Oligosaccharins Alleviate Heat Stress in Greenhouse-Grown Tomatoes during the Spring-Summer Season in a Semi-Arid Climate
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Gas Exchange Parameters
3.2. Yield Parameters
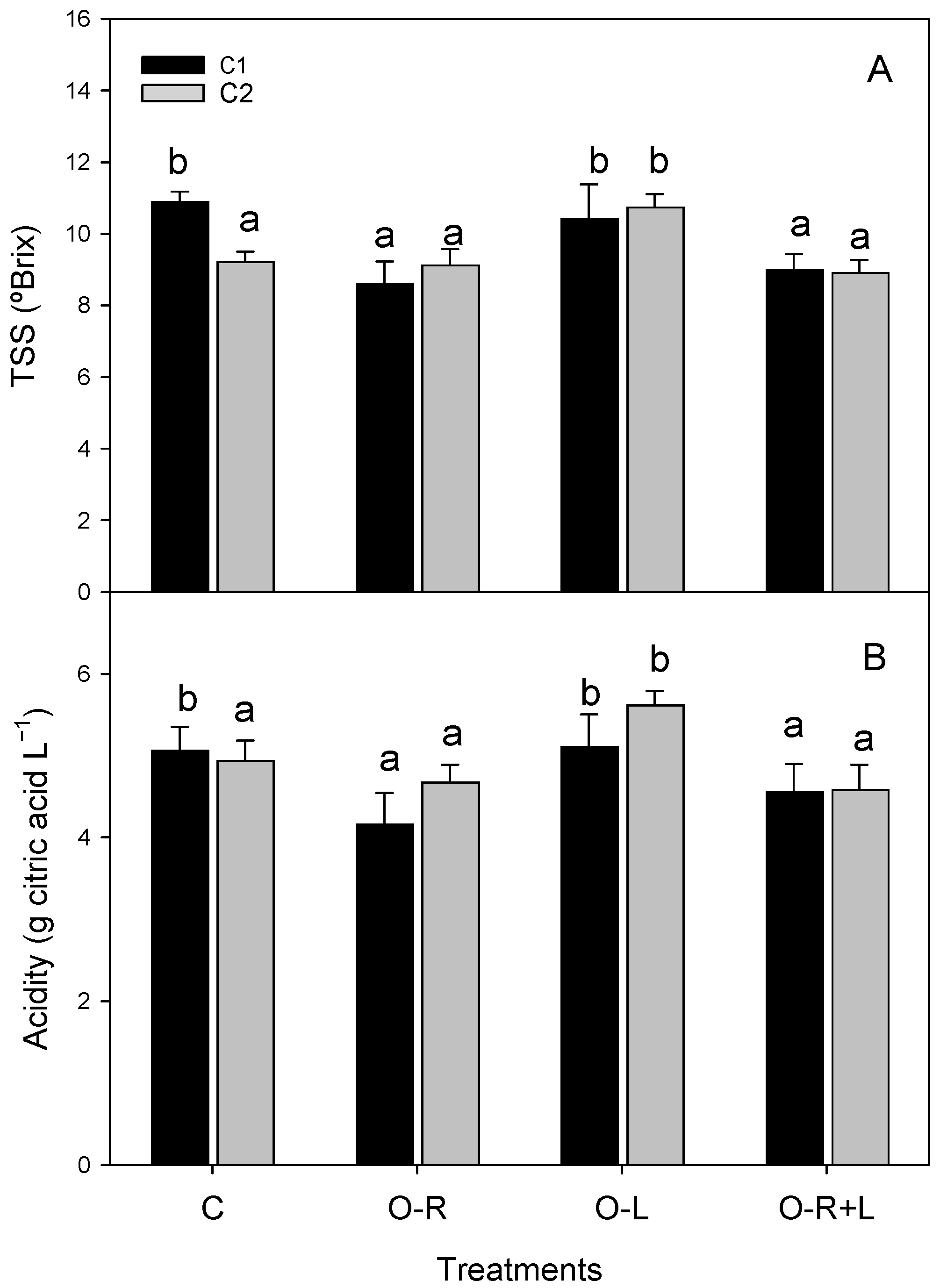
3.3. Fruit Organoleptic Properties
3.4. Phenolic Compounds
3.5. Vitamin C
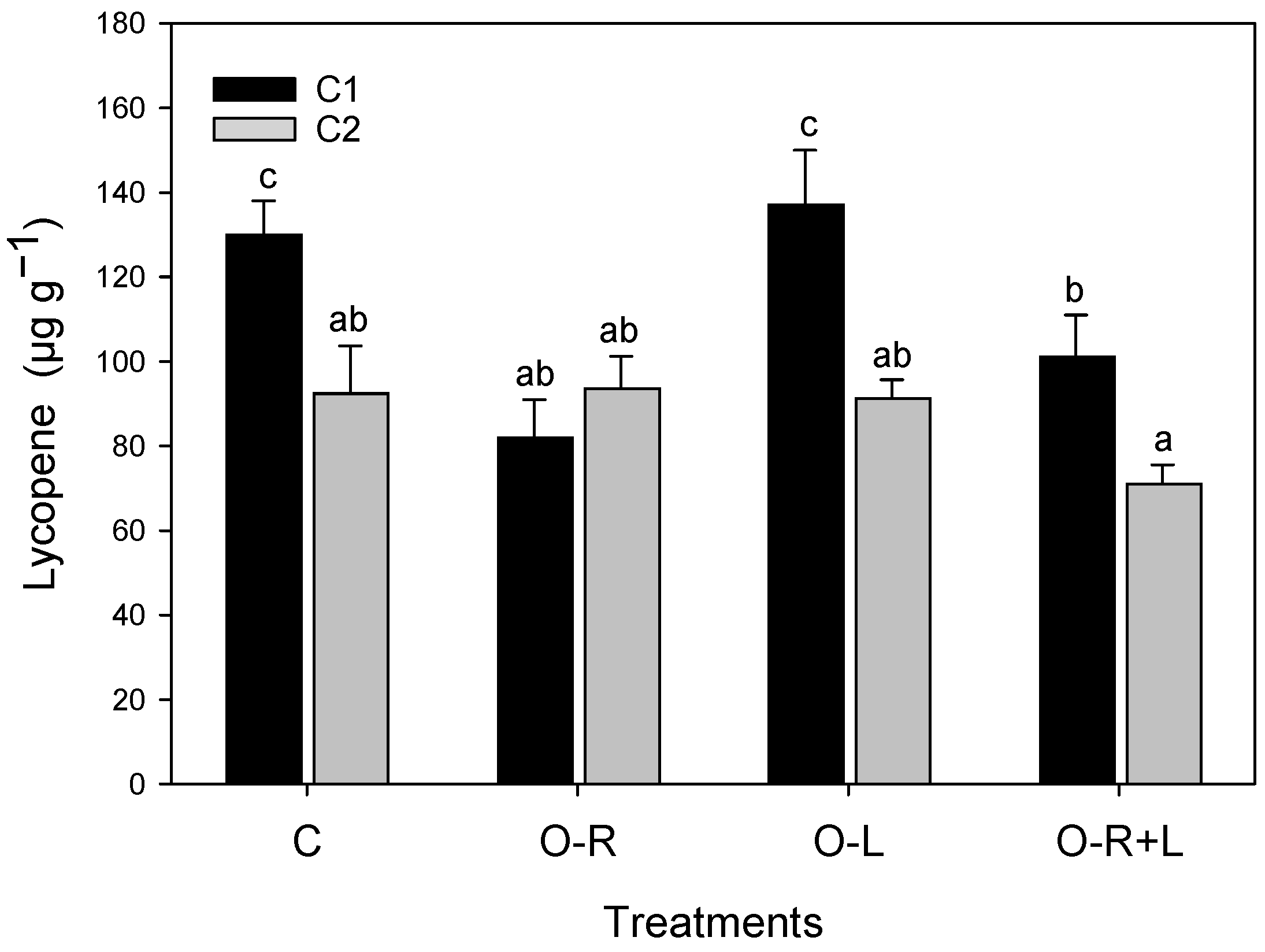
3.6. Carotenoids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nankishore, A.; Farrell, A.D. The response of contrasting tomato genotypes to combined heat and drought stress. J. Plant Physiol. 2016, 202, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Larkindale, J.; Hall, J.D.; Knight, M.R. Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol. 2005, 138, 882–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raja, M.M.; Vijayalakshmi, G.; Naik, M.L.; Basha, P.O.; Sergeant, K.; Hausman, J.F.; Khan, P.S.V. Pollen development and function under heat stress: From effects to responses. Acta Physiol. Plant. 2019, 41, 47. [Google Scholar] [CrossRef]
- Camejo, D.; Rodriguez, P.; Morales, A.; Dell’Amico, J.M.; Torrecillas, A.; Alarcon, J.J. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J. Plant Physiol. 2005, 162, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Alsamir, M.; Mahmood, T.; Trethowan, R.; Ahmad, N. An overview of heat stress in tomato (Solanum lycopersicum L.). Saudi J. Biol. Sci. 2021, 28, 1654–1663. [Google Scholar] [CrossRef]
- Adams, S.R.; Cockshull, K.E.; Cave, C.R.J. Effect of temperature on the growth and development of tomato fruits. Ann. Bot. 2001, 88, 869–877. [Google Scholar] [CrossRef]
- Islam, M.T. Effect of temperature on photosynthesis, yield attributes and yield of tomato genotypes. Int. J. Exp. Agric. 2011, 2, 8–11. [Google Scholar]
- Botella, M.A.; Hernandez, V.; Mestre, T.; Hellin, P.; Garcia-Legaz, M.F.; Rivero, R.M.; Martinez, V.; Fenoll, J.; Flores, P. Bioactive compounds of tomato fruit in response to salinity, heat and their combination. Agriculture 2021, 11, 534. [Google Scholar] [CrossRef]
- Gautier, H.; Diakou-Verdin, V.; Benard, C.; Reich, M.; Buret, M.; Bourgaud, F.; Poessel, J.L.; Caris-Veyrat, C.; Genard, M. How does tomato quality (sugar, acid, and nutritional quality) vary with ripening stage, temperature, and irradiance? J. Agric. Food Chem. 2008, 56, 1241–1250. [Google Scholar] [CrossRef]
- Rosales, M.A.; Cervilla, L.M.; Sanchez-Rodriguez, E.; del Mar Rubio-Wilhelmi, M.; Blasco, B.; Rios, J.J.; Soriano, T.; Castilla, N.; Romero, L.; Ruiz, J.M. The effect of environmental conditions on nutritional quality of cherry tomato fruits: Evaluation of two experimental Mediterranean greenhouses. J. Sci. Food Agric. 2011, 91, 152–162. [Google Scholar] [CrossRef]
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Tomas-Barberan, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in food and their health benefits. Plant Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Slimestad, R.; Verheul, M. Review of flavonoids and other phenolics from fruits of different tomato (Lycopersicon esculentum Mill) cultivars. J. Sci. Food Agric. 2009, 89, 1255–1270. [Google Scholar] [CrossRef]
- Carr, A.C.; Vissers, M.C. Synthetic or food-derived vitamin C are they equally bioavailable? Nutrients 2013, 28, 4284–4304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The role of polyphenols in human health and food systems: A mini-review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, H.L.; Thomas-Ahner, J.M.; Grainger, E.M.; Wan, L.; Francis, D.M.; Schwartz, S.J.; Erdman, J.W., Jr.; Clinton, S.K. Tomato-based food products for prostate cancer prevention: What have we learned? Cancer Metastasis Rev. 2010, 29, 553–568. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, V.; Hellín, P.; Fenoll, J.; Flores, P. Increased temperature produces changes in the bioactive composition of tomato, depending on its developmental stage. J. Agric. Food Chem. 2015, 63, 2378–2382. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Biostimulants in agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef] [Green Version]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Colla, G.; Rouphael, Y. Biostimulants in horticulture. Sci. Hortic. 2015, 196, 1–2. [Google Scholar] [CrossRef]
- Díaz, V.B. Oligosacarinas (Oligosacáridos): Producción Química y Aplicación a Los Cultivos Vegetales, 1st ed.; Autores de Argentina: Buenos Aires, Argentina, 2018. [Google Scholar]
- Falcón-Rodríguez, A.B.; Costales-Mené, D.; González-Peña, F.D.; Nápoles-García, M.-C. Nuevos productos naturales para la agricultura: Las oligosacarinas. Cultiv. Trop. 2015, 36, 111–129. [Google Scholar]
- Albersheim, P.; Darvill, A.; Augur, C.; Cheong, J.J.; Eberhard, S.; Hahn, M.G.; Marfa, V.; Mohnen, D.; Oneill, M.A.; Spiro, M.D. Oliosaccharins-oligosaccharide regulatory molecules. Acc. Chem. Res. 1992, 25, 77–83. [Google Scholar] [CrossRef]
- Darvill, A.; Augur, C.; Bergmann, C.; Carlson, R.W.; Cheong, J.J.; Eberhard, S.; Hahn, M.G.; Lo, V.M.; Marfa, V.; Meyer, B.; et al. Oligosaccharins-oligosaccharides that regulate growth, development and defense responses in plants. Glycobiology 1992, 2, 181–198. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, J.C.; Wegria, G.; Onderwater, R.C.A.; González, G.; Napoles, M.C.; Falcon-Rodriguez, A.B.; Costales, D.; Rogers, H.J.; Diosdado, E.; González, S.; et al. Practical use of oligosaccharins in agriculture. Acta Hortic. 2013, 1009, 195–212. [Google Scholar] [CrossRef]
- Costales, D.; Martínez, L.; Núñez, M. Efecto del tratamiento de semillas con una mezcla de oligogalacturónidos sobre el crecimiento de plántulas de tomate (Lycopersicum esculentum Mill). Cultiv. Trop. 2007, 28, 85–91. [Google Scholar]
- Ruiz, J.; Terry, E.; Tejeda, T.; Díaz, M. Aplicación de bioproductos a la producción ecológica de tomate. Cultiv. Trop. 2009, 30, 60–64. [Google Scholar]
- Mzibra, A.; Aasfar, A.; Khouloud, M.; Farrie, Y.; Boulif, R.; Kadmiri, I.M.; Bamouh, A.; Douira, A. Improving growth, yield, and quality of tomato plants (Solanum lycopersicum L.) by the application of Moroccan seaweed-based biostimulants under greenhouse conditions. Agronomy 2021, 11, 1373. [Google Scholar] [CrossRef]
- Ochoa-Villarreal, M.; Vargas-Arispuro, I.; Islas-Osuna, M.A.; Gonzalez-Aguilar, G.; Martínez-Tellez, A.M. Pectin-derived oligosaccharides increase color and anthocyanin content in flame seedless grapes. J. Sci. Food Agric. 2011, 91, 1928–1930. [Google Scholar] [CrossRef]
- He, Y.; Bose, S.K.; Wang, W.; Jia, X.; Lu, H.; Yin, H. Pre-harvest treatment of chitosan oligosaccharides improved strawberry fruit quality. Int. J. Mol. Sci. 2018, 19, 2194. [Google Scholar] [CrossRef] [Green Version]
- Flores, P.; Hernández, V.; Hellín, P.; Fenoll, J.; Cava, J.; Mestre, T.; Martínez, V. Metabolite profile of the tomato dwarf cultivar Micro-Tom and comparative response to saline and nutritional stresses with regard to a commercial cultivar. J. Sci. Food Agric. 2016, 96, 1562–1570. [Google Scholar] [CrossRef]
- Vallverdu-Queralt, A.; Jauregui, O.; Medina-Remon, A.; Andres-Lacueva, C.; Lamuela-Raventos, R.M. Improved characterization of tomato polyphenols using liquid chromatography/electrospray ionization linear ion trap quadrupole orbitrap mass spectrometry and liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 2986–2992. [Google Scholar] [CrossRef]
- Flores, P.; Hernández, V.; Fenoll, J.; Hellín, P. Pre-harvest application of ozonated water on broccoli crops: Effect on head quality. J. Food Compos. Anal. 2019, 83, 103260. [Google Scholar] [CrossRef]
- Fenoll, J.; Martínez, A.; Hellín, P.; Flores, P. Simultaneous determination of ascorbic and dehydroascorbic acids in vegetables and fruits by liquid chromatography with tandem-mass spectrometry. Food Chem. 2011, 127, 340–344. [Google Scholar] [CrossRef]
- Bohm, V. Use of column temperature to optimize carotenoid isomer separation by C30 high performance liquid chromatography. J. Sep. Sci. 2001, 24, 955–959. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Bernacchi, C.J.; Farquhar, G.D.; Singsaas, E.L. Fitting photosynthetic carbon dioxide response curves for c-3 leaves. Plant Cell Environ. 2007, 30, 1035–1040. [Google Scholar] [CrossRef]
- Wang, W.X.; Vinocur, B.; Shoseyov, O.; Altman, A. Biotechnology of plant osmotic stress tolerance: Physiological and molecular considerations. Acta Hortic. 2001, 560, 285–292. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Weis, E.; Berry, J.A. Plants and high temperature stress. Symp Soc. Exp. Biol. 1988, 42, 329–346. [Google Scholar]
- García-Sahagún, M.L.; Martínez-Juárez, V.; Avendaño-López, A.N.; Padilla-Sahagún, M.C.; Izquierdo-Oviedo, H. Acción de oligosacáridos en el rendimiento y calidad de tomate. Rev. Fitotec. Mex. 2009, 32, 295–301. [Google Scholar] [CrossRef]
- Benard, C.; Gautier, H.; Bourgaud, F.; Grasselly, D.; Navez, B.; Caris-Veyrat, C.; Weiss, M.; Genard, M. Effects of low nitrogen supply on tomato (Solanum lycopersicum) fruit yield and quality with special emphasis on sugars, acids, ascorbate, carotenoids, and phenolic compounds. J. Agric. Food Chem. 2009, 57, 4112–4123. [Google Scholar] [CrossRef]
- Hernández, V.; Hellín, P.; Fenoll, J.; Flores, P. Impact of nitrogen supply limitation on tomato fruit composition. Sci. Hortic. 2020, 264, 109173. [Google Scholar] [CrossRef]
- Tigist, M.; Workneh, T.S.; Woldetsadik, K. Effects of variety on the quality of tomato stored under ambient conditions. J. Food Sci. Technol. 2013, 50, 477–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehi, B.; Sharifi-Rad, R.; Sharopov, F.; Namiesnik, J.; Roointan, A.; Kamle, M.; Kumar, P.; Martins, N.; Sharifi-Rad, J. Beneficial effects and potential risks of tomato consumption for human health: An overview. Nutrition 2019, 62, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Lokesha, A.N.; Shivashankara, K.S.; Hunashikatti, L.A.G. Effect of high temperature on fruit quality parameters of contrasting tomato genotypes. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1019–1029. [Google Scholar] [CrossRef]
- Rosales, M.A.; Ruiz, J.M.; Hernández, J.; Soriano, T.; Castilla, N.; Romero, L. Antioxidant content and ascorbate metabolism in cherry tomato exocarp in relation to temperature and solar radiation. J. Sci. Food Agric. 2006, 86, 1545–1551. [Google Scholar] [CrossRef]


| AN | E | gs | WUEi | ||
|---|---|---|---|---|---|
| Treatment | |||||
| C | 17.7 a | 7.0 a | 0.30 a | 64.6 b | |
| O-R | 24.1 b | 9.6 b | 0.49 b | 54.1 a | |
| O-L | 21.1 b | 7.5 a | 0.35 a | 64.4 b | |
| O-R + L | 21.5 b | 8.6 b | 0.55 b | 47.4 a | |
| ** | *** | *** | * | ||
| Compartment | |||||
| C1 | 20.4 | 9.4 b | 0.45 b | 57.7 | |
| C2 | 22.2 | 7.4 a | 0.41 a | 56.8 | |
| n.s. | *** | * | n.s. | ||
| Interaction | |||||
| C1 | C | 18.1 | 8.1 cd | 0.32 | 62.8 |
| O-R | 21.5 | 10.5 ef | 0.53 | 52.2 | |
| O-F | 20.5 | 8.6 de | 0.33 | 64.4 | |
| O-R + L | 22.5 | 11.3 f | 0.71 | 47.4 | |
| C2 | C | 16.9 | 5.2 a | 0.25 | 67.4 |
| O-R | 25.3 | 9.2 de | 0.47 | 55.0 | |
| O-F | 21.8 | 6.3 ab | 0.38 | 64.3 | |
| O-R + L | 20.9 | 7.0 bc | 0.45 | 47.4 | |
| n.s | * | n.s. | n.s. | ||
| Yield | Fruit Number | Fruit Mean Weight | TSS | Acidity | |
|---|---|---|---|---|---|
| Treatment | |||||
| C | 1.59 a | 44.0 | 36.2 | 9.8 b | 4.9 b |
| O-R | 2.06 b | 48.1 | 42.6 | 8.9 a | 4.4 a |
| O-L | 2.09 b | 49.0 | 43.1 | 10.6 b | 5.4 b |
| O-R + L | 2.13 b | 49.6 | 43.7 | 9.0 a | 4.6 a |
| ** | n.s | n.s | *** | *** | |
| Compartment | |||||
| C1 | 2.31 b | 51.1 b | 44.9 | 10.4 | 4.9 |
| C2 | 1.75 a | 44.4 a | 39.9 | 9.6 | 5.0 |
| * | ** | n.s | n.s. | n.s | |
| Interaction | n.s. | n.s. | n.s | * | * |
| Glucose | Fructose | Glutamic | Citric | Malic | |
|---|---|---|---|---|---|
| Treatment | |||||
| C | 32.7 b | 28.1 b | 6.79 | 5.50 bc | 0.17 ab |
| O-R | 29.3 a | 25.9 a | 6.44 | 4.87 a | 0.15 a |
| O-L | 31.2 ab | 27.0 ab | 6.53 | 5.71 c | 0.18 b |
| O-R + L | 28.8 a | 25.1 a | 6.41 | 5.04 ab | 0.15 a |
| ** | * | n.s. | ** | * | |
| Compartment | |||||
| C1 | 31.7 | 28.5 b | 6.56 | 4.96 a | 0.14 a |
| C2 | 31.8 | 26.4 a | 6.59 | 5.77 b | 0.18 b |
| n.s. | * | n.s. | *** | *** | |
| Interaction | n.s. | n.s. | n.s. | n.s. | n.s. |
| Flvones | Flvnols | Hydroxy | Phl-C | Homoval | Vit C | |
|---|---|---|---|---|---|---|
| Treatments | ||||||
| C | 36 ab | 5.5 c | 31 b | 10.4 c | 90 b | 228 b |
| O-R | 37 ab | 4.0 ab | 26 a | 8.3 ab | 64 a | 211 a |
| O-L | 39 b | 4.7 bc | 28 ab | 9.3 bc | 62 a | 207 a |
| O-R + L | 33 a | 3.2 a | 25 a | 7.0 a | 58 a | 209 a |
| * | *** | ** | *** | *** | *** | |
| Compartment | ||||||
| C1 | 31 a | 4.0 a | 26 a | 7.4 a | 56 a | 219 b |
| C2 | 42 b | 4.7 b | 29 b | 9.9 b | 80 b | 210 a |
| *** | * | * | *** | *** | * | |
| Interaction | n.s. | *** | n.s. | * | *** | n.s. |
| β-carot | Lutein | Lycop | |
|---|---|---|---|
| Treatment | |||
| C | 7.51 a | 4.10 bc | 101 b |
| O-R | 8.26 a | 3.65 a | 89 a |
| O-L | 9.13 b | 4.27 c | 122 b |
| O-R + L | 8.39 a | 3.76 ab | 179 a |
| *** | * | * | |
| Compartment | |||
| C1 | 8.75 b | 3.49 a | 112 b |
| C2 | 7.59 a | 4.47 b | 86 a |
| *** | *** | *** | |
| Interaction | n.s. | n.s. | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández, V.; Hellín, P.; Botella, M.Á.; Vicente, E.; Fenoll, J.; Flores, P. Oligosaccharins Alleviate Heat Stress in Greenhouse-Grown Tomatoes during the Spring-Summer Season in a Semi-Arid Climate. Agronomy 2022, 12, 802. https://doi.org/10.3390/agronomy12040802
Hernández V, Hellín P, Botella MÁ, Vicente E, Fenoll J, Flores P. Oligosaccharins Alleviate Heat Stress in Greenhouse-Grown Tomatoes during the Spring-Summer Season in a Semi-Arid Climate. Agronomy. 2022; 12(4):802. https://doi.org/10.3390/agronomy12040802
Chicago/Turabian StyleHernández, Virginia, Pilar Hellín, M. Ángeles Botella, Elena Vicente, José Fenoll, and Pilar Flores. 2022. "Oligosaccharins Alleviate Heat Stress in Greenhouse-Grown Tomatoes during the Spring-Summer Season in a Semi-Arid Climate" Agronomy 12, no. 4: 802. https://doi.org/10.3390/agronomy12040802
APA StyleHernández, V., Hellín, P., Botella, M. Á., Vicente, E., Fenoll, J., & Flores, P. (2022). Oligosaccharins Alleviate Heat Stress in Greenhouse-Grown Tomatoes during the Spring-Summer Season in a Semi-Arid Climate. Agronomy, 12(4), 802. https://doi.org/10.3390/agronomy12040802






