Identification, Expression, and Functional Study of Seven NAC Transcription Factor Genes Involved in Stress Response in Tartary Buckwheat (Fagopyrum tataricum (L.) Gaertn.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth and Stress Treatment of Tartary Buckwheat
2.2. RNA Isolation, Identification, and Cloning of FtNACs
2.3. Bioinformatics Analyses of FtNACs
2.4. Quantitative Reverse Transcription PCR (qRT-PCR) Analyses of FtNACs
2.5. Arabidopsis Transformation and Stress Treatment of FtNAC70
3. Results
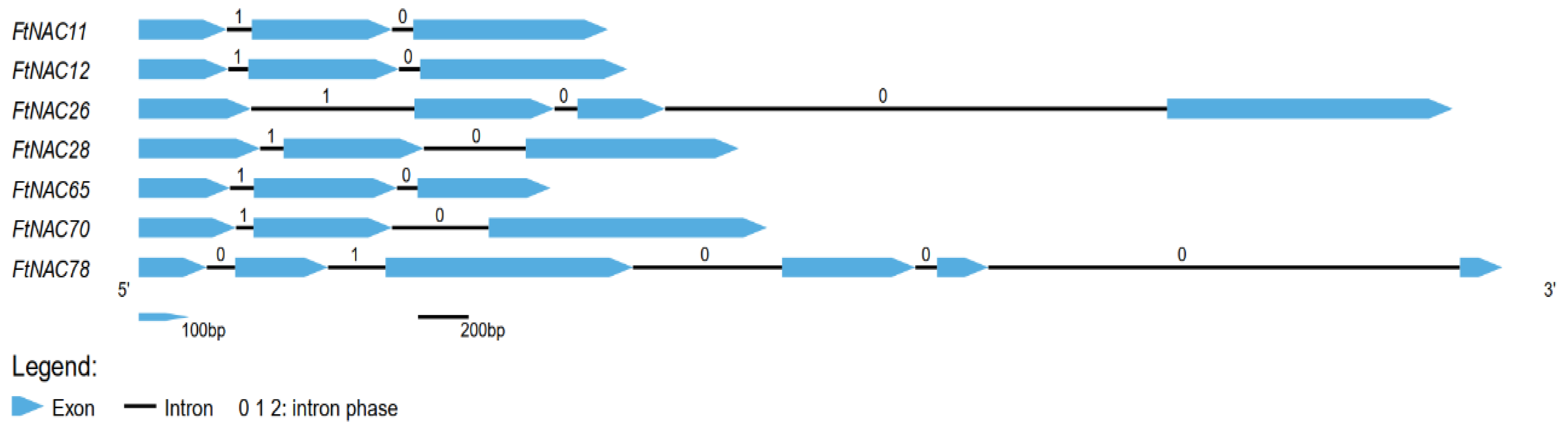
3.1. Isolation and Analyses of Seven NAC Genes from Tartary Buckwheat
3.2. Amino Acids and Phylogenetic Analyses of Seven FtNAC Proteins
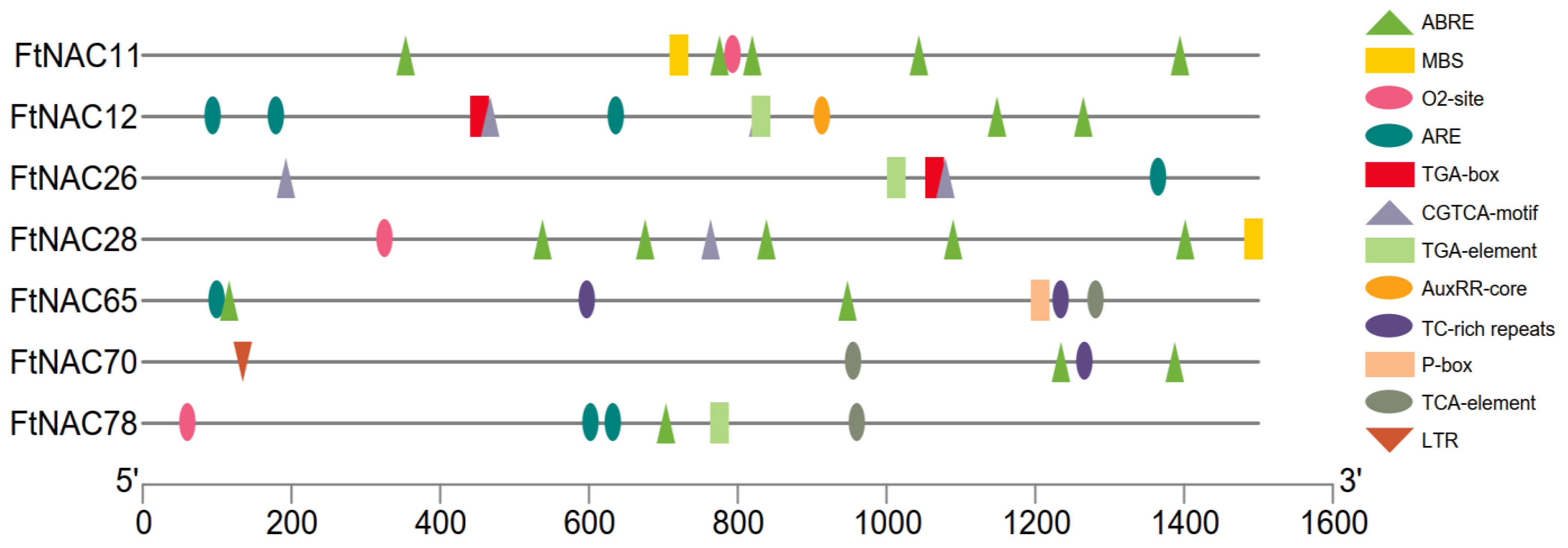
3.3. CARE Analyses of Seven FtNAC Promoters
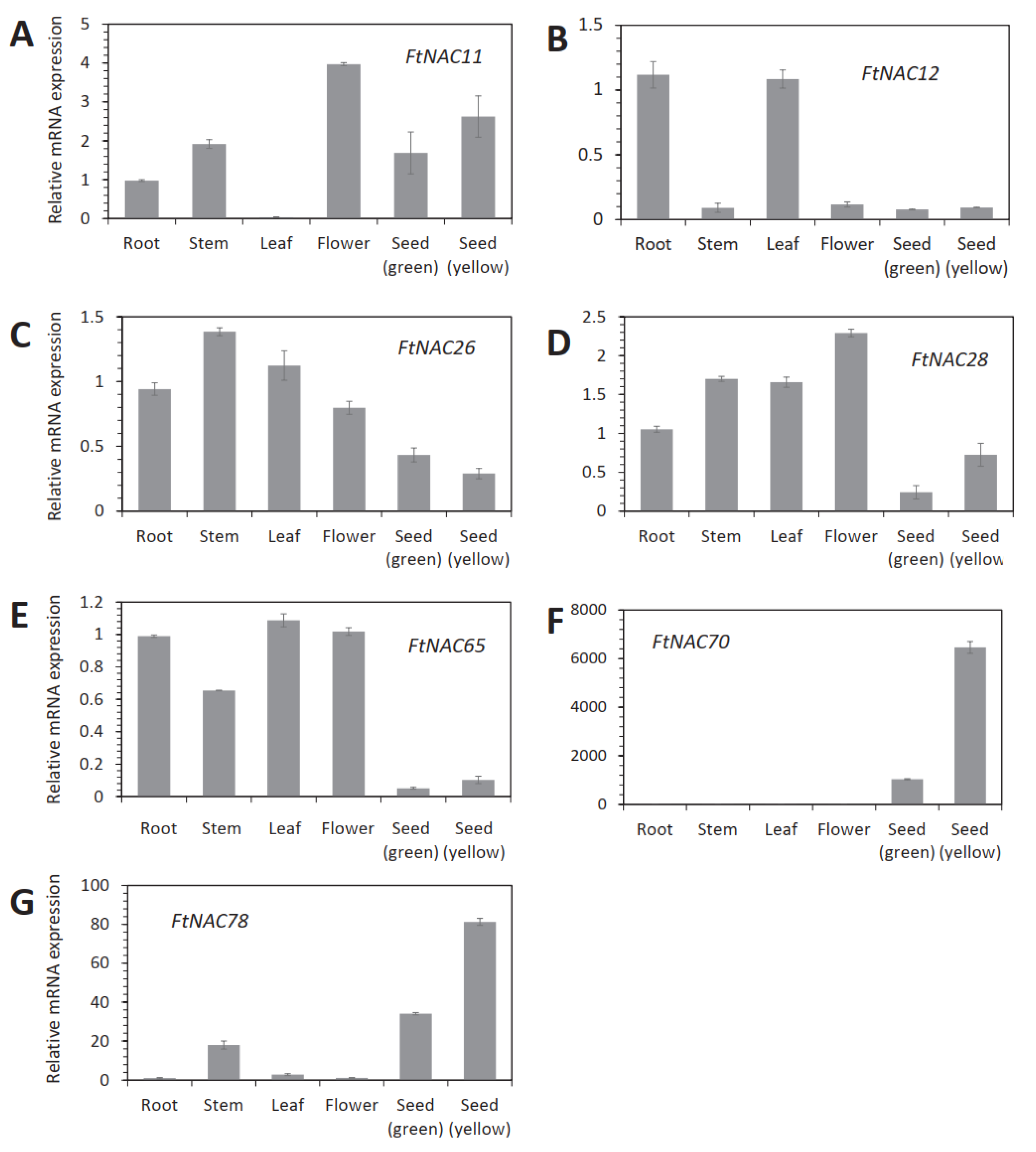
3.4. Tissue-Specific Analyses of Seven FtNAC Genes
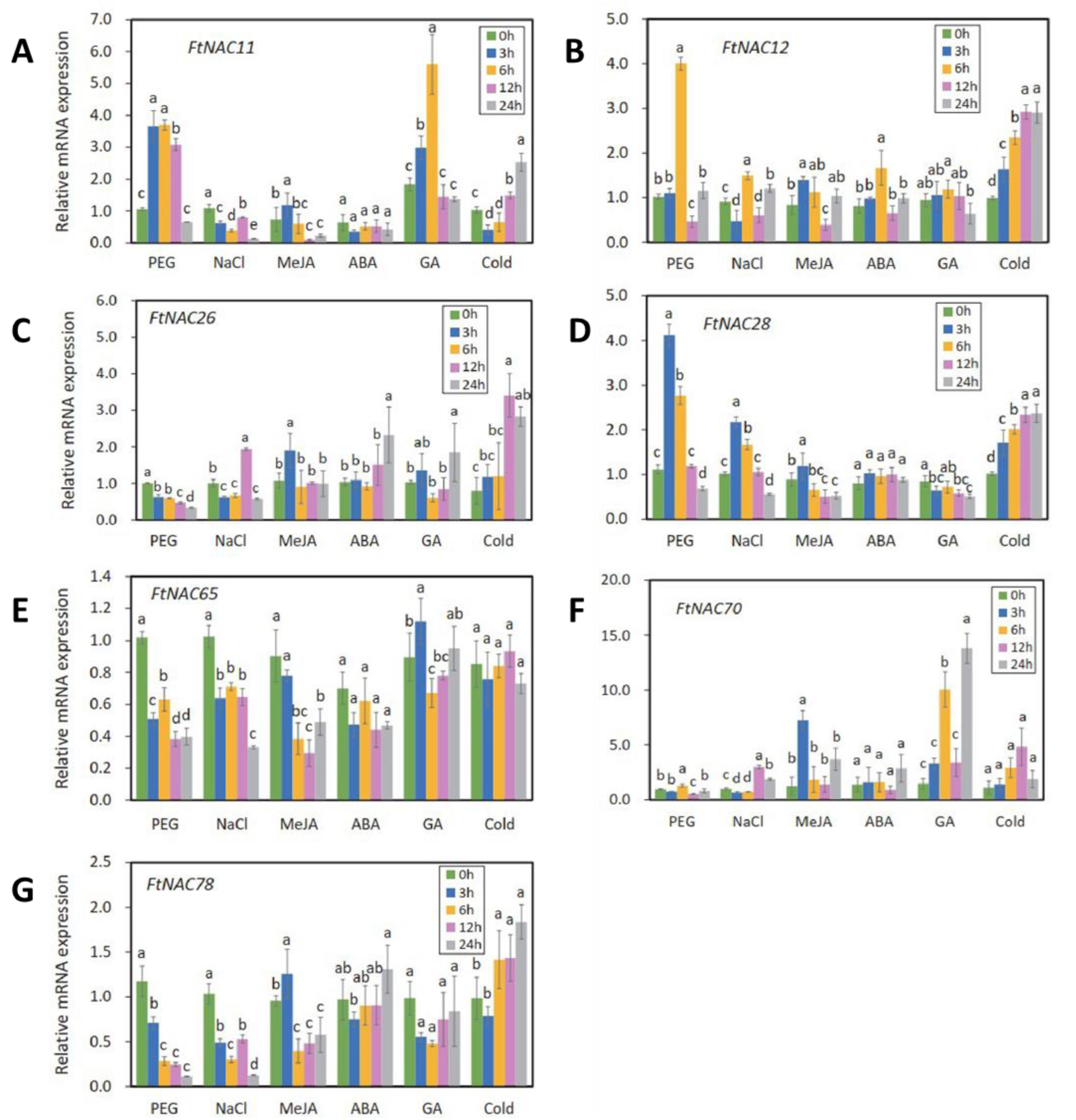
3.5. Expression Analyses of Seven FtNAC Genes under Abiotic Stress and Phytohormones Treatment
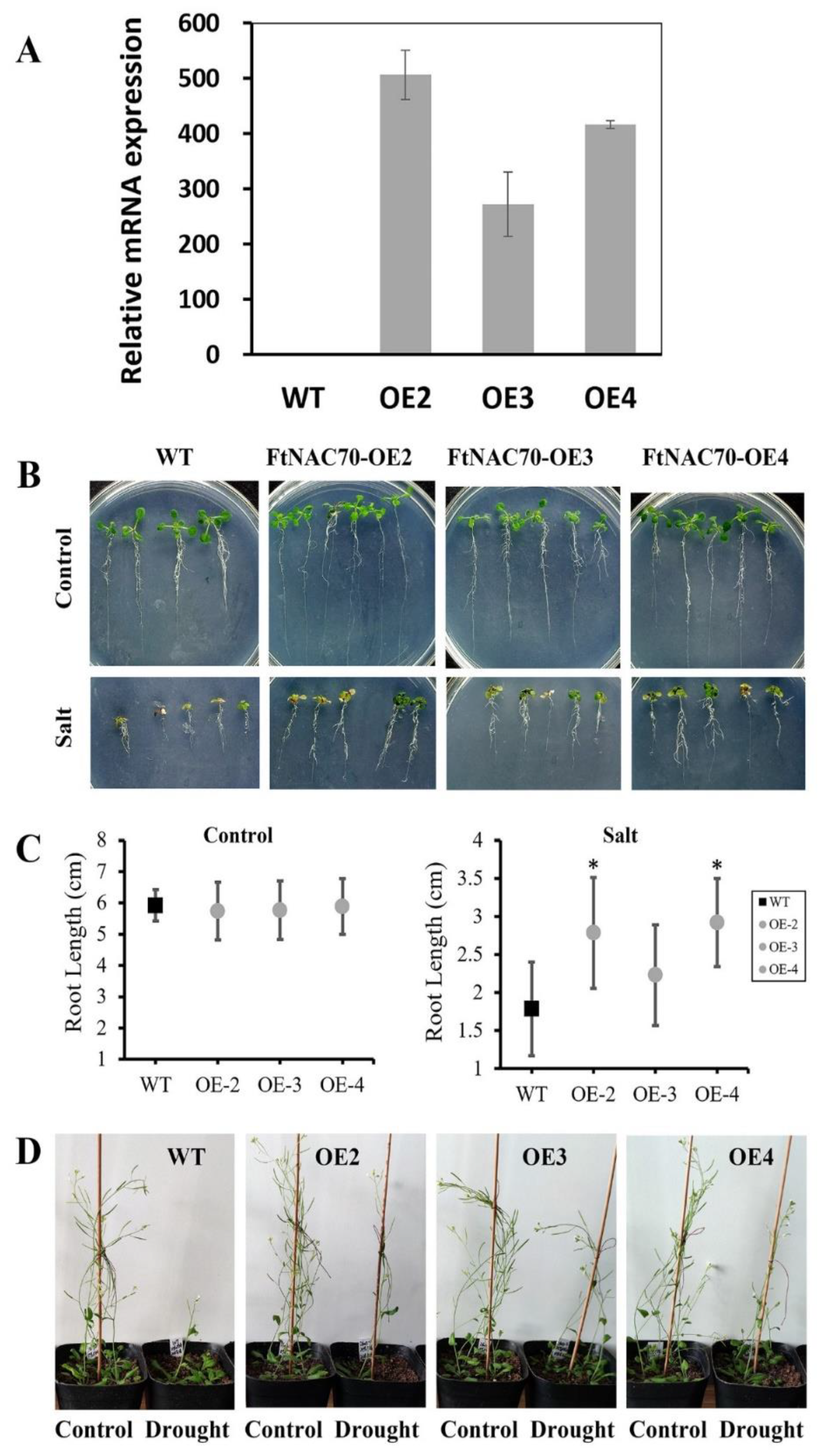
3.6. The Effects of FtNAC70 on Salt and Drought
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nakashima, K.; Takasaki, H.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. NAC transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta 2012, 1819, 97–103. [Google Scholar] [CrossRef]
- Diao, P.; Chen, C.; Zhang, Y.; Meng, Q.; Lv, W.; Ma, N. The role of NAC transcription factor in plant cold response. Plant Signal. Behav. 2020, 15, 1785668. [Google Scholar] [CrossRef] [PubMed]
- Munir, N.; Yukun, C.; Xiaohui, C.; Nawaz, M.A.; Iftikhar, J.; Rizwan, H.M.; Xu, S.; Yuling, L.; Xuhan, X.; Zhongxiong, L. Genome-wide identification and comprehensive analyses of NAC transcription factor gene family and expression patterns during somatic embryogenesis in Dimocarpus longan Lour. Plant Physiol. Biochem. 2020, 157, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Ooka, H.; Satoh, K.; Doi, K.; Nagata, T.; Kikuchi, S. Comprehensive Analysis of NAC Family Genes in Oryza sativa and Arabidopsis thaliana. DNA Res. 2004, 10, 239–247. [Google Scholar] [CrossRef]
- Satheesh, V.; Jagannadham, P.T.; Chidambaranathan, P.; Jain, P.K.; Srinivasan, R. NAC transcription factor genes: Genome-wide identification, phylogenetic, motif and cis-regulatory element analysis in pigeonpea (Cajanus cajan (L.) Millsp.). Mol. Biol. Rep. 2014, 41, 7763–7773. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Hu, M.; Li, J.; Chen, L.; Li, M.; Zhang, S.; Zhang, X.; Yang, X. Comprehensive analysis of NAC transcription factors uncovers their roles during fiber development and stress response in cotton. BMC Plant Biol. 2018, 18, 150. [Google Scholar] [CrossRef] [Green Version]
- Shiriga, K.; Sharma, R.; Kumar, K.; Yadav, S.K.; Hossain, F.; Thirunavukkarasu, N. Genome-wide identification and expression pattern of drought-responsive members of the NAC family in maize. Meta Gene 2014, 2, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ma, Z.; Sun, W.; Huang, L.; Wu, Q.; Tang, Z.; Bu, T.; Li, C.; Chen, H. Genome-wide analysis of the NAC transcription factor family in Tartary buckwheat (Fagopyrum tataricum). BMC Genom. 2019, 20, 113. [Google Scholar] [CrossRef] [Green Version]
- Rong, Y.; Zhang, W.; Deng, J.; Shi, T.; Liang, C.; Wang, Y.; Zhang, X.; Li, H.; Meng, Z.; Huang, J. Bioinformatics analysis of NAC gene family in Tartary Buckwheat. J. Hunan Agric. Univ. (Nat. Sci. China) 2019, 45, 273–280. [Google Scholar]
- Mitsuda, N.; Iwase, A.; Yamamoto, H.; Yoshida, M.; Seki, M.; Shinozaki, K.; Ohme-Takagi, M. NAC transcription factors, NST1 and NST3, are key regulators of the formation of secondary walls in woody tissues of Arabidopsis. Plant Cell 2007, 19, 270–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuruzzaman, M.; Sharoni, A.M.; Satoh, K.; Karim, M.; Harikrishna, J.; Shimizu, T.; Sasaya, T.; Oomura, T.; Haque, M.; Kikuchi, S. NAC transcription factor family genes are differentially expressed in rice during infections with Rice dwarf virus, Rice black-streaked dwarf virus, Rice grassy stunt virus, Rice ragged stunt virus, and Rice transitory yellowing virus. Front. Plant Sci. 2015, 6, 676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aida, M.; Ishida, T.; Fukaki, H.; Fujisawa, H.; Tasaka, M. Genes involved in organ separation in Arabidopsis: An analysis of the cup-shaped cotyledon mutant. Plant Cell 1997, 9, 841–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hickman, R.; Hill, C.; Penfold, C.A.; Breeze, E.; Bowden, L.; Moore, J.D.; Zhang, P.; Jackson, A.; Cooke, E.; Bewicke-Copley, F.; et al. A local regulatory network around three NAC transcription factors in stress responses and senescence in Arabidopsis leaves. Plant J. 2013, 75, 26–39. [Google Scholar] [CrossRef] [Green Version]
- John, I.; Hackett, R.; Cooper, W.; Drake, R.; Farrell, A.; Grierson, D. Cloning and characterization of tomato leaf senescence-related cDNAs. Plant Mol. Biol. 1997, 33, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Gladman, N.P.; Marshall, R.S.; Lee, K.H.; Vierstra, R.D. The Proteasome Stress Regulon Is Controlled by a Pair of NAC Transcription Factors in Arabidopsis. Plant Cell 2016, 28, 1279–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, C.C.; Sieber, P.; Wellmer, F.; Meyerowitz, E.M. The early extra petals1 mutant uncovers a role for microRNA miR164c in regulating petal number in Arabidopsis. Curr. Biol. 2005, 15, 303–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Alessandro, S.; Ksas, B.; Havaux, M. Decoding beta-Cyclocitral-Mediated Retrograde Signaling Reveals the Role of a Detoxification Response in Plant Tolerance to Photooxidative Stress. Plant Cell 2018, 30, 2495–2511. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Bayon, R.; Shen, Y.; Groszmann, M.; Zhu, A.; Wang, A.; Allu, A.D.; Dennis, E.S.; Peacock, W.J.; Greaves, I.K. Senescence and Defense Pathways Contribute to Heterosis. Plant Physiol. 2019, 180, 240–252. [Google Scholar] [CrossRef] [Green Version]
- Puranik, S.; Sahu, P.P.; Srivastava, P.S.; Prasad, M. NAC proteins: Regulation and role in stress tolerance. Trends Plant Sci. 2012, 17, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Nuruzzaman, M.; Sharoni, A.M.; Kikuchi, S. Roles of NAC transcription factors in the regulation of biotic and abiotic stress responses in plants. Front. Microbiol. 2013, 4, 248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takasaki, H.; Maruyama, K.; Kidokoro, S.; Ito, Y.; Fujita, Y.; Shinozaki, K.; Yamaguchi-Shinozaki, K.; Nakashima, K. The abiotic stress-responsive NAC-type transcription factor OsNAC5 regulates stress-inducible genes and stress tolerance in rice. Mol. Genet. Genom. 2010, 284, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, T.; Sugahara, S.; Yamada, T.; Kikuchi, K.; Yoshiba, Y.; Hirano, H.Y.; Tsutsumi, N. OsNAC6, a member of the NAC gene family, is induced by various stresses in rice. Genes Genet. Syst. 2005, 80, 135–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Deng, Z.; Lai, J.; Zhang, Y.; Yang, C.; Yin, B.; Zhao, Q.; Zhang, L.; Li, Y.; Yang, C.; et al. Dual function of Arabidopsis ATAF1 in abiotic and biotic stress responses. Cell Res. 2009, 19, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Souer, E.; van Houwelingen, A.; Kloos, D.; Mol, J.; Koes, R. The no apical meristem gene of Petunia is required for pattern formation in embryos and flowers and is expressed at meristem and primordia boundaries. Cell 1996, 85, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.H.; Jeon, H.S.; Kim, H.G.; Park, O.K. An Arabidopsis NAC transcription factor NAC4 promotes pathogen-induced cell death under negative regulation by microRNA164. New Phytol. 2017, 214, 343–360. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Vargas, F.; Godoy, T.; Tejos, R.; O’Brien, J.A. Overexpression of the Auxin Receptor AFB3 in Arabidopsis Results in Salt Stress Resistance and the Modulation of NAC4 and SZF1. Int. J. Mol. Sci. 2020, 21, 9528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, X.; Ma, B.; Gao, Q.; Du, H.; Han, Y.; Li, Y.; Cao, Y.; Qi, M.; Zhu, Y.; et al. The Tartary Buckwheat Genome Provides Insights into Rutin Biosynthesis and Abiotic Stress Tolerance. Mol. Plant 2017, 10, 1224–1237. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Chen, Q.; Rong, Y.; Tang, B.; Zhu, L.; Ren, R.; Shi, T.; Chen, Q. Transcriptome analysis revealed gene regulatory network involved in PEG-induced drought stress in Tartary buckwheat (Fagopyrum tararicum). PeerJ 2021, 9, e11136. [Google Scholar] [CrossRef]
- Wu, Q.; Bai, X.; Zhao, W.; Xiang, D.; Wan, Y.; Yan, J.; Zou, L.; Zhao, G. De Novo Assembly and Analysis of Tartary Buckwheat (Fagopyrum tataricum Garetn.) Transcriptome Discloses Key Regulators Involved in Salt-Stress Response. Genes 2017, 8, 255. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Campbell, C. Tartary buckwheat breeding (Fagopyrum tataricum L. Gaertn.) through hybridization with its Rice-Tartary type. Euphytica 2007, 156, 399–405. [Google Scholar] [CrossRef]
- Lv, B.; Wu, Q.; Wang, A.; Li, Q.; Dong, Q.; Yang, J.; Zhao, H.; Wang, X.; Chen, H.; Li, C. A WRKY transcription factor, FtWRKY46, from Tartary buckwheat improves salt tolerance in transgenic Arabidopsis thaliana. Plant Physiol. Biochem. 2020, 147, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhao, H.; Wang, X.; Kang, J.; Lv, B.; Dong, Q.; Li, C.; Chen, H.; Wu, Q. Tartary Buckwheat Transcription Factor FtbZIP5, Regulated by FtSnRK2.6, Can Improve Salt/Drought Resistance in Transgenic Arabidopsis. Int. J. Mol. Sci. 2020, 21, 1123. [Google Scholar] [CrossRef] [Green Version]
- Yao, P.F.; Li, C.L.; Zhao, X.R.; Li, M.F.; Zhao, H.X.; Guo, J.Y.; Cai, Y.; Chen, H.; Wu, Q. Overexpression of a Tartary Buckwheat Gene, FtbHLH3, Enhances Drought/Oxidative Stress Tolerance in Transgenic Arabidopsis. Front. Plant Sci. 2017, 8, 625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, P.; Sun, Z.; Li, C.; Zhao, X.; Li, M.; Deng, R.; Huang, Y.; Zhao, H.; Chen, H.; Wu, Q. Overexpression of Fagopyrum tataricum FtbHLH2 enhances tolerance to cold stress in transgenic Arabidopsis. Plant Physiol. Biochem. 2018, 125, 85–94. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Z.; Tang, B.; Yu, H.; Tang, Z.; Bu, T.; Wu, Q.; Chen, H. Tartary Buckwheat (Fagopyrum tataricum) NAC Transcription Factors FtNAC16 Negatively Regulates of Pod Cracking and Salinity Tolerant in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 3197. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Tang, B.; Li, P.; Zhang, J.; Chen, Q.; Zhu, L.; Deng, J.; Huang, J. Identification and Expression of NAC Transcription Factor FtNAC17 in Tartary Buckwheat. Biotechnol. Bull. 2021, 37, 174. [Google Scholar]
- Huang, J.; Rong, Y.; Meng, Z.; Tang, B.; Zhang, J.; Xia, Z.; Chen, Q. Cloning and Expression of FtNAC15 Transcription Factor in Fagopyrum tataricum. Acta Agric. Univ. Jiangxiensis 2019, 41, 1183–1191. [Google Scholar]
- Deng, R.Y.; Zhao, H.X.; Xiao, Y.H.; Huang, Y.J.; Yao, P.F.; Lei, Y.L.; Li, C.L.; Chen, H.; Wu, Q. Cloning, Characterization, and Expression Analysis of Eight Stress-Related NAC Genes in Tartary Buckwheat. Crop Sci. 2019, 59, 266–279. [Google Scholar] [CrossRef]
- Rychlik, W. OLIGO 7 primer analysis software. Methods Mol. Biol. 2007, 402, 35–60. [Google Scholar] [CrossRef]
- Huang, J.; Deng, J.; Shi, T.; Chen, Q.; Liang, C.; Meng, Z.; Zhu, L.; Wang, Y.; Zhao, F.; Yu, S.; et al. Global transcriptome analysis and identification of genes involved in nutrients accumulation during seed development of rice tartary buckwheat (Fagopyrum tararicum). Sci. Rep. 2017, 7, 11792. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Dehais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouze, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Crooks, G.E.; Hon, G.; Chandonia, J.M.; Brenner, S.E. WebLogo: A sequence logo generator. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Brauc, S.; De Vooght, E.; Claeys, M.; Geuns, J.M.; Hofte, M.; Angenon, G. Overexpression of arginase in Arabidopsis thaliana influences defence responses against Botrytis cinerea. Plant Biol. 2012, 14 (Suppl. 1), 39–45. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verslues, P.E.; Agarwal, M.; Katiyar-Agarwal, S.; Zhu, J.; Zhu, J.K. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 2006, 45, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.M.; Moyano, T.C.; Zhang, T.; Gras, D.E.; Herrera, F.J.; Araus, V.; O’Brien, J.A.; Carrillo, L.; Medina, J.; Vicente-Carbajosa, J.; et al. Local Changes in Chromatin Accessibility and Transcriptional Networks Underlying the Nitrate Response in Arabidopsis Roots. Mol. Plant 2019, 12, 1545–1560. [Google Scholar] [CrossRef] [Green Version]
- Kan, C.; Zhang, Y.; Wang, H.L.; Shen, Y.; Xia, X.; Guo, H.; Li, Z. Transcription Factor NAC075 Delays Leaf Senescence by Deterring Reactive Oxygen Species Accumulation in Arabidopsis. Front. Plant Sci. 2021, 12, 634040. [Google Scholar] [CrossRef]
- Meng, X.; Li, G.; Yu, J.; Cai, J.; Dong, T.; Sun, J.; Xu, T.; Li, Z.; Pan, S.; Ma, D. Isolation, Expression Analysis, and Function Evaluation of 12 Novel Stress-Responsive Genes of NAC Transcription Factors in Sweetpotato. Crop Sci. 2018, 58, 1328–1341. [Google Scholar] [CrossRef]
- Dekkers, B.J.; Pearce, S.; van Bolderen-Veldkamp, R.P.; Marshall, A.; Widera, P.; Gilbert, J.; Drost, H.G.; Bassel, G.W.; Muller, K.; King, J.R.; et al. Transcriptional dynamics of two seed compartments with opposing roles in Arabidopsis seed germination. Plant Physiol. 2013, 163, 205–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidal, E.A.; Alvarez, J.M.; Gutierrez, R.A. Nitrate regulation of AFB3 and NAC4 gene expression in Arabidopsis roots depends on NRT1.1 nitrate transport function. Plant Signal. Behav. 2014, 9, e28501. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Liu, N.; Virlouvet, L.; Riethoven, J.J.; Fromm, M.; Avramova, Z. Four distinct types of dehydration stress memory genes in Arabidopsis thaliana. BMC Plant Biol. 2013, 13, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winter, D.; Vinegar, B.; Nahal, H.; Ammar, R.; Wilson, G.V.; Provart, N.J. An “Electronic Fluorescent Pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS ONE 2007, 2, e718. [Google Scholar] [CrossRef] [PubMed]

| Gene Name | Genome Data Accession | CDS Length (bp) | DNA Length (bp) | Protein Length (Amino Acids) | Protein Molecular Weight (kDa) | Protein Theoretical pI | Protein Predicted Location | GenBank Accession | Chromosome |
|---|---|---|---|---|---|---|---|---|---|
| FtNAC11 | FtPinG0006563600 | 840 | 1023 | 279 | 31.82 | 6.91 | Nucleus | MW355481 | Ft1 |
| FtNAC12 | FtPinG0002560000 | 888 | 1144 | 295 | 33.25 | 8.86 | Nucleus | MW355482 | Ft1 |
| FtNAC26 | FtPinG0000059500 | 1242 | 4032 | 413 | 46.30 | 5.53 | Nucleus | MW355483 | Ft2 |
| FtNAC28 | FtPinG0002920400 | 942 | 1439 | 313 | 35.52 | 8.82 | Nucleus | MW355484 | Ft2 |
| FtNAC65 | FtPinG0005688900 | 729 | 922 | 242 | 28.01 | 8.97 | Nucleus | MW355485 | Ft7 |
| FtNAC70 | FtPinG0002339300 | 1020 | 1535 | 339 | 38.15 | 5.39 | Nucleus | MW355486 | Ft8 |
| FtNAC78 | FtPinG0005906600 | 1260 | 4908 | 419 | 47.14 | 6.43 | Nucleus | MW355487 | Ft8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Ren, R.; Rong, Y.; Tang, B.; Deng, J.; Chen, Q.; Shi, T. Identification, Expression, and Functional Study of Seven NAC Transcription Factor Genes Involved in Stress Response in Tartary Buckwheat (Fagopyrum tataricum (L.) Gaertn.). Agronomy 2022, 12, 849. https://doi.org/10.3390/agronomy12040849
Huang J, Ren R, Rong Y, Tang B, Deng J, Chen Q, Shi T. Identification, Expression, and Functional Study of Seven NAC Transcription Factor Genes Involved in Stress Response in Tartary Buckwheat (Fagopyrum tataricum (L.) Gaertn.). Agronomy. 2022; 12(4):849. https://doi.org/10.3390/agronomy12040849
Chicago/Turabian StyleHuang, Juan, Rongrong Ren, Yuping Rong, Bin Tang, Jiao Deng, Qingfu Chen, and Taoxiong Shi. 2022. "Identification, Expression, and Functional Study of Seven NAC Transcription Factor Genes Involved in Stress Response in Tartary Buckwheat (Fagopyrum tataricum (L.) Gaertn.)" Agronomy 12, no. 4: 849. https://doi.org/10.3390/agronomy12040849
APA StyleHuang, J., Ren, R., Rong, Y., Tang, B., Deng, J., Chen, Q., & Shi, T. (2022). Identification, Expression, and Functional Study of Seven NAC Transcription Factor Genes Involved in Stress Response in Tartary Buckwheat (Fagopyrum tataricum (L.) Gaertn.). Agronomy, 12(4), 849. https://doi.org/10.3390/agronomy12040849





