicon cycling and straw decomposition in a silicon deficient rice production system. Plant Soil 2016, 398, 153–163. [Google Scholar] [CrossRef]
- Flam-Shepherd, R.; Huynh, W.Q.; Coskun, D.; Hamam, A.M.; Britto, D.T.; Kronzucker, H.J. Membrane fluxes, bypass flows, and sodium stress in rice: The influence of silicon. J. Exp. Bot. 2018, 69, 1679–1692. [Google Scholar] [CrossRef]
- Ali, S.; Farooq, M.A.; Yasmeen, T.; Hussain, S.; Arif, M.S.; Abbas, F.; Bharwana, S.A.; Zhang, G. The influence of silicon on barley growth, photosynthesis and ultra-structure under chromium stress. Ecotoxicol. Environ. Saf. 2013, 89, 66–72. [Google Scholar] [CrossRef]
- Song, A.; Li, P.; Fan, F.; Li, Z.; Liang, Y. The Effect of Silicon on Photosynthesis and Expression of Its Relevant Genes in Rice (Oryza sativa L.) under High-Zinc Stress. PLoS ONE 2014, 9, e113782. [Google Scholar] [CrossRef]
- Xie, Z.; Song, R.; Shao, H.; Song, F.; Xu, H.; Lu, Y. Silicon improves maize photosynthesis in saline-alkaline soils. Sci. World J. 2015, 2015, 245072. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Apaolaza, L. Can silicon partially alleviate micronutrient deficiency in plants? A review. Planta 2014, 240, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Pontigo, S.; Ribera, A.; Gianfreda, L.; de la Luz Mora, M.; Nikolic, M.; Cartes, P. Silicon in vascular plants: Uptake, transport and its influence on mineral stress under acidic conditions. Planta 2015, 242, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Yamaji, N.; Shao, J.F.; Ma, J.F.; Shen, R.F. Silicon decreases both uptake and root-to-shoot translocation of manganese in rice. J. Exp. Bot. 2016, 67, 1535–1544. [Google Scholar] [CrossRef]
- Calero Hurtado, A.; Aparecida Chiconato, D.; de Mello Prado, R.; da Silveira Sousa Junior, G.; Felisberto, G. Silicon attenuates sodium toxicity by improving nutritional efficiency in sorghum and sunflower plants. Plant Physiol. Biochem. 2019, 142, 224–233. [Google Scholar] [CrossRef]
- Bowen, P.; Menzies, J.; Ehret, D.; Samuels, L.; Glass, A.D.M. Soluble Silicon Sprays Inhibit Powdery Mildew Development on Grape Leaves. J. Am. Soc. Hortic. Sci. Jashs 1992, 117, 906–912. [Google Scholar] [CrossRef]
- Mandlik, R.; Thakral, V.; Raturi, G.; Shinde, S.; Nikolić, M.; Tripathi, D.K.; Sonah, H.; Deshmukh, R. Significance of silicon uptake, transport, and deposition in plants. J. Exp. Bot. 2020, 71, 6703–6718. [Google Scholar] [CrossRef]
- Wei, G.; Zhu, Z.; Li, J.; Yao, Q. Effects of silicon supply and Sphaerotheca fuliginea inoculation on resistance of cucumber seedlings against powdery mildew. J. Appl. Ecol. 2004, 15, 2147–2151. [Google Scholar]
- Dallagnol, L.J.; Rodrigues, F.A.; DaMatta, F.M.; Mielli, M.V.B.; Pereira, S.C. Deficiency in Silicon Uptake Affects Cytological, Physiological, and Biochemical Events in the Rice–Bipolaris oryzae Interaction. Phytopathology 2011, 101, 92–104. [Google Scholar] [CrossRef]
- Kauss, H.; Seehaus, K.; Franke, R.; Gilbert, S.; Dietrich, R.A.; Kröger, N. Silica deposition by a strongly cationic proline-rich protein from systemically resistant cucumber plants. Plant J. 2003, 33, 87–95. [Google Scholar] [CrossRef]
- Manivannan, A.; Ahn, Y.K. Silicon Regulates Potential Genes Involved in Major Physiological Processes in Plants to Combat Stress. Front. Plant Sci. 2017, 8, 1346. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Wang, S.; Li, J.; Tanaka, K.; Oka, M. Application of silicon improves salt tolerance through ameliorating osmotic and ionic stresses in the seedling of Sorghum bicolor. Acta Physiol. Plant. 2013, 35, 3099–3107. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Xu, X.B.; Hu, Y.H.; Han, W.H.; Yin, J.L.; Li, H.L.; Gong, H.J. Silicon improves salt tolerance by increasing root water uptake in Cucumis sativus L. Plant Cell Rep. 2015, 34, 1629–1646. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, Y.; Han, W.; Feng, R.; Hu, Y.; Guo, J.; Gong, H. Silicon Enhances Water Stress Tolerance by Improving Root Hydraulic Conductance in Solanum lycopersicum L. Front. Plant Sci. 2016, 7, 196. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Khan, A.L.; Waqas, M.; Lee, I.-J. Silicon Regulates Antioxidant Activities of Crop Plants under Abiotic-Induced Oxidative Stress: A Review. Front. Plant Sci. 2017, 8, 510. [Google Scholar] [CrossRef]
- Lekklar, C.; Pongpanich, M.; Suriya-arunroj, D.; Chinpongpanich, A.; Tsai, H.; Comai, L.; Chadchawan, S.; Buaboocha, T. Genome-wide association study for salinity tolerance at the flowering stage in a panel of rice accessions from Thailand. BMC Genom. 2019, 20, 76. [Google Scholar] [CrossRef]
- Gao, X.; Zou, C.; Wang, L.; Zhang, F. Silicon Decreases Transpiration Rate and Conductance from Stomata of Maize Plants. J. Plant Nutr. 2006, 29, 1637–1647. [Google Scholar] [CrossRef]
- Liu, P.; Yin, L.; Deng, X.; Wang, S.; Tanaka, K.; Zhang, S. Aquaporin-mediated increase in root hydraulic conductance is involved in silicon-induced improved root water uptake under osmotic stress in Sorghum bicolor L. J. Exp. Bot. 2014, 65, 4747–4756. [Google Scholar] [CrossRef]
- Kang, J.; Zhao, W.; Zhu, X. Silicon improves photosynthesis and strengthens enzyme activities in the C3 succulent xerophyte Zygophyllum xanthoxylum under drought stress. J. Plant Physiol. 2016, 199, 76–86. [Google Scholar] [CrossRef]
- Alzahrani, Y.; Kusvuran, A.; Alharby, H.; Kusvuran, S.; Rady, M. The defensive role of silicon in wheat against stress conditions induced by drought, salinity or cadmium. Ecotoxicol. Environ. Saf. 2018, 154, 187–196. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, Y.; Gong, H.-J.; Zhao, H.-L.; Li, H.-L.; Hu, Y.-H.; Wang, Y.-C. Beneficial effects of silicon on photosynthesis of tomato seedlings under water stress. J. Integr. Agric. 2018, 17, 2151–2159. [Google Scholar] [CrossRef]
- Vaculík, M.; Lux, A.; Luxová, M.; Tanimoto, E.; Lichtscheidl, I. Silicon mitigates cadmium inhibitory effects in young maize plants. Environ. Exp. Bot. 2009, 67, 52–58. [Google Scholar] [CrossRef]
- Guo, B.; Liu, C.; Ding, N.; Fu, Q.; Lin, Y.; Li, H.; Li, N. Silicon Alleviates Cadmium Toxicity in Two Cypress Varieties by Strengthening the Exodermis Tissues and Stimulating Phenolic Exudation of Roots. J. Plant Growth Regul. 2016, 35, 420–429. [Google Scholar] [CrossRef]
- Wu, J.; Mock, H.-P.; Giehl, R.F.H.; Pitann, B.; Mühling, K.H. Silicon decreases cadmium concentrations by modulating root endodermal suberin development in wheat plants. J. Hazard. Mater. 2019, 364, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Keller, C.; Rizwan, M.; Davidian, J.C.; Pokrovsky, O.S.; Bovet, N.; Chaurand, P.; Meunier, J.D. Effect of silicon on wheat seedlings (Triticum turgidum L.) grown in hydroponics and exposed to 0 to 30 µM Cu. Planta 2015, 241, 847–860. [Google Scholar] [CrossRef]
- Bosnić, D.; Nikolić, D.; Timotijević, G.; Pavlović, J.; Vaculík, M.; Samardžić, J.; Nikolić, M. Silicon alleviates copper (Cu) toxicity in cucumber by increased Cu-binding capacity. Plant Soil 2019, 441, 629–641. [Google Scholar] [CrossRef]
- Farooq, M.A.; Detterbeck, A.; Clemens, S.; Dietz, K.-J. Silicon-induced reversibility of cadmium toxicity in rice. J. Exp. Bot. 2016, 67, 3573–3585. [Google Scholar] [CrossRef]
- Chen, D.; Chen, D.; Xue, R.; Long, J.; Lin, X.; Lin, Y.; Jia, L.; Zeng, R.; Song, Y. Effects of boron, silicon and their interactions on cadmium accumulation and toxicity in rice plants. J. Hazard. Mater. 2019, 367, 447–455. [Google Scholar] [CrossRef]
- Hossain, M.M.; Khatun, M.A.; Haque, M.N.; Bari, M.A.; Alam, M.F.; Mandal, A.; Kabir, A.H. Silicon alleviates arsenic-induced toxicity in wheat through vacuolar sequestration and ROS scavenging. Int. J. Phytoremediat. 2018, 20, 796–804. [Google Scholar] [CrossRef]
- Gu, H.-H.; Qiu, H.; Tian, T.; Zhan, S.-S.; Deng, T.-H.-B.; Chaney, R.L.; Wang, S.-Z.; Tang, Y.-T.; Morel, J.-L.; Qiu, R.-L. Mitigation effects of silicon rich amendments on heavy metal accumulation in rice (Oryza sativa L.) planted on multi-metal contaminated acidic soil. Chemosphere 2011, 83, 1234–1240. [Google Scholar] [CrossRef]
- Rizwan, M.; Meunier, J.-D.; Miche, H.; Keller, C. Effect of silicon on reducing cadmium toxicity in durum wheat (Triticum turgidum L. cv. Claudio W.) grown in a soil with aged contamination. J. Hazard. Mater. 2012, 209–210, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Feng Shao, J.; Che, J.; Yamaji, N.; Fang Shen, R.; Feng Ma, J. Silicon reduces cadmium accumulation by suppressing expression of transporter genes involved in cadmium uptake and translocation in rice. J. Exp. Bot. 2017, 68, 5641–5651. [Google Scholar] [CrossRef] [PubMed]
- Howladar, S.M.; Al-Robai, S.A.; Al-Zahrani, F.S.; Howladar, M.M.; Aldhebiani, A.Y. Silicon and its application method effects on modulation of cadmium stress responses in Triticum aestivum (L.) through improving the antioxidative defense system and polyamine gene expression. Ecotoxicol. Environ. Saf. 2018, 159, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Ujiie, K.; Ishimaru, K.; Hirotsu, N.; Nagasaka, S.; Miyakoshi, Y.; Ota, M.; Tokida, T.; Sakai, H.; Usui, Y.; Ono, K.; et al. How elevated CO2 affects our nutrition in rice, and how we can deal with it. PLoS ONE 2019, 14, e0212840. [Google Scholar] [CrossRef]
- Johnson, S.N.; Ryalls, J.M.W.; Gherlenda, A.N.; Frew, A.; Hartley, S.E. Benefits from Below: Silicon Supplementation Maintains Legume Productivity under Predicted Climate Change Scenarios. Front. Plant Sci. 2018, 9, 202. [Google Scholar] [CrossRef]
- Li, Z.; Song, Z.; Singh, B.P.; Wang, H. The impact of crop residue biochars on silicon and nutrient cycles in croplands. Science of Total Environ. 2019, 659, 673–680. [Google Scholar] [CrossRef]
- Coskun, D.; Deshmukh, R.; Sonah, H.; Menzies, J.G.; Reynolds, O.; Ma, J.F.; Kronzucker, H.J.; Bélanger, R.R. The controversies of silicon’s role in plant biology. New Phytol. 2019, 221, 67–85. [Google Scholar] [CrossRef]
- Rastogi, A.; Yadav, S.; Hussain, S.; Kataria, S.; Hajihashemi, S.; Kumari, P.; Yang, X.; Brestic, M. Does silicon really matter for the photosynthetic machinery in plants…? Plant Physiol. Biochem. 2021, 169, 40–48. [Google Scholar] [CrossRef]
- Choudri, B.S.; Charabi, Y.; Al-Nasiri, N.; Al-Awadhi, T. Pesticides and herbicides. Water Environ. Res. 2020, 92, 1425–1432. [Google Scholar] [CrossRef]
- Sathe, A.P.; Kumar, A.; Mandlik, R.; Raturi, G.; Yadav, H.; Kumar, N.; Shivaraj, S.M.; Jaswal, R.; Kapoor, R.; Gupta, S.K.; et al. Role of silicon in elevating resistance against sheath blight and blast diseases in rice (Oryza sativa L.). Plant Physiol. Biochem. 2021, 166, 128–139. [Google Scholar] [CrossRef]
- Liu, B.; Davies, K.; Hall, A. Silicon builds resilience in strawberry plants against both strawberry powdery mildew Podosphaera aphanis and two-spotted spider mites Tetranychus urticae. PLoS ONE 2020, 15, e0241151. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.U.P.; Rios, J.A.; Silva, E.T.; Rodrigues, F.Á. Silicon Alleviates Changes in the Source-Sink Relationship of Wheat Plants Infected by Pyricularia oryzae. Phytopathology 2019, 109, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Pazdiora, P.C.; da Rosa Dorneles, K.; Morello, T.N.; Nicholson, P.; Dallagnol, L.J. Silicon soil amendment as a complement to manage tan spot and fusarium head blight in wheat. Agron. Sustain. Dev. 2021, 41, 21. [Google Scholar] [CrossRef]
- Zhu, Y.; Gong, H. Beneficial effects of silicon on salt and drought tolerance in plants. Agron. Sustain. Dev. 2013, 34, 455–472. [Google Scholar] [CrossRef]
- Chen, D.; Wang, S.; Yin, L.; Deng, X. How Does Silicon Mediate Plant Water Uptake and Loss Under Water Deficiency? Front. Plant Sci. 2018, 9, 281. [Google Scholar] [CrossRef]
- Bokor, B.; Santos, C.; Kostoláni, D.; Machado, J.; Nunes da Silva, M.; Carvalho, S.; Vaculík, M.; Vasconcelos, M. Mitigation of climate change and environmental hazards in plants: Potential role of beneficial metalloid silicon. J. Hazard. Mater. 2021, 416, 126193. [Google Scholar] [CrossRef]
- Wang, M.; Wang, R.; Mur, L.; Ruan, J.Y.; Shen, Q.; Guo, S. Functions of silicon in plant drought stress responses. Hortic. Res. 2021, 8, 254. [Google Scholar] [CrossRef]
- Khan, I.; Awan, S.A.; Rizwan, M.; Ali, S.; Hassan, M.J.; Brestic, M.; Zhang, X.; Huang, L. Effects of silicon on heavy metal uptake at the soil-plant interphase: A review. Ecotoxicol. Environ. Saf. 2021, 222, 112510. [Google Scholar] [CrossRef]
- Oliveira, K.R.; Souza Junior, J.P.; Bennett, S.J.; Checchio, M.V.; Alves, R.d.C.; Felisberto, G.; Prado, R.d.M.; Gratão, P.L. Exogenous silicon and salicylic acid applications improve tolerance to boron toxicity in field pea cultivars by intensifying antioxidant defence systems. Ecotoxicol. Environ. Saf. 2020, 201, 110778. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Xie, Y.; Sangari, S. Silicon Mechanisms to Ameliorate Heavy Metal Stress in Plants. BioMed Res. Int. 2018, 2018, 8492898. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Alam, M.M.; Nahar, K.; Mohsin, S.M.; Bhuyan, M.H.M.B.; Parvin, K.; Hawrylak-Nowak, B.; Fujita, M. Silicon-induced antioxidant defense and methylglyoxal detoxification works coordinately in alleviating nickel toxicity in Oryza sativa L. Ecotoxicology 2019, 28, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Adrees, M.; Ali, S.; Rizwan, M.; Zia-Ur-Rehman, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Qayyum, M.F.; Irshad, M.K. Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: A review. Ecotoxicol. Environ. Saf. 2015, 119, 186–197. [Google Scholar] [CrossRef]
- Brownell, P.F. Sodium as an Essential Micronutrient Element for Plants and its Possible Role in Metabolism. In Advances in Botanical Research; Woolhouse, H.W., Ed.; Academic Press: Cambridge, MA, USA, 1980; Volume 7, pp. 117–224. [Google Scholar]
- Subbarao, G.V.; Ito, O.; Berry, W.L.; Wheeler, R.M. Sodium—A Functional Plant Nutrient. Crit. Rev. Plant Sci. 2003, 22, 391–416. [Google Scholar] [CrossRef]
- Almeida, D.M.; Oliveira, M.M.; Saibo, N.J.M. Regulation of Na+ and K+ homeostasis in plants: Towards improved salt stress tolerance in crop plants. Genet. Mol. Biol. 2017, 40, 326–345. [Google Scholar] [CrossRef] [PubMed]
- Nieves-Cordones, M.; Al-Burki, F.; Sentenac, H. Roles and Transport of Sodium and Potassium in Plants. In The Alkali Metal Ions: Thier Role for Life; Springer: New York, NY, USA, 2016; pp. 291–324. [Google Scholar]
- Larson, W.E.; Pierre, W.H. Interaction of sodium and potassium on yield and cation composition of selected crops. Soil Sci. Soc. Am. J. 1953, 76, 51–64. [Google Scholar] [CrossRef]
- Lehr, J.J. Sodium as a plant nutrient. J. Sci. Food Agric. 1953, 4, 460–471. [Google Scholar] [CrossRef]
- Ali, L.; Rahmatullah; Maqsood, M.A.; Kanwal, S.; Ashraf, M.; Hannan, A. Potassium Substitution by Sodium in Root Medium Influencing Growth Behavior and Potassium Efficiency in Cotton Genotypes. J. Plant Nutr. 2009, 32, 1657–1673. [Google Scholar] [CrossRef]
- Wakeel, A.; Abd-El-Motagally, F.; Steffens, D.; Schubert, S. Sodium-induced calcium deficiency in sugar beet during substitution of potassium by sodium. J. Plant Nutr. Soil Sci. 2009, 172, 254–260. [Google Scholar] [CrossRef]
- Wakeel, A.; Steffens, D.; Schubert, S. Potassium substitution by sodium in sugar beet (Beta vulgaris) nutrition on K-fixing soils. J. Plant Nutr. Soil Sci. 2010, 173, 127–134. [Google Scholar] [CrossRef]
- Maathuis, F.J.M. Sodium in plants: Perception, signalling, and regulation of sodium fluxes. J. Exp. Bot. 2013, 65, 849–858. [Google Scholar] [CrossRef]
- Cui, Y.N.; Li, X.T.; Yuan, J.Z.; Wang, F.Z.; Wang, S.M.; Ma, Q. Nitrate transporter NPF7.3/NRT1.5 plays an essential role in regulating phosphate deficiency responses in Arabidopsis. Biochem. Biophys. Res. Commun. 2019, 508, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Horie, T.; Costa, A.; Kim, T.H.; Han, M.J.; Horie, R.; Leung, H.-Y.; Miyao, A.; Hirochika, H.; An, G.; Schroeder, J.I. Rice OsHKT2;1 transporter mediates large Na+ influx component into K+-starved roots for growth. EMBO J. 2007, 26, 3003–3014. [Google Scholar] [CrossRef] [PubMed]
- Erel, R.; Ben-Gal, A.; Dag, A.; Schwartz, A.; Yermiyahu, U. Sodium replacement of potassium in physiological processes of olive trees (var. Barnea) as affected by drought. Tree Physiol. 2014, 34, 1102–1117. [Google Scholar] [CrossRef] [PubMed]
- Hampe, T.; Marschner, H. Effect of Sodium on Morphology, Water Relations and Net Photosynthesis of Sugar Beet Leaves. Z. Für Pflanzenphysiol. 1982, 108, 151–162. [Google Scholar] [CrossRef]
- Wakeel, A.; Farooq, M.; Qadir, M.; Schubert, S. Potassium Substitution by Sodium in Plants. Crit. Rev. Plant Sci. 2011, 30, 401–413. [Google Scholar] [CrossRef]

| Plant Species | Se Supply | Se Amount Supplemented/Se Amount Present * | Application Method | Effects | Reference | |
|---|---|---|---|---|---|---|
| Green Fertilizer | Triticum aestivum, Secale cereale | Na₂SeO₃ | 10 ppm | Soil fertilization | ↑ bioavailability of Se in the final yield | [108] |
| Pisum sativum L. | 3 ppm | Foliar fertilization | ↑ photosynthetic ability; ↓ lipid peroxidation rate | [109] | ||
| Camellia sinensis | 150 ppm | Foliar fertilization | ↑ Vit C and plant antioxidant activity | [110] | ||
| Phaseolus vulgaris Zea mays | 10 and 31 g ha−1 | Foliar fertilization | ↑ bioavailability of Se in the final yield | [111] | ||
| Ocimum basilicum L. | 8 ppm | Foliar fertilization | ↑ number of germinated seeds and longer roots | [112] | ||
| Biofortification | Triticum durum | Na2SeO4 | 40 g ha−1 | Foliar fertilization | Uptake avg: 1.540–5.532 ppm DW (SeMet) | [113] |
| Lens culinaris L. | SeO₃ and SeO4 | 0.037 to 0.301 ppm * | Natural soil Se concentration | Uptake avg: 0.425–0.673 ppm DW (SeMet) | [114] | |
| Allium porrum | SeO₃ | 3.8 ppm | Soil fertilization | Uptake avg: 0.167 ppm DW (SeMet) and 0.068 ppm DW (MeSeCys) | [115] | |
| Triticum aestivum | SeO4 | 10 g ha−1 | Soil fertilization | Uptake avg: >0.100 ppm DW (SeMet) | [116] | |
| Ocimum basilicum L. | SeO₃ | 8 ppm | Foliar fertilization | Uptake avg: 0.203 ppm DW (SeMet) | [112] | |
| Phytoremediation | Oryza sativa | SeO4 | 0.25–4.55 ppm * | Soil fertilization | ↑ water pH from 29 to 92% | [117] |
| Oryza sativa, Brassica oleracea | 20 µM | Volatilization | ↑ volatilization of SeO4 and SeO₃ by rhizosphere bacteria | [118] | ||
| Lolium perenne | Na₂SeO₃ | 150 ppm | Soil fertilization | ↑ aerial part weight and plant development by 59% and 27% respectively | [119] | |
| Olea europaea L. | Na2SeO4 | 150 ppm | Foliar fertilization | ↑ fruit yield, photosynthesis and leaf water level | [120] | |
| Cucumis sativus L. | Na2SeO4 | 8 µM | Foliar fertilization | ↓ membrane damage; ↑ plant biomass and fruit yield | [121] | |
| Triticum aestivum L. | Na₂SeO₃ | 1–2 ppm | Foliar fertilization | ↑ biomass and root activity, ↑ proline content and peroxidase and catalase activity | [122] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes da Silva, M.; Machado, J.; Osorio, J.; Duarte, R.; Santos, C.S. Non-Essential Elements and Their Role in Sustainable Agriculture. Agronomy 2022, 12, 888. https://doi.org/10.3390/agronomy12040888
Nunes da Silva M, Machado J, Osorio J, Duarte R, Santos CS. Non-Essential Elements and Their Role in Sustainable Agriculture. Agronomy. 2022; 12(4):888. https://doi.org/10.3390/agronomy12040888
Chicago/Turabian StyleNunes da Silva, Marta, Joana Machado, Jazmin Osorio, Rafael Duarte, and Carla S. Santos. 2022. "Non-Essential Elements and Their Role in Sustainable Agriculture" Agronomy 12, no. 4: 888. https://doi.org/10.3390/agronomy12040888
APA StyleNunes da Silva, M., Machado, J., Osorio, J., Duarte, R., & Santos, C. S. (2022). Non-Essential Elements and Their Role in Sustainable Agriculture. Agronomy, 12(4), 888. https://doi.org/10.3390/agronomy12040888










