A Comprehensive Literature Review on Cadmium (Cd) Status in the Soil Environment and Its Immobilization by Biochar-Based Materials
Abstract
:1. Introduction
2. Literature Search
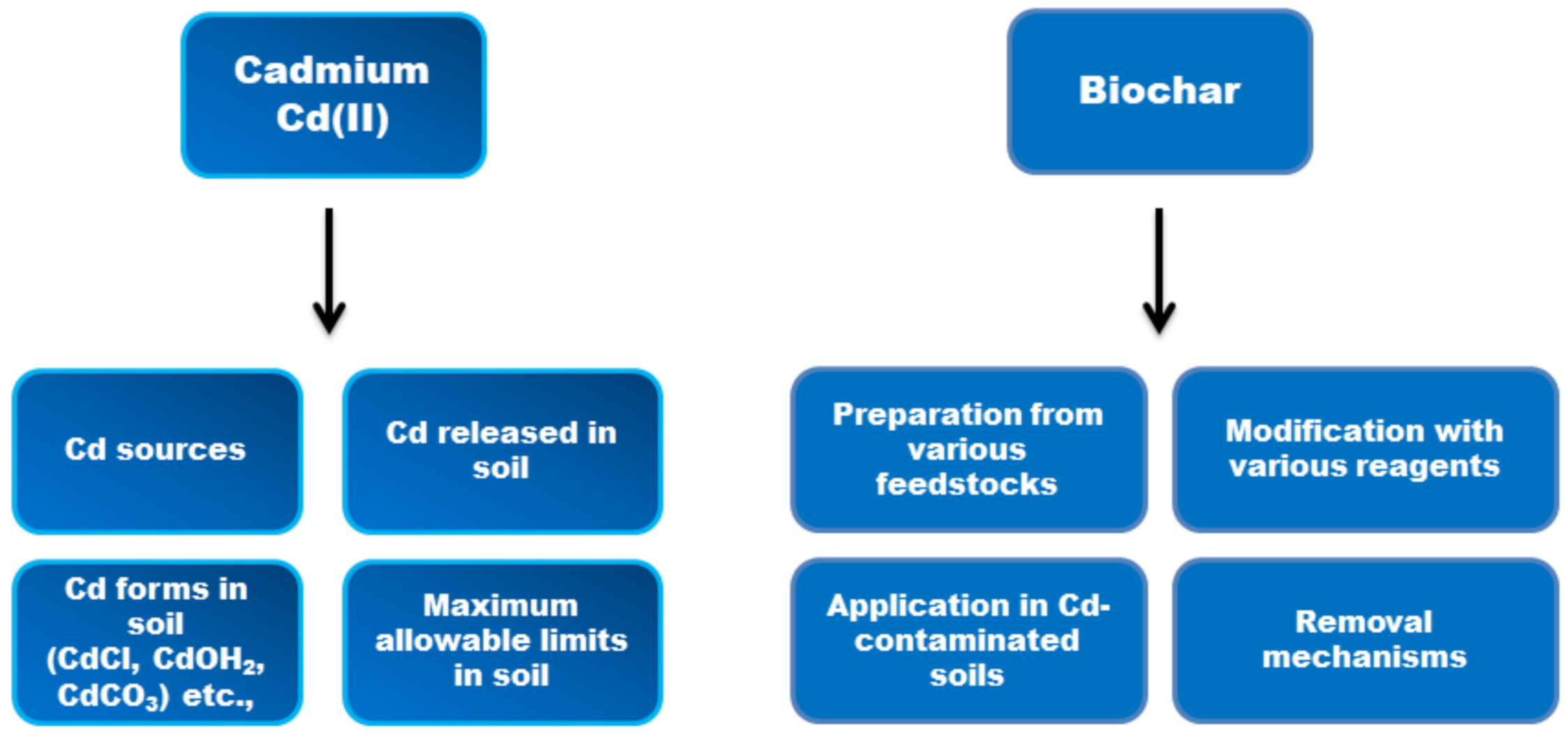
3. Cadmium (Cd) in the Soil Environment
3.1. Chemical Forms of Cd in Soil
3.2. Sources of Cd in Soil
3.3. Permissible Limits of Cd
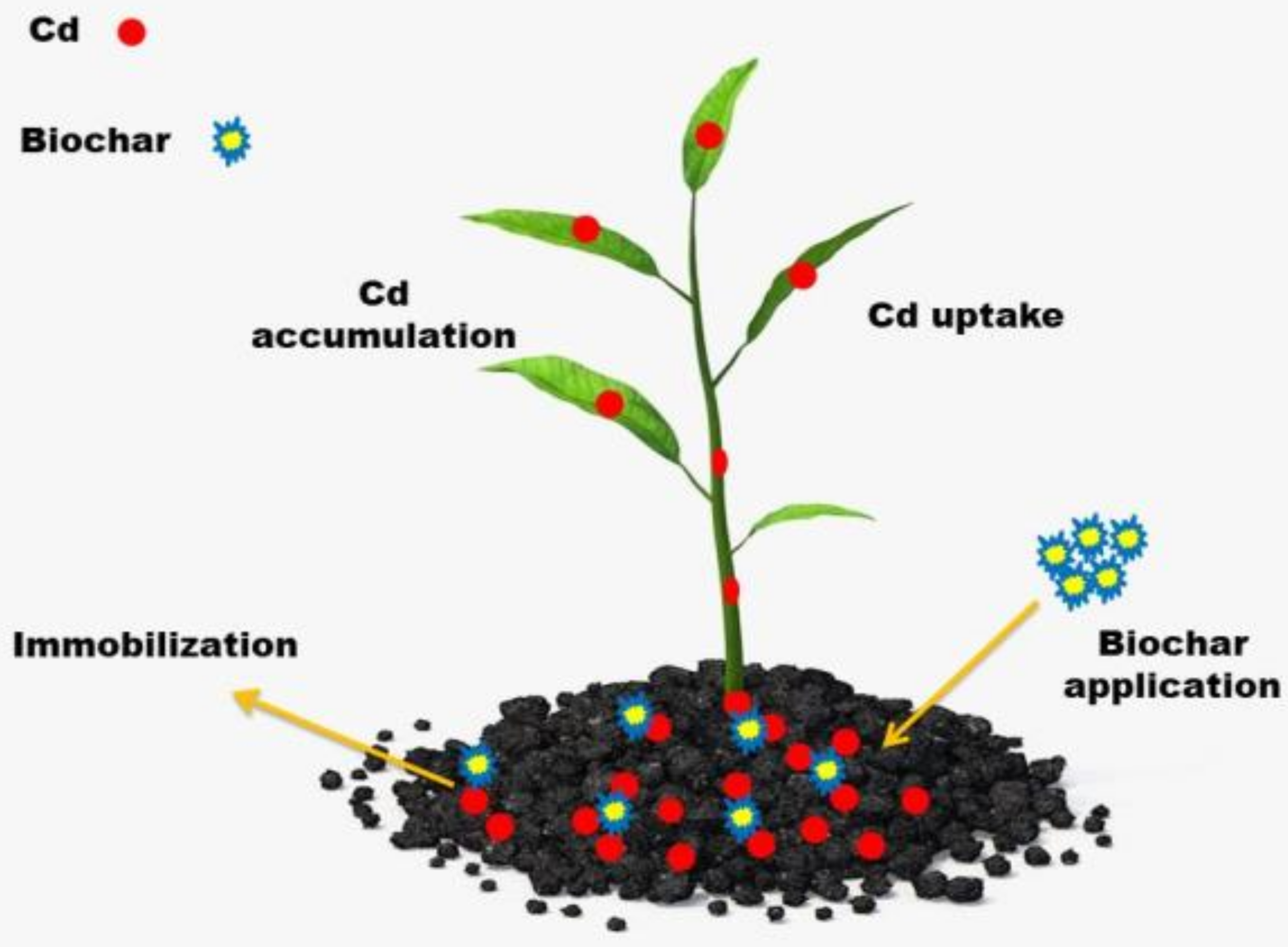
4. Biochar and Cd (II) Immobilization in Soil: Recent Progress
4.1. Description and Main Findings of Experiments with Pristine Biochar in Cd-Contaminated Soil
4.2. Description and Main Findings of Experiments with Metal-Modified Biochar in Cd-Contaminated Soil
4.3. Description and Main Findings of Experiments with Metal Oxide-Modified Biochar in Cd-Contaminated Soil
4.4. Description and Main Findings of Experiments of Biochar Combined with Other Amendments in Cd-Contaminated Soil
4.5. Cd(II) Removal Mechanisms

| Feedstock Used for Biochar | Surface Modifier for Raw Biochar | Test Crop | Dose | Removal Efficiency | Ref. |
|---|---|---|---|---|---|
| Wheat straw biochar | Pristine | Green pepper and eggplant | 0 and 5% (w/w) | 0–5% (w/w) = 6.8–11.5% (green pepper) 0–5% (w/w) = 15.1–15.4% (eggplant) | [66] |
| Corn stalk | Manganese (Mn) | Wheat | 1, 2 and 3% (w/w) | Among all the Mn-modified biochar treatments, 1%, 2%, and 3% treatments of MBC2-500-5:1, showed the potential to convert the mild acid-soluble fraction Cd to the reducible, oxidizable, residual fraction Cd, thereby controlling the migration, transformation, and enrichment of Cd in the soil. | [90] |
| Rice straw | Pristine | Legume–grass mixture | 0, 1, 2.5, and 5% | Biochar addition did not reduce Cd uptake when increased the amount of legumes in the legume–grass mixture | [68] |
| Sporobolus alterniflora-derived biochar | Pristine | Pak choi | 0, 2.5, 5, and 10% in pots | Biochar facilitated Cd immobilization in soil, which decreased Cd bioavailability and enhanced Cd recalcitrance. | [79] |
| Coconut shell | Bacillus sp. TZ5 | Perennial ryegrass | 5 g in 100 mL suspension + 100 mL bacteria suspension | The application of biochemical composite material (BCM) significantly decreased the Cd concentration of ryegrass by 48.49% in soil, thus providing a practical approach for bioremediation of Cd-contaminated soil. | [80] |
| Wheat straw | Bare wheat straw-derived biochar | Rice | 0, 10, 20, 30, and 40 t/ha | Biochar at 40 t/ha decreased the available Cd (49.4 and 51.7) significantly, compared with 0 t/ha | [70] |
| Wheat straw | Phosphoric acid (H3PO4) | Quinoa | 1 and 2% (w/w) | H3PO4-treated biochar effectively alleviated Cd toxicity in quinoa by reducing Cd(II) accumulation and regulating Cd-induced oxidative stress by the antioxidant enzymatic system. | [91] |
| Wheat straw | Pristine | Paddy rice field | 0, 10, 20 and 40 t/ha | Biochar at 40 t/ha altered the chemical properties of soil and reduced the mobility of Cd along with Pb in paddy soil. | [92] |
| Cattle carcass biochar | Carbonate-bearing hydroxyapatite (CHAP) | NR (Sorption test) | 0.1 g sample | Cattle-derived biochar from cattle carcasses containing a substantial amount of naturally occurring mineral form of carbonate-bearing hydroxyapatite (CHAP) allowed a 97.% reduction in Cd in soil. | [93] |
| Bamboo hardwood | Sulfur-modified and S–Fe-modified biochar | Rice | 1% | Addition of S–Fe-modified biochar to Cd-contaminated paddy soil reduced Cd(II) accumulation in rice grain by 0.018 mg kg−1. | [75] |
| Rice straw biochar | Zinc oxide (ZnO) | Rice seedlings | 0, 50, 75, and 100 mg/L of ZnO, alone or with 1.0% (w/w) biochar | Cd content in shoots was reduced by 30% and in roots by 31% at a dose of 100 mg/L ZnO; Cd content in shoots was reduced by 39% and in roots by 38% at a dose of 100 mg/L ZnO + biochar. | [94] |
| Ferromanganese binary oxide–corn straw–biochar composite (FMBC) | KMNO4 and Fe(NO3)3 | NR (Adsorption experiment) | 0.5, 1, 2, and 4% wt/wt FMBC and biochar | The adsorption capacity of FMBC was the highest (6.72 mg g−1) when compared to those of pristine biochar (4.85 mg g−1) and control (2.28 mg g−1). | [95] |
| Sugar cane bagasse | Pristine | NR (Adsorption experiment) | 0, 2, and 4% (wt/wt). | A 2% biochar application reduced Cd(II) contamination in saline–sodic soils, but increasing the biochar rate from 2 to 4% decreased Cd adsorption. | [96] |
| Bamboo hardwood | Sulfur-modified and S–Fe- modified biochar | NR (Incubation experiment) | 1% (wt/wt). | Treatments with BC, S–BC, and SF–BC significantly reduced the exchangeable Cd by 12.54%, 29.71%, and 18.53%, respectively. | [97] |
| Rice straw | Pristine | Pak choi | 0, 2.5 and 5% (wt/wt). | Rice straw-derived biochar at a dose of 5% showed potential to reduce the bioavailability of Cd(II) in soil by 16.64%, and increased pak choi yield. | [98] |
| Fe–Mn oxide-modified BC composite(FMBC) | Fe and Mn | Indica rice | 0.5–2.0% (wt/wt). | A 2% FMBC application reduced Cd(II) accumulation in rice grain by 66.7–74.1% and improved grain quality. | [99] |
5. Concluding Remarks and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.-H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Rahim, H.U.; Qaswar, M.; Wang, M.; Jing, X.; Cai, X. Environmental Applications of Reduced Sulfur Species and Composites in Transformation and Detoxification of Contaminants. J. Environ. Chem. Eng. 2021, 9, 106696. [Google Scholar] [CrossRef]
- Ur Rahim, H.; Qaswar, M.; Uddin, M.; Giannini, C.; Herrera, M.L.; Rea, G. Nano-enable materials promoting sustainability and resilience in modern agriculture. Nanomaterials 2021, 11, 2068. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhang, Y.; Huang, B.; Teng, Y. Soil environmental quality in greenhouse vegetable production systems in eastern China: Current status and management strategies. Chemosphere 2017, 170, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Xu, J.; Jiang, X.; Liu, C.; McCall, W.; Lu, J. Stabilization of heavy metals in soil using two organo-bentonites. Chemosphere 2017, 184, 884–891. [Google Scholar] [CrossRef]
- Li, N.; Kang, Y.; Pan, W.; Zeng, L.; Zhang, Q.; Luo, J. Concentration and transportation of heavy metals in vegetables and risk assessment of human exposure to bioaccessible heavy metals in soil near a waste-incinerator site, South China. Sci. Total Environ. 2015, 521, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, L.; Tsang, D.C.; Li, J.-S.; Baek, K.; Hou, D.; Ding, S.; Poon, C.-S. Recycling dredged sediment into fill materials, partition blocks, and paving blocks: Technical and economic assessment. J. Clean. Prod. 2018, 199, 69–76. [Google Scholar] [CrossRef]
- United Nations Human Settlements Programme. The State of the World’s Cities 2004/2005: Globalization and Urban Culture; UN-HABITAT: Nairobi, Kenya, 2004; Volume 2. [Google Scholar]
- Sagbara, G.; Zabbey, N.; Sam, K.; Nwipie, G.N. Heavy metal concentration in soil and maize (Zea mays L.) in partially reclaimed refuse dumpsite ‘borrow-pit’in Port Harcourt, Nigeria. Environ. Technol. Innov. 2020, 18, 100745. [Google Scholar] [CrossRef]
- Xu, J.; Hu, C.; Wang, M.; Zhao, Z.; Zhao, X.; Cao, L.; Lu, Y.; Cai, X. Changeable effects of coexisting heavy metals on transfer of cadmium from soils to wheat grains. J. Hazard. Mater. 2022, 423, 127182. [Google Scholar] [CrossRef]
- Kumar, V.; Pandita, S.; Sharma, A.; Bakshi, P.; Sharma, P.; Karaouzas, I.; Bhardwaj, R.; Thukral, A.K.; Cerda, A. Ecological and human health risks appraisal of metal (loid) s in agricultural soils: A review. Geol. Ecol. Landsc. 2021, 5, 173–185. [Google Scholar] [CrossRef]
- Grant, C.; Clarke, J.; Duguid, S.; Chaney, R. Selection and breeding of plant cultivars to minimize cadmium accumulation. Sci. Total Environ. 2008, 390, 301–310. [Google Scholar] [CrossRef]
- Grant, C.; Buckley, W.; Bailey, L.; Selles, F. Cadmium accumulation in crops. Can. J. Plant Sci. 1998, 78, 1–17. [Google Scholar] [CrossRef]
- Roberts, T.L. Cadmium and phosphorous fertilizers: The issues and the science. Procedia Eng. 2014, 83, 52–59. [Google Scholar] [CrossRef]
- Wang, M.; Hu, C.; Xu, J.; Jing, X.; Rahim, H.U.; Cai, X. Facile combinations of thiosulfate and zerovalent iron synergically immobilize cadmium in soils through mild extraction and facilitated immobilization. J. Hazard. Mater. 2021, 407, 124806. [Google Scholar] [CrossRef] [PubMed]
- Arao, T.; Kawasaki, A.; Baba, K.; Mori, S.; Matsumoto, S. Effects of water management on cadmium and arsenic accumulation and dimethylarsinic acid concentrations in Japanese rice. Environ. Sci. Technol. 2009, 43, 9361–9367. [Google Scholar] [CrossRef] [PubMed]
- Meharg, A.A.; Norton, G.; Deacon, C.; Williams, P.; Adomako, E.E.; Price, A.; Zhu, Y.; Li, G.; Zhao, F.-J.; McGrath, S. Variation in rice cadmium related to human exposure. Environ. Sci. Technol. 2013, 47, 5613–5618. [Google Scholar] [CrossRef] [PubMed]
- Faroon, O.; Ashizawa, A.; Wright, S.; Tucker, P.; Jenkins, K.; Ingerman, L.; Rudisill, C. Toxicological Profile of Cadmium; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2012; pp. 273–274. [Google Scholar]
- Yu, Y.; Murthy, B.N.; Shapter, J.G.; Constantopoulos, K.T.; Voelcker, N.H.; Ellis, A.V. Benzene carboxylic acid derivatized graphene oxide nanosheets on natural zeolites as effective adsorbents for cationic dye removal. J. Hazard. Mater. 2013, 260, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Debrassi, A.; Corrêa, A.F.; Baccarin, T.; Nedelko, N.; Ślawska-Waniewska, A.; Sobczak, K.; Dłużewski, P.; Greneche, J.-M.; Rodrigues, C.A. Removal of cationic dyes from aqueous solutions using N-benzyl-O-carboxymethylchitosan magnetic nanoparticles. Chem. Eng. J. 2012, 183, 284–293. [Google Scholar] [CrossRef]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Hamby, D. Site remediation techniques supporting environmental restoration activities—A review. Sci. Total Environ. 1996, 191, 203–224. [Google Scholar] [CrossRef]
- Pera-Titus, M.; García-Molina, V.; Baños, M.A.; Giménez, J.; Esplugas, S. Degradation of chlorophenols by means of advanced oxidation processes: A general review. Appl. Catal. B Environ. 2004, 47, 219–256. [Google Scholar] [CrossRef]
- Huang, X.; Chen, T.; Zou, X.; Zhu, M.; Chen, D.; Pan, M. The adsorption of Cd (II) on manganese oxide investigated by batch and modeling techniques. Int. J. Environ. Res. Public Health 2017, 14, 1145. [Google Scholar] [CrossRef]
- Huang, J.; Wu, Z.; Chen, L.; Sun, Y. Surface complexation modeling of adsorption of Cd (II) on graphene oxides. J. Mol. Liq. 2015, 209, 753–758. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, J.; Meng, Y.; Aihemaiti, A.; Xu, Y.; Xiang, H.; Gao, Y.; Chen, X. Preparation, environmental application and prospect of biochar-supported metal nanoparticles: A review. J. Hazard. Mater. 2020, 388, 122026. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Qayyum, M.F.; Ibrahim, M.; Zia-ur-Rehman, M.; Abbas, T.; Ok, Y.S. Mechanisms of biochar-mediated alleviation of toxicity of trace elements in plants: A critical review. Environ. Sci. Pollut. Res. 2016, 23, 2230–2248. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for environmental management: An introduction. In Biochar for Environmental Management–Science and Technology; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2009. [Google Scholar]
- Sepehri, A.; Sarrafzadeh, M.-H. Effect of nitrifiers community on fouling mitigation and nitrification efficiency in a membrane bioreactor. Chem. Eng. Process. Process Intensif. 2018, 128, 10–18. [Google Scholar] [CrossRef]
- Rahim, H.U. Field-based investigation of aged biochar coupled with summer legumes effect on wheat yield in Pakistan. Bul. Agroteknol. 2020, 28, 1–6. [Google Scholar] [CrossRef]
- O’Laughlin, J.; McElligott, K. Biochar for Environmental Management: Science and Technology; Lehmann, J., Joseph, S.M., Eds.; Earthscan: London, UK; Elsevier: Amsterdam, The Netherlands, 2009; p. 448. [Google Scholar]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation; Routledge: Oxfordshire, UK, 2015. [Google Scholar]
- Hu, B.; Ai, Y.; Jin, J.; Hayat, T.; Alsaedi, A.; Zhuang, L.; Wang, X. Efficient elimination of organic and inorganic pollutants by biochar and biochar-based materials. Biochar 2020, 2, 47–64. [Google Scholar] [CrossRef]
- Rahim, H.U.; Mian, I.A.; Arif, M.; Rahim, Z.U.; Ahmad, S.; Khan, Z.; Zada, L.; Khan, M.A.; Haris, M. 3. Residual effect of biochar and summer legumes on soil physical properties and wheat growth. Pure Appl. Biol. 2019, 8, 16–26. [Google Scholar] [CrossRef]
- ur Rahim, H.; Mian, I.A.; Arif, M.; Ahmad, S.; Khan, Z. Soil fertility status as influenced by the carryover effect of biochar and summer legumes. Asian J. Agric. Biol. 2020, 8, 11–16. [Google Scholar] [CrossRef]
- Azadi, N.; Raiesi, F. Biochar alleviates metal toxicity and improves microbial community functions in a soil co-contaminated with cadmium and lead. Biochar 2021, 3, 485–498. [Google Scholar] [CrossRef]
- Gu, S.; Kang, X.; Wang, L.; Lichtfouse, E.; Wang, C. Clay mineral adsorbents for heavy metal removal from wastewater: A review. Environ. Chem. Lett. 2019, 17, 629–654. [Google Scholar] [CrossRef]
- Duan, C.; Ma, T.; Wang, J.; Zhou, Y. Removal of heavy metals from aqueous solution using carbon-based adsorbents: A review. J. Water Process Eng. 2020, 37, 101339. [Google Scholar] [CrossRef]
- Lu, L.; Yu, W.; Wang, Y.; Zhang, K.; Zhu, X.; Zhang, Y.; Wu, Y.; Ullah, H.; Xiao, X.; Chen, B. Application of biochar-based materials in environmental remediation: From multi-level structures to specific devices. Biochar 2020, 2, 1–31. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, X.; Khan, A.; Wang, P.; Liu, Y.; Alsaedi, A.; Hayat, T.; Wang, X. Environmental remediation and application of nanoscale zero-valent iron and its composites for the removal of heavy metal ions: A review. Environ. Sci. Technol. 2016, 50, 7290–7304. [Google Scholar] [CrossRef]
- Liu, X.; Ma, R.; Wang, X.; Ma, Y.; Yang, Y.; Zhuang, L.; Zhang, S.; Jehan, R.; Chen, J.; Wang, X. Graphene oxide-based materials for efficient removal of heavy metal ions from aqueous solution: A review. Environ. Pollut. 2019, 252, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Oh, S.; Park, Y.-K. Overview of biochar production from preservative-treated wood with detailed analysis of biochar characteristics, heavy metals behaviors, and their ecotoxicity. J. Hazard. Mater. 2020, 384, 121356. [Google Scholar] [CrossRef] [PubMed]
- Bandara, T.; Franks, A.; Xu, J.; Bolan, N.; Wang, H.; Tang, C. Chemical and biological immobilization mechanisms of potentially toxic elements in biochar-amended soils. Crit. Rev. Environ. Sci. Technol. 2020, 50, 903–978. [Google Scholar] [CrossRef]
- Mandal, S.; Pu, S.; Adhikari, S.; Ma, H.; Kim, D.-H.; Bai, Y.; Hou, D. Progress and future prospects in biochar composites: Application and reflection in the soil environment. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1148–1175. [Google Scholar] [CrossRef]
- Templeton, D.M.; Ariese, F.; Cornelis, R.; Danielsson, L.-G.; Muntau, H.; van Leeuwen, H.P.; Lobinski, R. Guidelines for terms related to chemical speciation and fractionation of elements. Definitions, structural aspects, and methodological approaches (IUPAC Recommendations 2000). Pure Appl. Chem. 2000, 72, 1453–1470. [Google Scholar] [CrossRef]
- Wu, Q.; Hendershot, W.H.; Marshall, W.D.; Ge, Y. Speciation of cadmium, copper, lead, and zinc in contaminated soils. Commun. Soil Sci. Plant Anal. 2000, 31, 1129–1144. [Google Scholar] [CrossRef]
- Sauvé, S.; Hendershot, W.; Allen, H.E. Solid-solution partitioning of metals in contaminated soils: Dependence on pH, total metal burden, and organic matter. Environ. Sci. Technol. 2000, 34, 1125–1131. [Google Scholar] [CrossRef]
- Holm, P.E.; Andersen, S.; Christensen, T.H. Speciation of dissolved cadmium: Interpretation of dialysis, ion exchange and computer (GEOCHEM) methods. Water Res. 1995, 29, 803–809. [Google Scholar] [CrossRef]
- Gerritse, R.; Van Driel, W. The relationship between adsorption of trace metals, organic matter, and pH in temperate soils. J. Environ. Qual. 1984, 13, 197–204. [Google Scholar] [CrossRef]
- Crea, F.; Foti, C.; Milea, D.; Sammartano, S. Speciation of cadmium in the environment. Cadmium Toxic. Essent. 2013, 11, 63–83. [Google Scholar]
- Hu, Y.; Cheng, H.; Tao, S. The challenges and solutions for cadmium-contaminated rice in China: A critical review. Environ. Int. 2016, 92, 515–532. [Google Scholar] [CrossRef]
- Taylor, M. Accumulation of cadmium derived from fertilisers in New Zealand soils. Sci. Total Environ. 1997, 208, 123–126. [Google Scholar] [CrossRef]
- de Vries, W.; Römkens, P.F.; Schütze, G. Critical soil concentrations of cadmium, lead, and mercury in view of health effects on humans and animals. Rev. Environ. Contam. Toxicol. 2007, 191, 91–130. [Google Scholar]
- Tian, H.; Cheng, K.; Wang, Y.; Zhao, D.; Lu, L.; Jia, W.; Hao, J. Temporal and spatial variation characteristics of atmospheric emissions of Cd, Cr, and Pb from coal in China. Atmos. Environ. 2012, 50, 157–163. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for the Study of Dietary Intakes of Chemical Contaminants; World Health Organization: Geneva, Switzerland, 1985; p. 100. [Google Scholar]
- Cai, K.; Yu, Y.; Zhang, M.; Kim, K. Concentration, source, and total health risks of cadmium in multiple media in densely populated areas, China. Int. J. Environ. Res. Public Health 2019, 16, 2269. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Environmental Health Criteria 134: Cadmium; World Health Organization: Geneva, Switzerland, 1992; pp. 17–35. [Google Scholar]
- Bernard, A.; Lauwerys, R. Effects of Cadmium Exposure in Humans. In Cadmium; Springer: Berlin/Heidelberg, Germany, 1986; pp. 135–177. [Google Scholar]
- Yu, Z.; Qiu, W.; Wang, F.; Lei, M.; Wang, D.; Song, Z. Effects of manganese oxide-modified biochar composites on arsenic speciation and accumulation in an indica rice (Oryza sativa L.) cultivar. Chemosphere 2017, 168, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Fang, Z.; Zheng, L.; Cheng, W.; Tsang, P.E.; Fang, J.; Zhao, D. Remediation of lead contaminated soil by biochar-supported nano-hydroxyapatite. Ecotoxicol. Environ. Saf. 2016, 132, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Li, Z.; Gascó, G.; Méndez, A.; Shen, Y.; Paz-Ferreiro, J. Use of magnetic biochars for the immobilization of heavy metals in a multi-contaminated soil. Sci. Total Environ. 2018, 622, 892–899. [Google Scholar] [CrossRef]
- Wu, C.; Cui, M.; Xue, S.; Li, W.; Huang, L.; Jiang, X.; Qian, Z. Remediation of arsenic-contaminated paddy soil by iron-modified biochar. Environ. Sci. Pollut. Res. 2018, 25, 20792–20801. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.-C.; Beiyuan, J.; Wang, L.; Tsang, D.C.; Baek, K.; Bolan, N.S.; Ok, Y.S.; Li, X.-D. A combination of ferric nitrate/EDDS-enhanced washing and sludge-derived biochar stabilization of metal-contaminated soils. Sci. Total Environ. 2018, 616, 572–582. [Google Scholar] [CrossRef]
- Beiyuan, J.; Awad, Y.M.; Beckers, F.; Tsang, D.C.; Ok, Y.S.; Rinklebe, J. Mobility and phytoavailability of As and Pb in a contaminated soil using pine sawdust biochar under systematic change of redox conditions. Chemosphere 2017, 178, 110–118. [Google Scholar] [CrossRef]
- Sun, J.; Fan, Q.; Ma, J.; Cui, L.; Quan, G.; Yan, J.; Wu, L.; Hina, K.; Abdul, B.; Wang, H. Effects of biochar on cadmium (Cd) uptake in vegetables and its natural downward movement in saline-alkali soil. Environ. Pollut. Bioavailab. 2020, 32, 36–46. [Google Scholar] [CrossRef]
- Tan, Z.; Yuan, S.; Hong, M.; Zhang, L.; Huang, Q. Mechanism of negative surface charge formation on biochar and its effect on the fixation of soil Cd. J. Hazard. Mater. 2020, 384, 121370. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wang, L.; Zhao, Z.; Che, Y. Biochar shifts biomass and element allocation of legume-grass mixtures in Cd-contaminated soils. Environ. Sci. Pollut. Res. 2020, 27, 10835–10845. [Google Scholar] [CrossRef] [PubMed]
- Ramezanzadeh, H.; Reyhanitabar, A.; Oustan, S.; Mohammadi, M.; van der Zee, S. Enhanced Sorption of Cadmium by using Biochar Nanoparticles from Ball Milling in a Sandy Soil. Eurasian Soil Sci. 2021, 54, 201–211. [Google Scholar] [CrossRef]
- Jing, F.; Chen, C.; Chen, X.; Liu, W.; Wen, X.; Hu, S.; Yang, Z.; Guo, B.; Xu, Y.; Yu, Q. Effects of wheat straw derived biochar on cadmium availability in a paddy soil and its accumulation in rice. Environ. Pollut. 2020, 257, 113592. [Google Scholar] [CrossRef] [PubMed]
- Moradi, N.; Karimi, A. Fe-Modified common reed biochar reduced cadmium (Cd) mobility and enhanced microbial activity in a contaminated calcareous soil. J. Soil Sci. Plant Nutr. 2021, 21, 329–340. [Google Scholar] [CrossRef]
- Tang, X.; Shen, H.; Chen, M.; Yang, X.; Yang, D.; Wang, F.; Chen, Z.; Liu, X.; Wang, H.; Xu, J. Achieving the safe use of Cd-and As-contaminated agricultural land with an Fe-based biochar: A field study. Sci. Total Environ. 2020, 706, 135898. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Li, C.; Parikh, S.J. Simultaneous removal of arsenic, cadmium, and lead from soil by iron-modified magnetic biochar. Environ. Pollut. 2020, 261, 114157. [Google Scholar] [CrossRef]
- Chen, D.; Wang, X.; Wang, X.; Feng, K.; Su, J.; Dong, J. The mechanism of cadmium sorption by sulphur-modified wheat straw biochar and its application cadmium-contaminated soil. Sci. Total Environ. 2020, 714, 136550. [Google Scholar] [CrossRef]
- Rajendran, M.; Shi, L.; Wu, C.; Li, W.; An, W.; Liu, Z.; Xue, S. Effect of sulfur and sulfur-iron modified biochar on cadmium availability and transfer in the soil–rice system. Chemosphere 2019, 222, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Li, Z.; Yang, D.; Xu, J.; Liu, X. MgO-laden biochar enhances the immobilization of Cd/Pb in aqueous solution and contaminated soil. Biochar 2021, 3, 175–188. [Google Scholar] [CrossRef]
- Xiang, J.; Lin, Q.; Yao, X.; Yin, G. Removal of Cd from aqueous solution by chitosan coated MgO-biochar and its in-situ remediation of Cd-contaminated soil. Environ. Res. 2021, 195, 110650. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Hussain, Q.; Jun, Z.; Qingling, F.; Houben, D.; Hongqing, H. Efficiency of KOH-modified rice straw-derived biochar for reducing cadmium mobility, bioaccessibility and bioavailability risk index in red soil. Pedosphere 2020, 30, 874–882. [Google Scholar] [CrossRef]
- Qiu, Z.; Tang, J.; Chen, J.; Zhang, Q. Remediation of cadmium-contaminated soil with biochar simultaneously improves biochar’s recalcitrance. Environ. Pollut. 2020, 256, 113436. [Google Scholar] [CrossRef]
- Ma, H.; Wei, M.; Wang, Z.; Hou, S.; Li, X.; Heng, X. Bioremediation of cadmium polluted soil using a novel cadmium immobilizing plant growth promotion strain Bacillus sp. TZ5 loaded on biochar. J. Hazard. Mater. 2020, 388, 122065. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Wei, J.; Guan, F.; Liu, Y.; Sun, Y.; Luo, Y. Biochar and bacteria inoculated biochar enhanced Cd and Cu immobilization and enzymatic activity in a polluted soil. Environ. Int. 2020, 137, 105576. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, L.; Zhang, J.; Ren, L.; Zhou, Y.; Zheng, Y.; Luo, L.; Yang, Y.; Huang, H.; Chen, A. Physicochemical features, metal availability and enzyme activity in heavy metal-polluted soil remediated by biochar and compost. Sci. Total Environ. 2020, 701, 134751. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhao, J.; Yang, W.; He, L.; Wei, W.; Tan, X.; Wang, J.; Lin, A. Evaluation of biochar pyrolyzed from kitchen waste, corn straw, and peanut hulls on immobilization of Pb and Cd in contaminated soil. Environ. Pollut. 2020, 261, 114133. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Shen, R.F.; Shao, J.F. Transport of cadmium from soil to grain in cereal crops: A review. Pedosphere 2021, 31, 3–10. [Google Scholar]
- Qiu, Z.; Chen, J.; Tang, J.; Zhang, Q. A study of cadmium remediation and mechanisms: Improvements in the stability of walnut shell-derived biochar. Sci. Total Environ. 2018, 636, 80–84. [Google Scholar] [CrossRef]
- Liu, L.; Fan, S. Removal of cadmium in aqueous solution using wheat straw biochar: Effect of minerals and mechanism. Environ. Sci. Pollut. Res. 2018, 25, 8688–8700. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, W.; Hao, X.; Zhou, D. Transport of biochar particles in saturated granular media: Effects of pyrolysis temperature and particle size. Environ. Sci. Technol. 2013, 47, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Niu, J.; Morales, V.L.; Chen, X.; Hay, A.G.; Lehmann, J.; Steenhuis, T.S. Transport and retention of biochar particles in porous media: Effect of pH, ionic strength, and particle size. Ecohydrology 2010, 3, 497–508. [Google Scholar] [CrossRef]
- Chi, T.; Zuo, J.; Liu, F. Performance and mechanism for cadmium and lead adsorption from water and soil by corn straw biochar. Front. Environ. Sci. Eng. 2017, 11, 15. [Google Scholar] [CrossRef]
- Tan, X.; Wei, W.; Xu, C.; Meng, Y.; Bai, W.; Yang, W.; Lin, A. Manganese-modified biochar for highly efficient sorption of cadmium. Environ. Sci. Pollut. Res. 2020, 27, 9126–9134. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.A.; Shabbir, A.; Amjad, M.; Abbas, G.; Imran, M.; Murtaza, B.; Tahir, M.; Ahmad, A. Acid treated biochar enhances cadmium tolerance by restricting its uptake and improving physio-chemical attributes in quinoa (Chenopodium quinoa Willd.). Ecotoxicol. Environ. Saf. 2020, 191, 110218. [Google Scholar] [CrossRef] [PubMed]
- Jing, F.; Chen, X.; Wen, X.; Liu, W.; Hu, S.; Yang, Z.; Guo, B.; Luo, Y.; Yu, Q.; Xu, Y. Biochar effects on soil chemical properties and mobilization of cadmium (Cd) and lead (Pb) in paddy soil. Soil Use Manag. 2020, 36, 320–327. [Google Scholar] [CrossRef]
- Lei, S.; Zhu, L.; Xue, C.; Hong, C.; Wang, J.; Che, L.; Hu, Y.; Qiu, Y. Mechanistic insights and multiple characterizations of cadmium binding to animal-derived biochar. Environ. Pollut. 2020, 258, 113675. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Noureen, S.; Anwar, S.; Ali, B.; Naveed, M.; Abd_Allah, E.F.; Alqarawi, A.A.; Ahmad, P. Combined use of biochar and zinc oxide nanoparticle foliar spray improved the plant growth and decreased the cadmium accumulation in rice (Oryza sativa L.) plant. Environ. Sci. Pollut. Res. 2019, 26, 11288–11299. [Google Scholar] [CrossRef]
- Zhou, Q.; Liao, B.; Lin, L.; Song, Z.; Khan, Z.H.; Lei, M. Characteristic of adsorption cadmium of red soil amended with a ferromanganese oxide-biochar composite. Environ. Sci. Pollut. Res. 2019, 26, 5155–5163. [Google Scholar] [CrossRef] [PubMed]
- Zahedifar, M.; Moosavi, A.A. Assessing cadmium availability of contaminated saline-sodic soils as influenced by biochar using the adsorption isotherm models. Arch. Agron. Soil Sci. 2019, 66, 1735–1752. [Google Scholar] [CrossRef]
- Wu, C.; Shi, L.; Xue, S.; Li, W.; Jiang, X.; Rajendran, M.; Qian, Z. Effect of sulfur-iron modified biochar on the available cadmium and bacterial community structure in contaminated soils. Sci. Total Environ. 2019, 647, 1158–1168. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.; Malik, Z.; Parveen, A.; Zong, Y.; Abbasi, G.H.; Rafiq, M.T.; Shaaban, M.; Mustafa, A.; Bashir, S.; Rafay, M. Biochar alleviates Cd phytotoxicity by minimizing bioavailability and oxidative stress in pak choi (Brassica chinensis L.) cultivated in Cd-polluted soil. J. Environ. Manag. 2019, 250, 109500. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Lin, L.; Qiu, W.; Song, Z.; Liao, B. Supplementation with ferromanganese oxide–impregnated biochar composite reduces cadmium uptake by indica rice (Oryza sativa L.). J. Clean. Prod. 2018, 184, 1052–1059. [Google Scholar] [CrossRef]
| Material | Summary of Review Paper Content | Ref. |
|---|---|---|
| Biochar-supported metal nanoparticles | This paper reviewed the synthesis, characterization, environmental applications, and underlying mechanisms in the removal of contaminants from soil and water by biochar-supported metal nanoparticles. | [26] |
| Preservative-treated wood biochar | In this paper, the authors discussed the synthesis of biochar from preservative-treated wood, with particular focus on feedstock, synthesis method, characterization, application in pollutant removal, and ecotoxicity. | [43] |
| Biochar | This review paper discussed the molecular interaction mechanisms between biochar and potentially toxic elements such as those in soil systems. | [44] |
| Biochar-based composites | This paper reviewed the role of biochar-based composites with metal oxides, surface agents, and nanoparticles in the remediation of contaminated soil. Future research directions to verify the underlying mechanisms involved in biochar composite–soil microbial interactions and remediation of heavy metals. | [45] |
| Biochar and Biochar-based materials | This paper discussed the potential applications of biochar and its composite materials for the removal of organic and inorganic contaminants from soil and water. | [34] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahim, H.U.; Akbar, W.A.; Alatalo, J.M. A Comprehensive Literature Review on Cadmium (Cd) Status in the Soil Environment and Its Immobilization by Biochar-Based Materials. Agronomy 2022, 12, 877. https://doi.org/10.3390/agronomy12040877
Rahim HU, Akbar WA, Alatalo JM. A Comprehensive Literature Review on Cadmium (Cd) Status in the Soil Environment and Its Immobilization by Biochar-Based Materials. Agronomy. 2022; 12(4):877. https://doi.org/10.3390/agronomy12040877
Chicago/Turabian StyleRahim, Hafeez Ur, Waqas Ali Akbar, and Juha M. Alatalo. 2022. "A Comprehensive Literature Review on Cadmium (Cd) Status in the Soil Environment and Its Immobilization by Biochar-Based Materials" Agronomy 12, no. 4: 877. https://doi.org/10.3390/agronomy12040877
APA StyleRahim, H. U., Akbar, W. A., & Alatalo, J. M. (2022). A Comprehensive Literature Review on Cadmium (Cd) Status in the Soil Environment and Its Immobilization by Biochar-Based Materials. Agronomy, 12(4), 877. https://doi.org/10.3390/agronomy12040877






