Effects of Cultivar, Nitrogen Rate and Biostimulant Application on the Chemical Composition of Perennial Ryegrass (Lolium perenne L.) Biomass
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Experiment
- k—Selyaninov hydrothermal coefficient
- P—total monthly precipitation,
- ∑t—mean monthly temperature (sum of mean daily temperatures) >0.
2.2. Plant Materials
2.3. Statistical Analysis
3. Results and Discussion
3.1. Weather Conditions
3.2. Crude Protein (CP)
3.3. Crude Fiber (CF)
3.4. Leaf Greenness (SPAD)
3.5. Phosphorus (P)
3.6. Potassium (K)
3.7. Calcium (Ca)
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taweel, H.Z.; Tas, T.B.M.; Smit, H.J.; Elgersma, A.; Dijkstra, J.; Tamminga, S. Improving the quality of perennial ryegrass (Lolium perenne L.) for dairy cows by selecting for fast clearing and/or degradable neutral detergent fiber. Livest. Prod. Sci. 2005, 96, 239–248. [Google Scholar] [CrossRef]
- Daccord, R.; Arrigo, Y.; Kessler, J.; Jeangros, B.; Scehovic, J.; Schubiger, F.X.; Lehmann, J. Nutritive value of grassland plants. Contents of calcium, phosphorus, magnesium and potassium. Agrarforschung 2001, 8, 264–269. [Google Scholar]
- Schlegel, P.; Wyss, U.; Arrigo, Y.; Hess, H.D. Mineral concentrations of fresh herbage from mixed grassland as influenced by botanical composition, harvest time and growth stage. Anim. Feed Sci. Technol. 2016, 219, 226–233. [Google Scholar] [CrossRef]
- Wyłupek, T.; Harkot, W.; Czarnecki, Z. The content of selected macroelements in the dry weight of permanent grassland sward, grass yields and its agricultural value. J. Elem. 2014, 19, 853–864. [Google Scholar] [CrossRef]
- Pirhofer-Walzl, K.; Søegaard, K.; Høgh-Jensen, H.; Eriksen, J.; Sanderson, M.A.; Rasmussen, J.; Rasmussen, J. Forage herbs improve mineral composition of grassland herbage. Grass Forage Sci. 2011, 66, 415–423. [Google Scholar] [CrossRef]
- Delagarde, R.; Peyraud, J.L.; Delaby, L.; Faverdin, P. Vertical distribution of biomass, chemical composition and pepsin-cellulase digestibility in a perennial ryegrass sward: Interaction with month of year, regrowth age and time of day. Anim. Feed Sci. Technol. 2000, 84, 49–68. [Google Scholar] [CrossRef]
- Moot, D.J.; Scott, W.D.; Roy, A.M.; Nicholls, A.C. Base temperature and thermal time requirements fir germination and emergence of temperate pasture species. N. Z. J. Agric. Res. 2000, 43, 15–25. [Google Scholar] [CrossRef]
- Guy, C.; Hennessy, D.; Gilliland, T.; Coughlan, F.; McCarthy, B. Growth, morphology and biological nitrogen fixation potential of perennial ryegrass-white clover swards throughout the grazing season. J. Agric. Sci. 2018, 156, 188–199. [Google Scholar] [CrossRef]
- Nair, R. Developing tetraploid perennial ryegrass (Lolium perenne L.) populations. N. Z. J. Agric. Res. 2004, 47, 45–49. [Google Scholar] [CrossRef]
- Olszewska, M. Effects of Cultivar, Nitrogen Rate and Harvest Time on the Content of Carbohydrates and Protein in the Biomass of Perennial Ryegrass. Agronomy 2021, 11, 468. [Google Scholar] [CrossRef]
- Shahabivand, S.; Padash, A.; Aghaee, A.; Nasiri, Y.; Rezaei, P.F. Plant biostimulants (Funneliformis mosseae and humic substances) rather than chemical fertilizer improved biochemical responses in peppermint. Iran. J. Plant Physiol. 2018, 8, 2333–2344. [Google Scholar] [CrossRef]
- Perrino, E.V.; Calabrese, G. Endangered segetal species in southern Italy: Distribution, conservation status, trends, actions and ethnobotanical notes. Genet. Resour. Crop Evol. 2018, 65, 2107–2134. [Google Scholar] [CrossRef]
- Perrino, E.V.; Wagensommer, R.P. Crop Wild Relatives (CWRs) Threatened and Endemic to Italy: Urgent Actions for Protection and Use. Biology 2022, 11, 193. [Google Scholar] [CrossRef] [PubMed]
- Godlewska, A.; Ciepiela, G.A. Italian Ryegrass (Lolium multiflorum Lam.) Fiber Fraction Content and Dry Matter Digestibility Following Biostimulant Application against the Background of Varied Nitrogen Regime. Agronomy 2021, 11, 39. [Google Scholar] [CrossRef]
- Godlewska, A.; Ciepiela, G. Carbohydrate and lignin contents in perennial ryegrass (Lolium perenne L.) treated with sea bamboo (Ecklonia maxima) extract against the background of nitrogen fertilization regime. Appl. Ecol. Environ. Res. 2020, 18, 6087–6097. [Google Scholar] [CrossRef]
- Godlewska, A.; Ciepiela, G.A. The effect of growth regulator on dry matter yield and some chemical components in selected grass species and cultivars. Soil Sci. Plant Nutr. 2016, 62, 297–302. [Google Scholar] [CrossRef]
- Godlewska, A.; Ciepiela, G.A. The effect of natural growth regulators obtained from ecklonia maxima and mineral nitrogen on true protein and simple sugar contents of Dactylis glomerata L. and Festulolium braunii (K. Richt.) A. Camus. Turk. J. Field Crop. 2013, 18, 247–253. [Google Scholar]
- Murawska, B.; Gabrowska, M.; Spychaj-Fabisiak, E.; Wszelaczyńska, E.; Chmielewski, J. Production and environmental aspects of the application of biostimulators Asahi SL, Kelpak SL and stymulator Tytanit with limited doses of nitrogen. Environ. Protec. Nat. Resour. 2017, 28, 10–15. [Google Scholar] [CrossRef][Green Version]
- Sosnowski, J.; Malinowska, E.; Jankowski, K.; Redzik, P. Morpho-chemical diversity in Festuca pratensis and Lolium perenne depending on concentrations of Ecklonia maxima extract. Appl. Ecol. Env. Res. 2016, 14, 369–379. [Google Scholar] [CrossRef]
- Sharma, H.S.S.; Fleming, C.; Selby, C.; Rao, J.R.; Martin, T. Plant biostimulants: A review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. J. Appl. Phycol. 2014, 26, 465–490. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Alam, M.Z.; Braun, G.; Norrie, J.; Hodges, D.M. Ascophyllum extract application can promote plant growth and root yield in carrot associated with increased root-zone soil microbial activity. Can. J. Plant Sci. 2014, 94, 337–348. [Google Scholar] [CrossRef]
- Kleiber, T.; Markiewicz, B. Application of “Tytanit” in greenhouse tomato growing. Acta Sci. Pol. Hortorum Cult. 2013, 12, 117–126. [Google Scholar]
- Matysiak, K.; Kaczmarek, S.; Krawczyk, R. Influence of seaweed extracts and mixture of humic acid fulvic acids on germination and growth of Zea mays L. Acta Sci. Pol. Agric. 2011, 10, 33–45. [Google Scholar]
- Sivasankari, S.; Venkatesalu, V.; Anantharaj, M.; Chandrasekaran, M. Effect of seaweed extracts on the growth and biochemical constituents of Vigna sinensis. Bioresour. Technol. 2005, 97, 1745–1751. [Google Scholar] [CrossRef]
- Karr-Lilienthal, L.; Kadzere, C.; Grieshop, C.; Fahey, G. Chemical and nutritional properties of soybean carbohydrates as related to nonruminants: A review. Livest. Prod. Sci. 2005, 97, 1–12. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No. 1455/1999 of 1 July 1999 as Last Amended by Regulations (EC) No. 2706/2000 and No. 46/2003. Available online: https://eur-lex.europa.eu/ (accessed on 7 March 2022).
- Official Website of Agrosystemy, a Supplier of Effective Microorganisms (EM) Products Manufactured by the Austrian Company Multikraft. Available online: https://agrosystemy.com/ (accessed on 7 March 2022).
- Majkowska-Gadomska, J.; Dobrowolski, A.; Jadwisieńczak, K.K.; Kaliniewicz, Z.; Francke, A. Effect of biostimulants on the growth, yield and nutritional value of Capsicum annuum grown in an unheated plastic tunnel. Sci. Rep. 2021, 11, 22335. [Google Scholar] [CrossRef]
- Skowera, B.; Puła, J. Pluviometric extreme conditions in spring season in Poland in the years 1971–2000. Acta Agroph. 2004, 3, 171–177. (In Polish) [Google Scholar]
- Henneberg, W.; Stohmann, F. Über das Ernährungsfutter volljährigen Rindviehs. J. Landwirtsch 1859, 3, 485–551. [Google Scholar]
- TIBCO Software Inc. Statistica (Data Analysis Software System, Palo Alto, USA). 2017, Version 13.3. Available online: https://docs.tibco.com/products/tibco-statistica-13-3-0 (accessed on 7 January 2022).
- Alende, M.; Fluck, A.C.; Volpi-Lagreca, G.; Andrae, J.G. Chemical composition and in vitro digestibility of annual ryegrass varieties grown in greenhouse conditions. RIA 2020, 46, 50–55. [Google Scholar]
- Stewart, A.; Hayes, R. Ryegrass breeding-balancing trait priorities. Ir. J. Agric. Food Res. 2011, 50, 31–46. [Google Scholar]
- Smith, K.F.; Simpson, R.J.; Culvenor, R.A.; Humphreys, M.O.; Prud’Homme, M.P.; Oram, R.N. The effects of ploidy and a phenotype conferring a high water-soluble carbohydrate concentration on carbohydrate accumulation, nutritive value and morphology of perennial ryegrass (Lolium perenne L.). J. Agric. Sci. 2001, 136, 65–74. [Google Scholar] [CrossRef]
- Cosgrove, G.P.; Burke, J.L.; Death, A.F.; Hickey, M.J.; Pacheco, D.; Lane, G.A. Ryegrasses with increased water soluble carbohydrate: Evaluating the potential for grazing dairy cows in New Zealand. Proc. N. Z. Grassl. Assoc. 2007, 69, 179–185. [Google Scholar] [CrossRef]
- Wims, C.M.; Mcevoy, M.; Delaby, T.M.; Boland, T.M.; Donovan, M.O. Effect of perennial ryegrass (Lolium perenne L.) cultivars on the milk yield of grazing dairy cows. Animal 2012, 7, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, S. Responses of shoot growth and survival to water stress gradient in diploid and tetraploid populations of Lolium multiflorum and L. perenne. Grassl. Sci. 2006, 52, 155–160. [Google Scholar] [CrossRef]
- Balocchi, O.A.; López, I.F. Herbage production, nutritive value and grazing preference of diploid and tetraploid perennial ryegrass cultivars (Lolium perenne L.). Chil. J. Agric. Res. 2009, 69, 331–339. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Veloso, A.; Vaz, E.; Almeida, J.F. Production and nutritional composition of two annual ryegrass cultivars (diploid and tetraploid). Curr. Investig. Agric. Curr. Res. 2019, 7, 914–916. [Google Scholar] [CrossRef][Green Version]
- Solomon, J.K.Q.; Macoon, B.; Lang, D.J.; Vann, R.C.; Ward, S. Cattle grazing preference among tetraploid and diploid annual ryegrass cultivars. Crop Sci. 2014, 54, 430–438. [Google Scholar] [CrossRef]
- Ihtisham, M.; Fahad, S.; Luo, T.; Larkin, R.M.; Yin, S.; Chen, L. Optimization of nitrogen, phosphorus and potassium fertilization rates for overseeded perennial ryegrass turfon dormant bermudagrass in atransitional climate. Front. Plant Sci. 2018, 9, 487. [Google Scholar] [CrossRef]
- Purwin, C.; Stanek, M.; Lipiński, K.; Wierzbowska, J.; Nogalska, A.; Fijalkowska, M. Effect of a harvest time and cultivar on the chemical composition and in vitro ruminal dry matter degradability of perennial ryegrass (Lolium perenne L.). J. Elem. 2016, 21, 811–822. [Google Scholar] [CrossRef]
- Belanger, G.; Virkajarvi, P.; Duru, M.; Tremblay, G.F.; Saarijarvi, K. Herbage nutritive in less—Favoured areas of cool regions. Grassl. Sci. Eur. 2013, 18, 57–70. [Google Scholar]
- Baert, J.; Van Waes, C. Improvement of the digestibility of tall fescue (Festuca arundinacea Schreb.) inspired by perennial ryegrass (Lolium perenne L.). Grassl. Sci. Eur. 2014, 19, 172–174. [Google Scholar]
- Staniak, M.; Księżak, J. Chemical composition of Festulolium braunii-Trifolium pratense mixtures in relations to nitrogen fertilisation and the share of components. Water-Environ.-Rural Areas 2008, 8, 163–173. (In Polish) [Google Scholar]
- Szkutnik, J.; Kacorzyk, P.; Szewczyk, W. The content change of total protein and crude fibre depending on the dose of fertilization and phonological phase of grasses. Grassl. Sci. Pol. 2012, 15, 185–191. [Google Scholar]
- Zielewicz, W.; Wróbel, B.; Niedbała, G. Quantification of Chlorophyll and Carotene Pigments Content in Mountain Melick (Melica nutans L.) in Relation to Edaphic Variables. Forests 2020, 11, 1197. [Google Scholar] [CrossRef]
- Selzer, L.J.; Busso, C.A. Pigments and photosynthesis of understory grasses: Light irradiance and soil moisture effects. Russ. J. Plant Phys. 2016, 63, 224–234. [Google Scholar] [CrossRef]
- Olszewska, M.; Grzegorczyk, S.; Olszewski, J.; Bałuch-Małecka, A. A comparison of the response of selected grass species to water stress. Grassl. Sci. Pol. 2010, 13, 127–136. [Google Scholar]
- Golińska, B. Chlorophyll as an indicator of nitrogen status of Poa pratensis (Poaceae) in conditions of repeated defoliation of its sward. Fragm. Florist. Geobot. Pol. 2007, 9, 137–145. (In Polish) [Google Scholar]
- Olszewska, M.; Grzegorczyk, S. Effect of manganese deficiency on gas exchange parameters, leaf greenness (SPAD) and yield of perennial ryegrass (Lolium perenne L.) and orchard grass (Dactylis glomerata L.). J. Elem. 2008, 13, 589–596. [Google Scholar]
- Olszewska, M.; Grzegorczyk, S.; Olszewski, J.; Bałuch-Małecka, A. Effect of phosphorus deficiency on gas exchange parameters, leaf greenness (SPAD) and yield of perennial ryegrass (Lolium perenne L.) and orchard grass (Dactylis glomerata L.). J. Elem. 2008, 13, 91–99. [Google Scholar]
- Kozłowski, S.; Swędrzyński, A. Variability in the occurrence of chlorophyll and carotenoids dyes in Lolium perenne (Poaceae). Fragm. Flor. Geobot. Pol. 2007, 9, 163–171. (In Polish) [Google Scholar]
- Çelebi, Z.; Andiç, N.; Yilmaz, H. Determination of proper species mixtures for established turfgrass field in Van region. YYU J. Agric. Sci. 2010, 20, 16–25. [Google Scholar]
- Wood, C.W.; Reeves, D.W.; Himelrick, D.G. Relationships between chlorophyll meter readings and leaf chlorophyll concentration, N status, and crop yield: A review. Proc. Agron. Soc. N. Z. 1993, 23, 1–9. [Google Scholar]
- Hudson, D.; Guevara, D.; Yaish, M.W.; Hannam, C.; Long, N.; Clarke, J.D.; Bi, Y.M.; Rothstein, S.J. GNC and CGA1 modulate chlorophyll biosynthesis and glutamate synthase (GLU1/Fd-GOGAT) expression in Arabidopsis. PLoS ONE 2011, 6, e26765. [Google Scholar] [CrossRef]
- Havé, M.; Marmagne, A.; Chardon, F.; Masclaux-Daubresse, C. Nitrogen remobilization during leaf senescence: Lessons from Arabidopsis to crops. J. Exp. Bot. 2016, 68, 2513–2529. [Google Scholar] [CrossRef]
- Lyu, S.; Wei, X.; Chen, J.; Wang, C.; Wang, X.-M.; Pan, D. Titanium as a Beneficial Element for Crop Production. Front. Plant Sci. 2017, 8, 597. [Google Scholar] [CrossRef]
- Kovacik, P.; Hudec, J.; Ondrisik, P.; Poliakova, N. The effect of liquid Mg-Titanit on creation of winter wheat phytomass. Res. J. Agric. Sci. 2014, 46, 125–131. [Google Scholar]
- Ciepiela, G.A.; Godlewska, A.; Jankowska, J. The effect of biostimulant on yields of mixed grass/red clover stands and chlorophyll kontent in crop plant leaves under different nitrogen fertilisation regimes. Fresenius Environ. Bull. 2013, 22, 3700–3708. [Google Scholar]
- Zielewicz, W.; Kozłowski, S. Occurrence of chlorophyll and carotene dyes in forest grasses. Grassld. Sci. Pol. 2011, 14, 161–170. [Google Scholar]
- Kozłowski, S.; Kubiak, T.; Swędrzyński, A. Variability of chlorophyll dyes occurrence in leaf blades of Phragmites australis (Cav.) Trin. ex Steud. Grassl. Sci. Pol. 2014, 17, 53–60. (In Polish) [Google Scholar]
- Jodełka, J.; Sosnowski, J. Evaluation of nitrogen delivery methods to some grass species depending on the phosphorus and potassium fertilization. Fragm. Agronom. 2010, 27, 53–61. (In Polish) [Google Scholar]
- Gaborcik, N.; Zmetakova, Z. Chlorophyll (SPAD readings) and nitrogen concentrations in leaves of some forage grasses and legumes. Grassl. Sci. Pol. 2001, 4, 43–48. [Google Scholar]
- Olszewska, M. Effects of magnesium deficiency on gas exchange parameters, leaf greenness index (SPAD) and yields of Lolium perenne and Dactylis glomerata. Grassl. Sci. Pol. 2005, 6, 141–148. (In Polish) [Google Scholar]
- Falkowski, M.; Kukułka, I.; Kozłowski, S. Chemical Properties of Meadow Plants; Wydawnictwo Uniwersytetu Przyrodniczego w Poznaniu: Poznań, Poland, 2000; p. 132. (In Polish) [Google Scholar]
- Wyss, U.; Kessler, J. The intensity of grassland management influences the mineral contents of the grass. Agrarforschung 2002, 9, 292–297. [Google Scholar]
- Gaj, R.; Maciejewski, T.; Rębarz, K. Effect of irrigation and nitrogen fertilization on potassium content in the three grasses cultivated in field cultivation. Zesz. Nauk. Uniw. Przyr. Wroc. 2014, 599, 7–16. (In Polish) [Google Scholar]
- Grzebisz, W.; Bandurska, H. Potassium uptake by plants from soil—Mechanisms and conditions. J. Elem. 2004, 9, 27–36. (In Polish) [Google Scholar]
- Neto, G.B.; Reis, R.A.; Ruggieri, A.C. Impacts of limestone and nitrogen top dressing application on the potassium content in the soil profile and marandu-grass leaf concentration. Rev. Bras. Zootec. 2009, 38, 1170–1175. [Google Scholar] [CrossRef][Green Version]
- Ciepiela, G.A.; Godlewska, A. The effect of the biostimulant Kelpak SL on selected macroelements in two grass species. Fresenius Environ. Bull. 2017, 26, 2779–2784. [Google Scholar]

| Years of the Study | Month | ||||||
|---|---|---|---|---|---|---|---|
| April | May | June | July | August | September | October | |
| 2016 | 1.3 (fd) | 1.3 (fd) | 1.3 (fd) | 2.5 (w) | 1.3 (fd) | 0.5 (vd) | 5.54 (ew) |
| 2017 | 3.4 (ew) | 0.7 (vd) | 1.6 (o) | 2.1(w) | 1.2 (fd) | 4.44 (ew) | 4.34 (ew) |
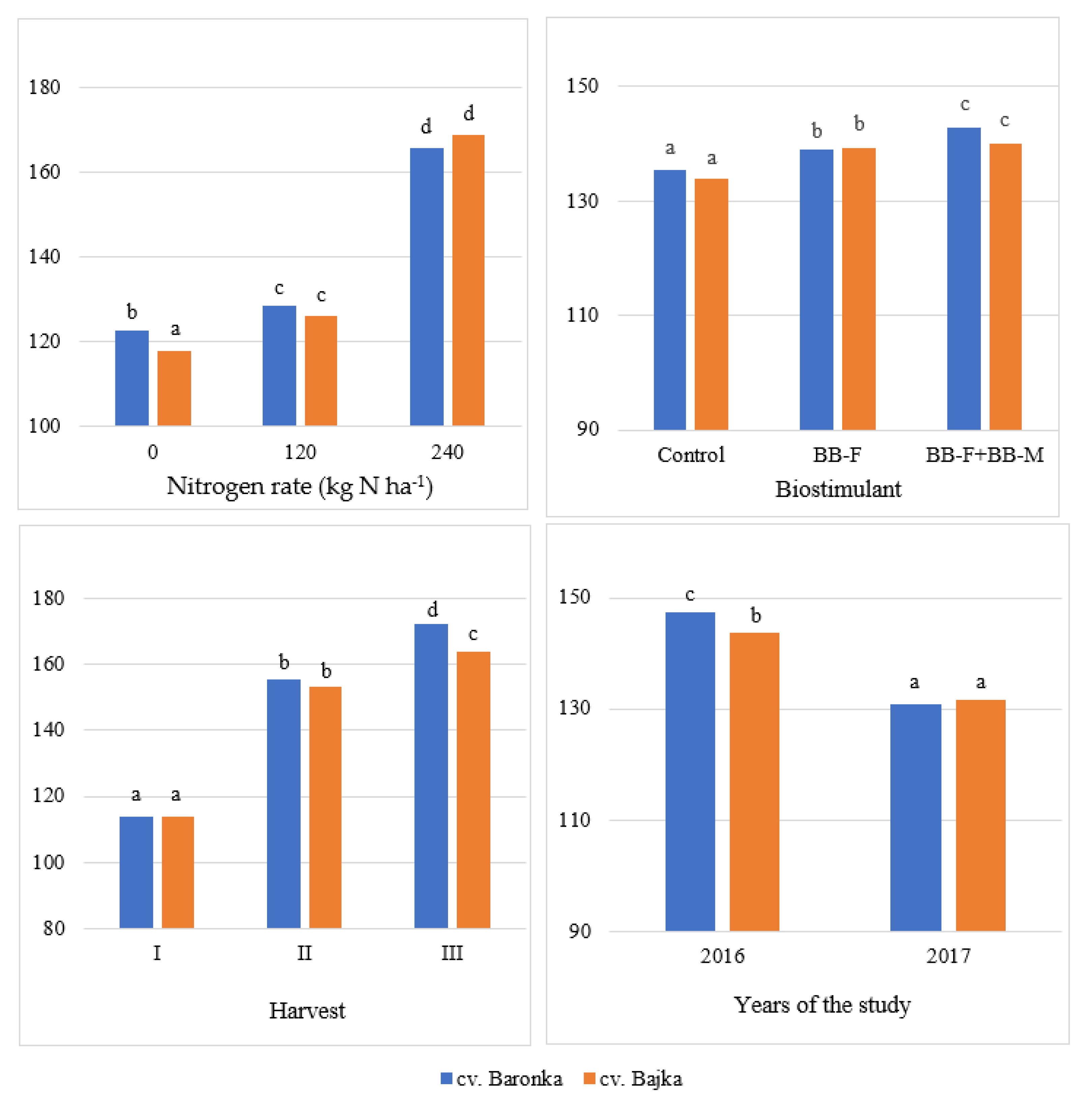
| Factor | Years of the Study | Mean | |
|---|---|---|---|
| 2016 | 2017 | ||
| Cultivar | |||
| Baronka | 147.37 b | 130.77 a | 139.07 b |
| Bajka | 143.58 a | 131.65 a | 137.62 a |
| Nitrogen rate (kg ha−1) | |||
| 0 | 123.45 a | 117.19 a | 120.32 a |
| 120 | 131.97 b | 122.69 b | 127.33 b |
| 240 | 181.01 c | 153.76 c | 167.39 c |
| Biostimulant | |||
| Control | 140.26 a | 128.99 a | 134.63 a |
| BB-F | 145.95 b | 132.23 b | 139.09 b |
| BB-F + BB-M | 150.22 c | 132.42 b | 141.32 c |
| Harvest | |||
| I | 113.97 a | 107.96 a | 110.97 a |
| II | 154.33 b | 120.06 b | 137.20 b |
| III | 168.13 c | 165.62 c | 166.88 c |
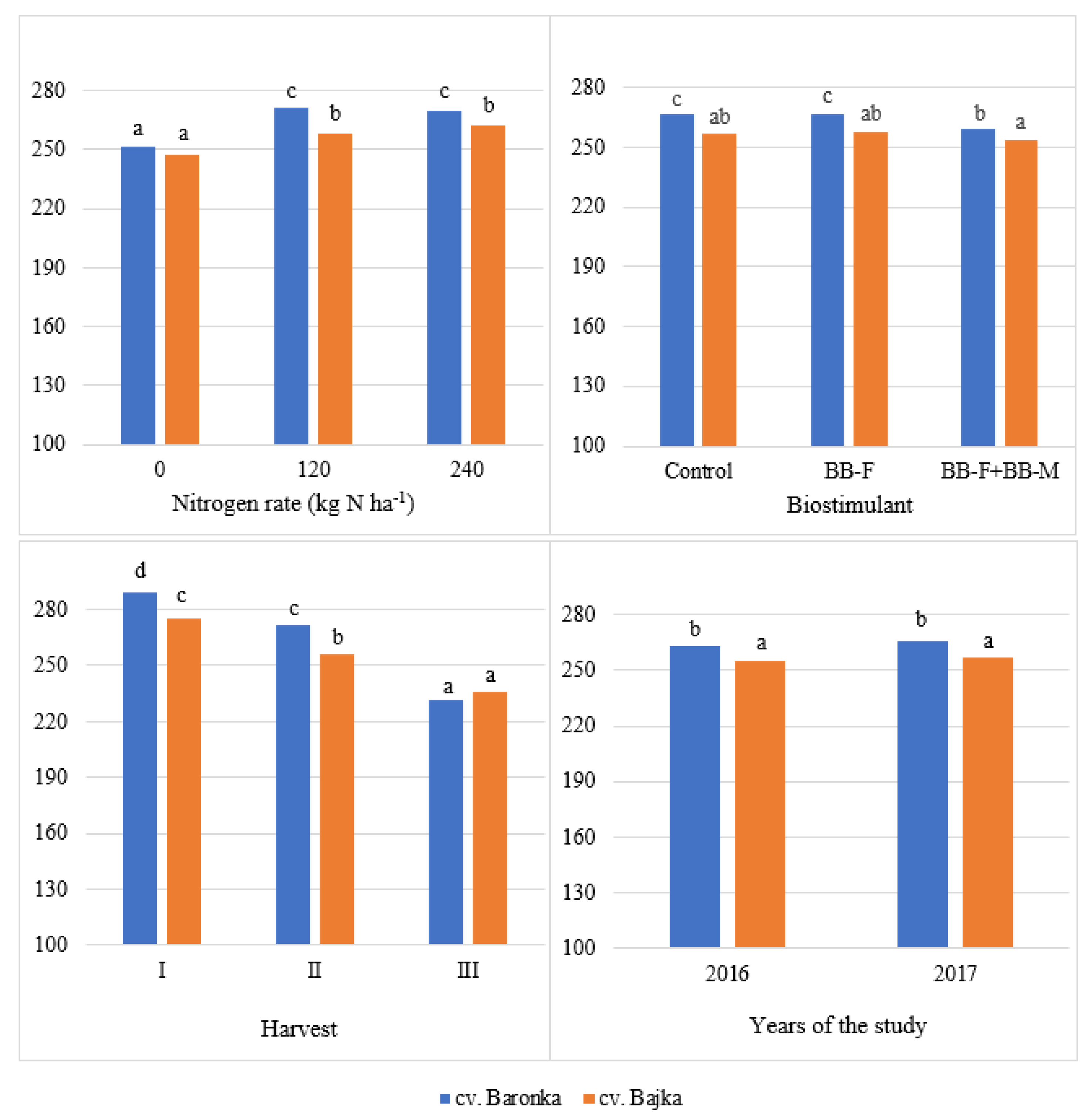
| Factor | Years of the Study | Mean | |
|---|---|---|---|
| 2016 | 2017 | ||
| Cultivar | |||
| Baronka | 263.03 b | 265.64 b | 264.21 b |
| Bajka | 255.17 a | 256.64 a | 255.91 a |
| Nitrogen rate (kg ha−1) | |||
| 0 | 248.60 a | 250.60 a | 249.59 a |
| 120 | 265.00 b | 264.23 b | 264.62 b |
| 240 | 263.70 b | 268.22 b | 265.96 b |
| Biostimulant | |||
| Control | 261.06 b | 262.38 b | 261.72 b |
| BB-F | 262.46 b | 261.75 b | 262.11 b |
| BB-F + BB-M | 253.78 a | 258.92 a | 256.35 a |
| Harvest | |||
| I | 298.38 c | 271.96 c | 285.17 c |
| II | 256.39 b | 266,27 b | 261.33 b |
| III | 222.53 a | 244.82 a | 233.68 a |
| Factor | Years of the Study | Mean | |
|---|---|---|---|
| 2016 | 2017 | ||
| Cultivar | |||
| Baronka | 37.18 b | 35.37 b | 36.28 b |
| Bajka | 34.81 a | 32.95 a | 33.88 a |
| Nitrogen rate (kg ha−1) | |||
| 0 | 33.37 a | 31.21 a | 32.29 a |
| 120 | 35.61 b | 33.69 b | 34.65 b |
| 240 | 39.01 c | 37.58 c | 38.29 c |
| Biostimulant | |||
| Control | 34.56 a | 33.41 a | 33.99 a |
| BB-F | 35.88 b | 33.22 a | 34.55 a |
| BB-F + BB-M | 37.54 c | 35.85 b | 36.70 b |
| Harvest | |||
| I | 37.18 b | 36.46 c | 36.82 c |
| II | 35.26 a | 31.55 a | 33.40 a |
| III | 35.55 a | 34.47 b | 35.01 b |
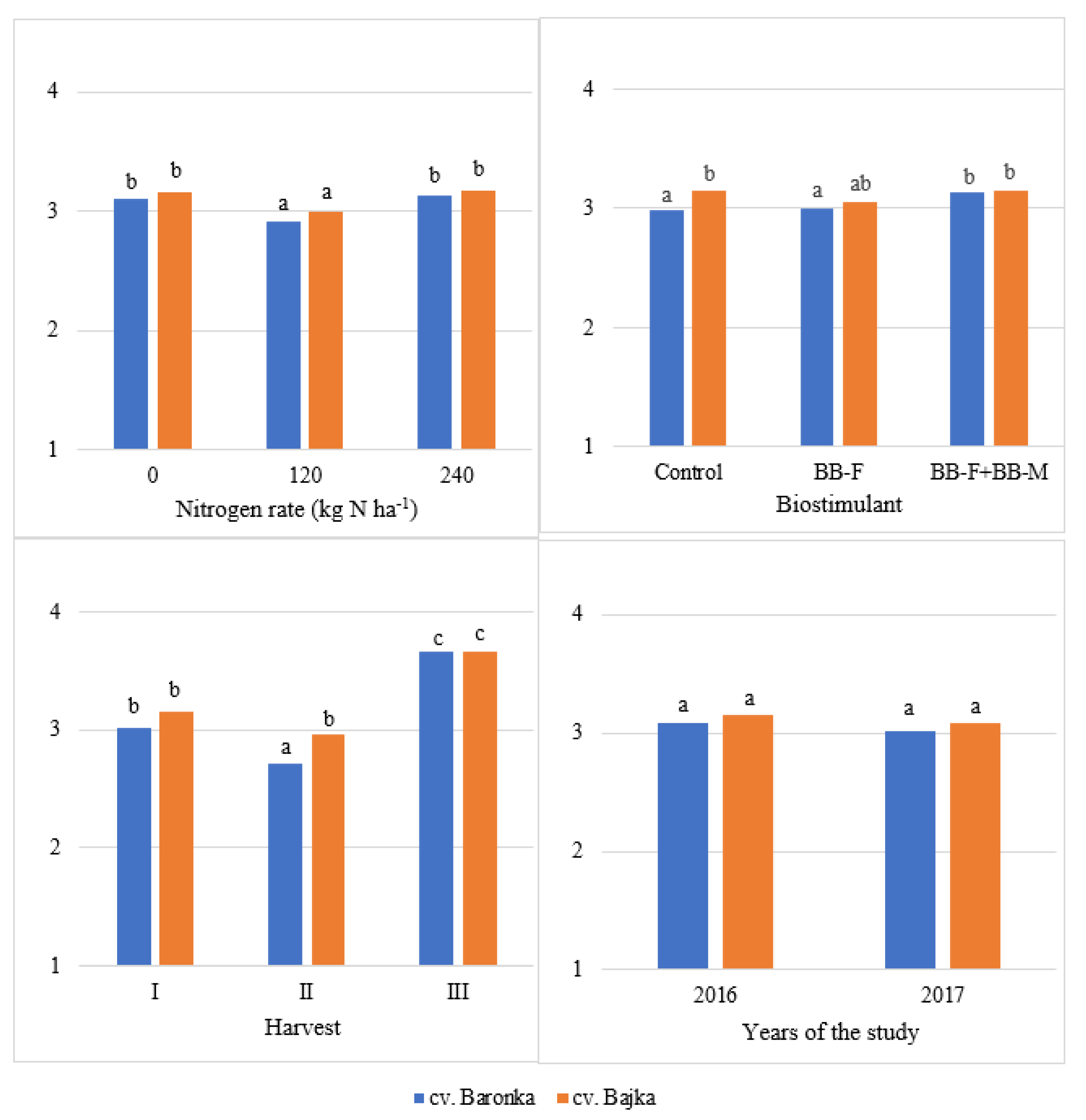
| Factor | Years of the Study | Mean | |
|---|---|---|---|
| 2016 | 2017 | ||
| Cultivar | |||
| Baronka | 3.09 a | 3.19 a | 3.14 a |
| Bajka | 3.15 b | 3.37 b | 3.26 b |
| Nitrogen rate (kg ha−1) | |||
| 0 | 3.18 b | 3.20 b | 3.19 b |
| 120 | 2.99 a | 3.06 a | 3.03 a |
| 240 | 3.19 b | 3.57 c | 3.38 b |
| Biostimulant | |||
| Control | 3.09 a | 3.31 b | 3.20 ab |
| BB-F | 3.08 a | 3.33 b | 3.21 b |
| BB-F + BB-M | 3.18 b | 3.19 a | 3.19 a |
| Harvest | |||
| I | 3.32 b | 2.86 a | 3.09 b |
| II | 2.64 a | 3.04 b | 2.84 a |
| III | 3.39 b | 3.94 c | 3.67 c |
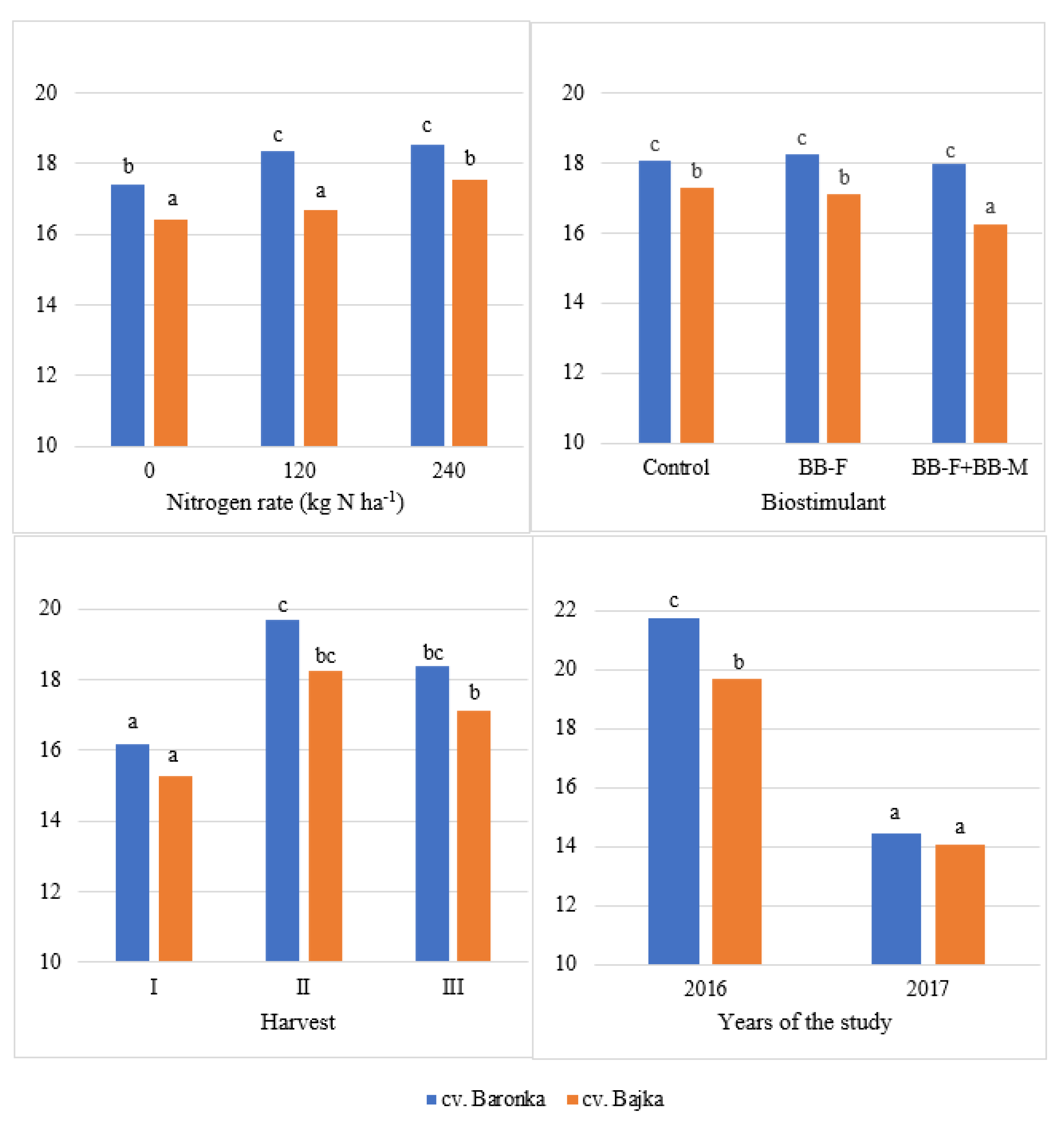
| Factor | Years of the Study | Mean | |
|---|---|---|---|
| 2016 | 2017 | ||
| Cultivar | |||
| Baronka | 21.74 b | 14.45 b | 18.10 b |
| Bajka | 19.67 a | 14.09 a | 16.88 a |
| Nitrogen rate (kg ha−1) | |||
| 0 | 19.88 a | 13.93 a | 16.90 a |
| 120 | 20.75 b | 14.27 b | 17.51 b |
| 240 | 21.48 c | 14.61 c | 18.05 c |
| Biostimulant | |||
| Control | 21.09 b | 14.29 a | 17.69 b |
| BB-F | 21.03 b | 14.28 a | 17.66 b |
| BB-F + BB-M | 19.99 a | 14.24 a | 17.11 a |
| Harvest | |||
| I | 17.79 a | 13.66 b | 15.73 a |
| II | 24.88 c | 13.06 a | 18.97 c |
| III | 19.44 b | 16.09 c | 17.76 b |
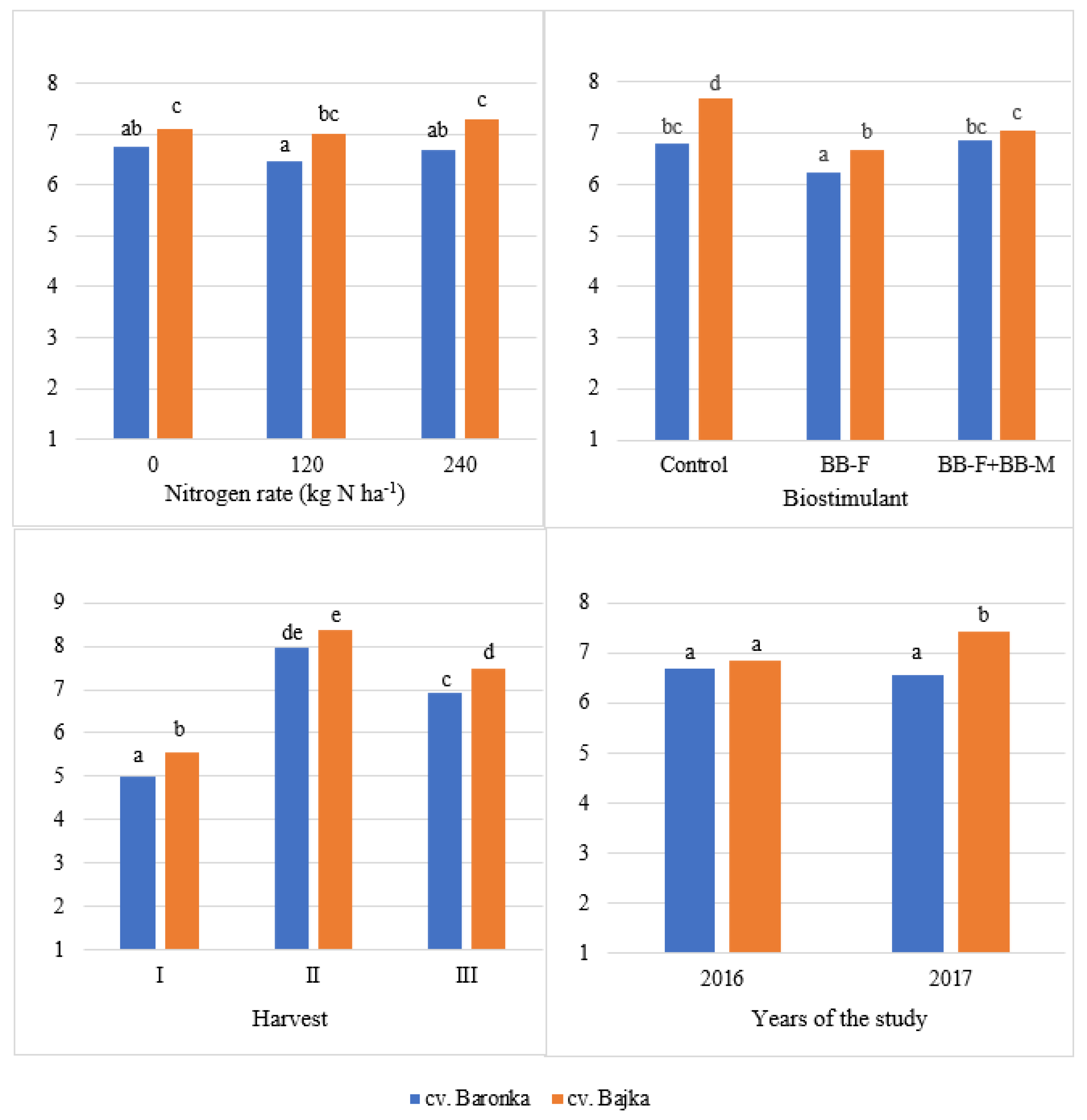
| Factor | Years of the Study | Mean | |
|---|---|---|---|
| 2016 | 2017 | ||
| Cultivar | |||
| Baronka | 6.68 a | 6.57 a | 6.62 a |
| Bajka | 6.84 b | 7.42 b | 7.13 b |
| Nitrogen rate (kg ha−1) | |||
| 0 | 7.03 b | 6.83 a | 6.93 b |
| 120 | 6.27 a | 7.18 b | 6.72 a |
| 240 | 6.98 b | 6.99 ab | 6.99 b |
| Biostimulant | |||
| Control | 6.88 b | 7.60 c | 7.24 c |
| BB-F | 6.42 a | 6.49 a | 6.46 a |
| BB-F + BB-M | 6.98 b | 6.91 b | 6.95 b |
| Harvest | |||
| I | 5.14 a | 5.42 a | 5.28 a |
| II | 8.46 c | 7.86 b | 8.16 c |
| III | 6.69 b | 7.71 b | 7.20 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olszewska, M. Effects of Cultivar, Nitrogen Rate and Biostimulant Application on the Chemical Composition of Perennial Ryegrass (Lolium perenne L.) Biomass. Agronomy 2022, 12, 826. https://doi.org/10.3390/agronomy12040826
Olszewska M. Effects of Cultivar, Nitrogen Rate and Biostimulant Application on the Chemical Composition of Perennial Ryegrass (Lolium perenne L.) Biomass. Agronomy. 2022; 12(4):826. https://doi.org/10.3390/agronomy12040826
Chicago/Turabian StyleOlszewska, Marzenna. 2022. "Effects of Cultivar, Nitrogen Rate and Biostimulant Application on the Chemical Composition of Perennial Ryegrass (Lolium perenne L.) Biomass" Agronomy 12, no. 4: 826. https://doi.org/10.3390/agronomy12040826
APA StyleOlszewska, M. (2022). Effects of Cultivar, Nitrogen Rate and Biostimulant Application on the Chemical Composition of Perennial Ryegrass (Lolium perenne L.) Biomass. Agronomy, 12(4), 826. https://doi.org/10.3390/agronomy12040826






