Nutrient Release Dynamics in Argentinean Pampean Soils Amended with Composts under Laboratory Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sampling and Compost Analyses
2.2. Analytical Methods
2.3. Incubation Experiment
2.3.1. N and P Mineralization
2.3.2. C Mineralization
2.4. Data Analysis and Statistical Methods
3. Results and Discussion
3.1. Compost Characteristics
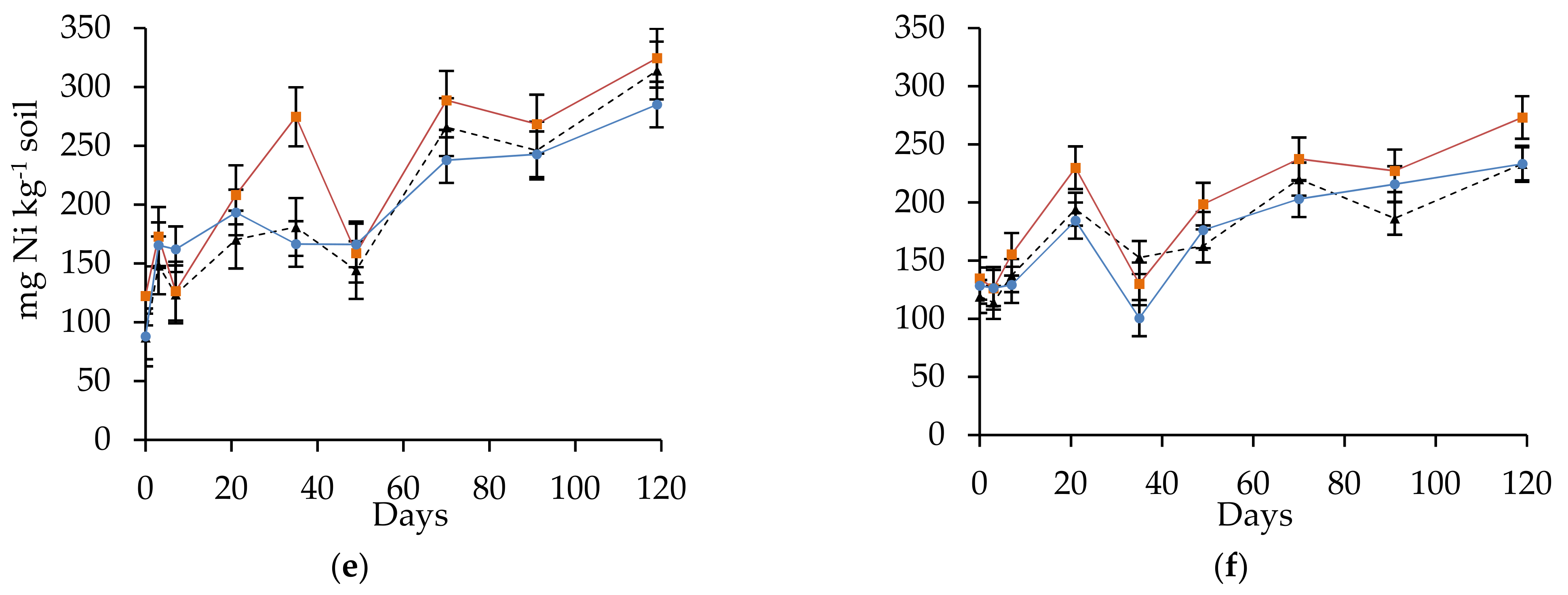
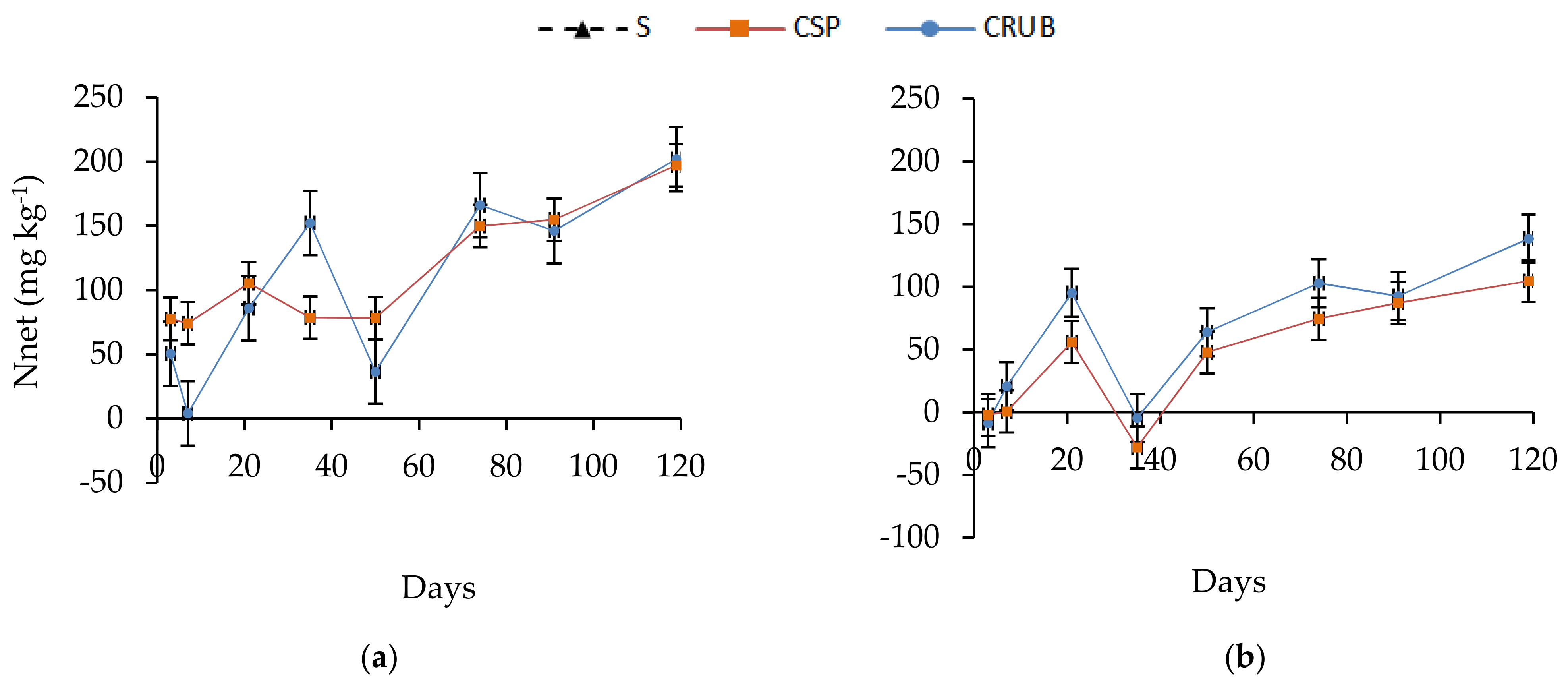
3.2. Nitrogen Mineralization Dynamics
3.3. P Mineralization Dynamics
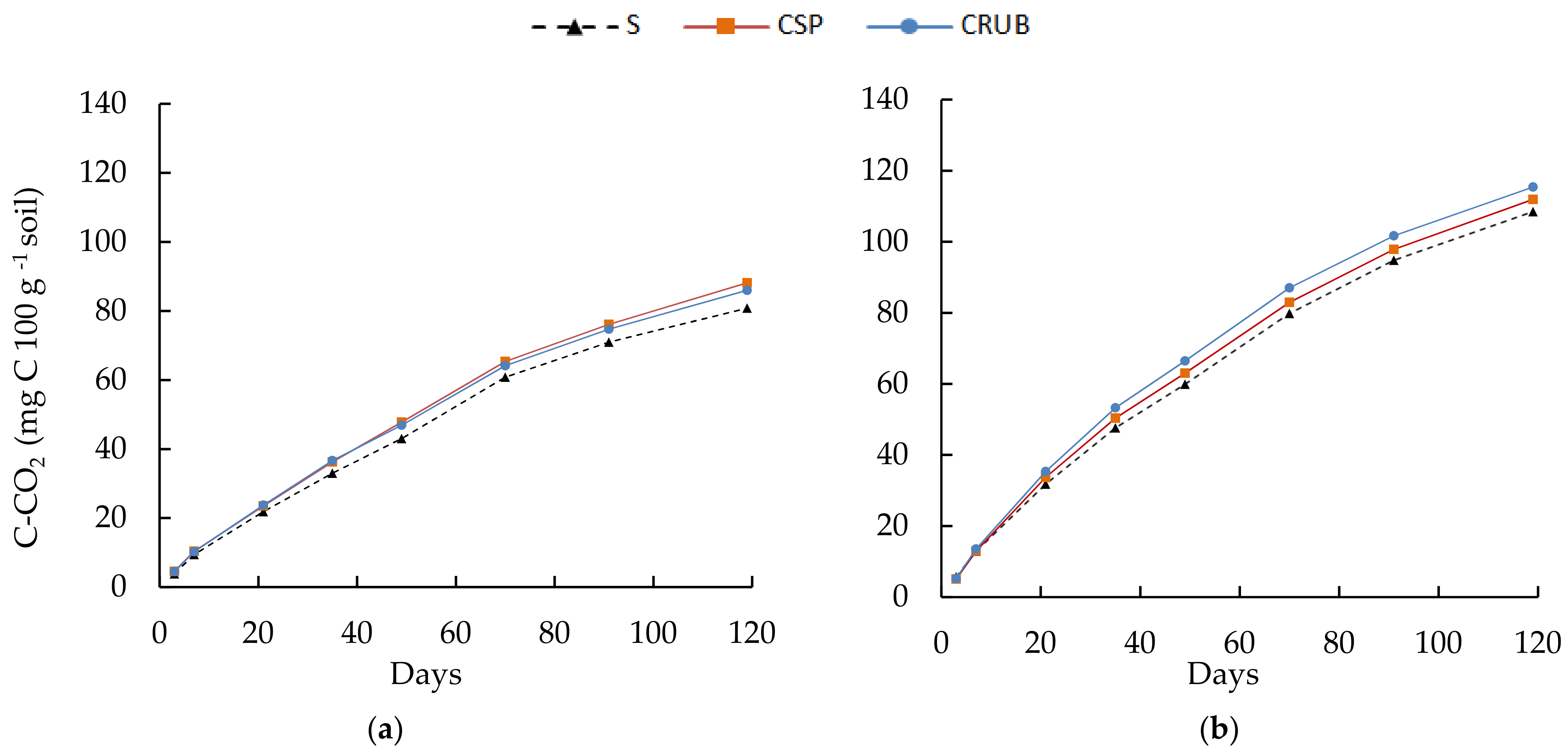
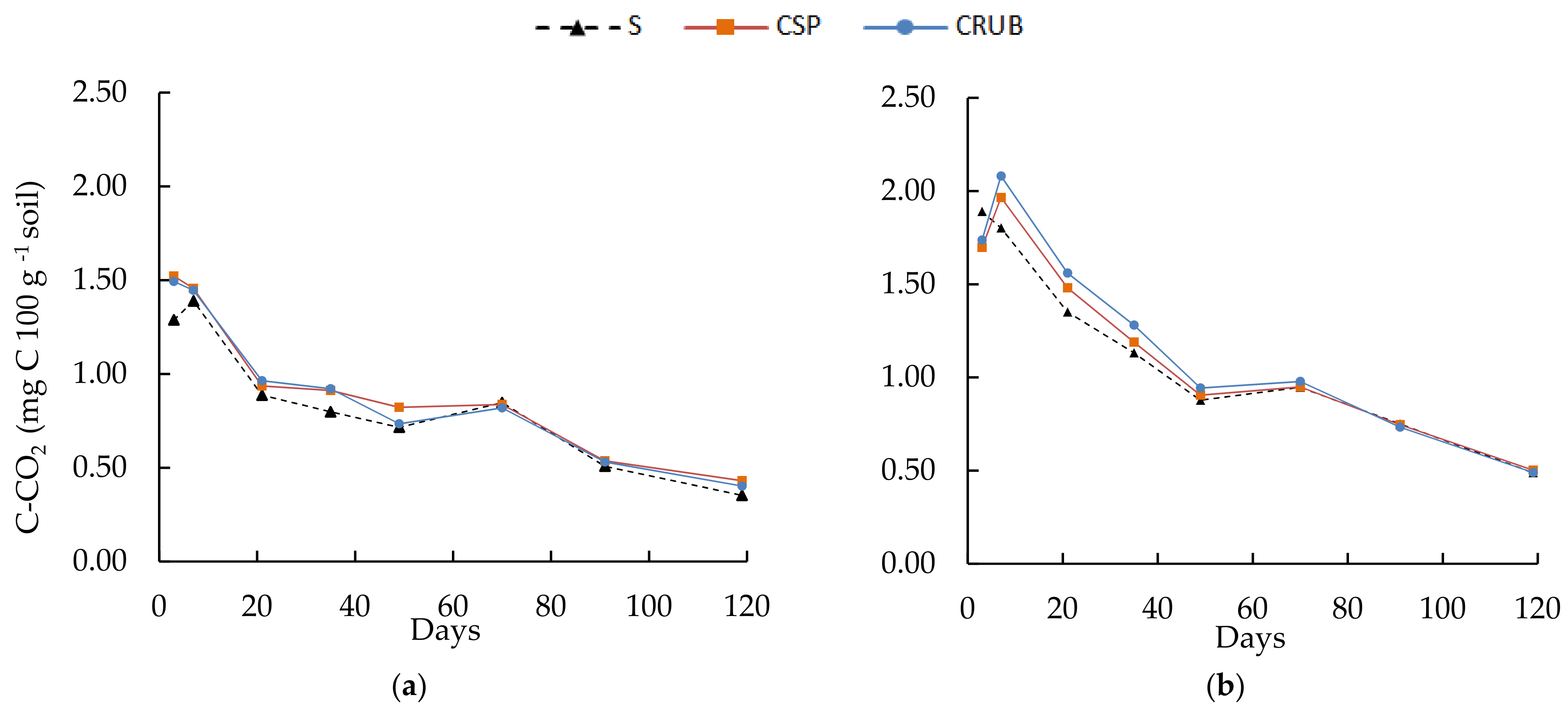
3.4. Carbon Mineralization Dynamics
- Ct = cumulative C-CO2 at time t (mg 100 g−1 of soil)
- Co = potentially mineralizable C stock (mg 100 g−1 of soil)
- ko = first-order constant (day−1)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sayara, T.; Basheer-Salimia, R.; Hawamde, F.; Sánchez, A. Recycling of Organic Wastes through Composting: Process Performance and Compost Application in Agriculture. Agronomy 2020, 10, 1838. [Google Scholar] [CrossRef]
- Muhammad, J.; Khan, S.; Su, J.Q.; Hesham, A.E.; Ditta, A.; Nawab, J.; Ali, A. Antibiotics in poultry manure and their associated health issues: A systematic review. J. Soils Sediments 2020, 20, 486–497. [Google Scholar] [CrossRef]
- Goss, M.J.; Tubeileh, A.M.; Goorahoo, D. A Review of the Use of Organic Amendments and the Risk to Human Health. Adv. Agron. 2013, 130, 275–379. [Google Scholar]
- Lazicki, P.; Geisseler, D.; Lloyd, M. Nitrogen mineralization from organic amendments is variable but predictable. J. Environ. Qual. 2019, 49, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Melo Araújo, M.D.; Monteiro Feitosa, M.; Alves Primo, A.; Kenji Taniguchi, C.A.; Antunes De Souza, H. Mineralization of nitrogen anda carbon from organic compost from animal production waste. Rev. Caatinga 2020, 33, 310–320. [Google Scholar] [CrossRef]
- Pinto, R.; Brito, L.M.; Coutinho, J. Nitrogen Mineralization from Organic Amendments Predicted by Laboratory and Field Incubations. Commun. Soil Sci. Plant Anal. 2020, 51, 515–526. [Google Scholar] [CrossRef]
- Bastida, F.; Kandeler, E.; Moreno, J.L.; Ros, M.; Garcia, C.; Hernandez, T. Application of fresh and composted organic wastes modifies structure, size and activity of soil microbial community under semiarid climate. Appl. Soil Ecol. 2008, 40, 318–329. [Google Scholar] [CrossRef]
- Laos, F.; Satti, P.; Walter, I.; Mazzarino, M.J.; Moyano, S. Nutrient availability of composted and non-composted residues in a Patagonian Xeric Mollisol. Biol. Fertil. Soils 2000, 31, 462–469. [Google Scholar] [CrossRef]
- Campitelli, P.; Ceppi, S. Chemical. physical and biological compost and vermicompost characterization: A chemometric study. Chemometr. Intell. Lab. 2008, 90, 64–71. [Google Scholar] [CrossRef]
- Benítez, C.; Tejada, M.; González, J.L. Kinetics of the Mineralization of Nitrogen in a Pig Slurry Compost Applied to Soils. Compost. Sci. Util. 2003, 11, 72–80. [Google Scholar] [CrossRef]
- De Neve, S.; Sleutel, S.; Hofman, G. Carbon mineralization from composts and food industry wastes added to soil. Nutr. Cycl. Agroecosystems 2003, 67, 13–20. [Google Scholar] [CrossRef]
- Hernandez, T.; Moral, R.; Perez-Espinosa, A.; Moreno-Caselles, J.; Perez-Murcia, M.D.; Garcia, C. Nitrogen mineralisation potential in calcareous soils amended with sewage sludge. Bioresour. Technol. 2002, 83, 213–219. [Google Scholar] [CrossRef]
- Bustamante, M.A.; Said-Pullicino, D.; Paredes, C.; Cecilia, J.A.; Moral, R. Influences of winery–distillery waste compost stability and soil type on soil carbon dynamics in amended soils. Waste Manag. 2010, 30, 1966–1975. [Google Scholar] [CrossRef] [PubMed]
- Griffin, T.S.; He, Z.; Honeycutt, C.W. Manure composition affects net transformation of nitrogen from dairy manures. Plant Soil 2005, 273, 29–38. [Google Scholar] [CrossRef]
- Bowden, C.; Spargo, J.; Evanylo, G. Mineralization and N Fertilizer Equivalent Value of Composts as Assessed by Tall Fescue (Festuca arundinacea). Compost Sci. Util. 2007, 15, 111–118. [Google Scholar] [CrossRef]
- Chadwick, D.R.; John, F.; Pain, B.F.; Chambers, B.J.; Williams, J. Plant uptake of nitrogen from the organic nitrogen fraction of animal manures: A laboratory experiment. J. Agric. Sci. 2000, 134, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Amlinger, F.; Götz, B.; Dreher, P.; Geszti, J.; Weissteiner, C. Nitrogen in biowaste and yard waste compost: Dynamics of mobilisation and availability—A review. Eur. J. Soil Biol. 2003, 39, 107–116. [Google Scholar] [CrossRef]
- Castán, E.; Satti, P.; González-Polo, M.; Iglesias, M.; Mazzarino, M.J. Managing the value of composts as organic amendments and fertilizers in sandy soils. Agric. Ecosyst. Environ. 2016, 224, 29–38. [Google Scholar] [CrossRef]
- Kaur, G.; Reddy, M.S. Role of phosphate-solubilizing bacteria in improving the soil fertility and crop productivity in organic farming. Arch. Agron. Soil Sci. 2014, 60, 549–564. [Google Scholar] [CrossRef]
- Bustamante, M.A.; Ceglie, F.G.; Aly, A.; Mihreteab, H.T.; Ciaccia, C.; Tittarelli, F. Phosphorus availability from rock phosphate: Combined effect of green waste composting and sulfur addition. J. Environ. Manag. 2016, 182, 557–563. [Google Scholar] [CrossRef]
- Chi, R.; Xiao, C.; Huang, X.; Wang, C.; Wu, Y. Bio-decomposition of rock phosphate containing pyrites by Acidithiobacillus ferrooxidans. J. Cent. South Univ. Technol. 2007, 14, 170–175. [Google Scholar] [CrossRef]
- Rick, T.L.; Jones, C.A.; Engel, R.E.; Miller, P.R. Green manure and phosphate rock effects on phosphorus availability in a northern Great Plains dryland organic cropping system. Org. Agric. 2011, 1, 81–90. [Google Scholar] [CrossRef]
- Stanisławska-Glubiak, E.; Korzeniowska, J.; Hoffmann, J.; Górecka, H.; Józwiak, W.; Wísniewska, G. Effect of sulphur added to phosphate rock on solubility and phytoavailability of phosphorus. Pol. J. Chem. Technol. 2014, 16, 81–85. [Google Scholar] [CrossRef]
- Lerch, R.N.; Barbarick, K.A.; Sommers, L.E.; Westfall, D.G. Sewage sludge proteins as labile C and N sources. Soil Sci. Soc. Am. J. 1992, 56, 1470–1476. [Google Scholar] [CrossRef]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H.; Soltanpour, P.N.; Tabatabai, M.A.; Johnston, C.T.; Sumner, M.E. Methods of Soil Analysis, Part 3. Chemical Methods; Soil Science Society of America: Madison, WI, USA, 1996; Volume 5, p. 1390. [Google Scholar]
- Bremner, J.M.; Keeney, D.R. Steam distillation methods for determination of ammonium, nitrate and nitrite. Anal. Chim. Acta. 1965, 32, 485–495. [Google Scholar] [CrossRef]
- Martin, T.D.; Brockhoff, C.A.; Creed, J.T. EMMC Methods Work Group. Method 200.7; Revision 4.4; In Determination of Metals and Trace Elements in Water and Wastes by Inductively Coupled Plasma-Atomic Emission Spectrometry; USEPA: Cincinnati, OH, USA, 1994; p. 58. [Google Scholar]
- Honeycutt, C.W.; Griffin, T.S.; He, Z. Manure nitrogen availability: Dairy manure in northeast and central US soils. Biol. Agric. Hortic. 2005, 23, 199–214. [Google Scholar] [CrossRef]
- de la Fuente, C.; Clemente, R.; Martínez, J.; Bernal, M.P. Optimization of pig slurry application to heavy metal polluted soils monitoring nitrification processes. Chemosphere 2010, 81, 603–610. [Google Scholar] [CrossRef]
- Pell, M.; Stenström, J.; Granhall, U. Soil Respiration. In Microbiological Methods for Assessing Soil Quality; Bloem, J., Hopkin, D.W., Benedetti, A., Eds.; CABI International: Wallingford, CT, USA, 2005; pp. 117–126. [Google Scholar]
- Zibilske, L.M. Carbon Mineralization. In Methods of soil analysis. Part 2. Microbiological and Biochemical Properties; Weaver, R.W., Angle, J.S., Bottomley, P.S., Eds.; Soil Science Society of America Madison: Madison, WI, USA, 1994; pp. 835–863. [Google Scholar]
- Hyams, D.G. Curve Expert Software v.2.4.0. 2016. Available online: http://www.curveexpert.net (accessed on 28 February 2022).
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; González, L.; Tablada, M.; Robledo, C.W. InfoStat Software 2020; Universidad Nacional de Córdoba: Córdoba, Argentina; Available online: https://www.infostat.com.ar/ (accessed on 28 February 2022).
- Compost: Marco normativo para la producción, Registro y aplicación de compost. In Resolución Conjunta 1/2019; Servicio Nac. de Sanidad y Calidad Agroalimentaria-Secretaria de Control y Monitoreo Ambiental: Buenos Aires, Argentina, 2019; Available online: https://www.argentina.gob.ar/normativa/nacional/resoluci%C3%B3n-1-2019-318692 (accessed on 28 February 2022). (In Spanish)
- Satti, P.S. Biodisponibilidad de Nitrógeno y Fósforo en Suelos Volcánicos Bajo Bosque Nativo. Disturbados y Enmendados. Ph.D. Thesis, Universidad Nacional del Comahue, San Carlos de Bariloche, Argentina, 2007. (In Spanish). [Google Scholar]
- Tognetti, C. Compostaje de Residuos Orgánicos Urbanos: Optimización Del Proceso Para Una Mayor Calidad Del Producto Final. Ph.D. Thesis, Universidad Nacional del Comahue, San Carlos de Bariloche, Argentina, 2007. (In Spanish). [Google Scholar]
- Griffin, T.S.; Honeycutt, C.W. Using growing degree days to predict nitrogen availability from livestock manures. Soil Sci. Soc. Am. J. 2000, 64, 1876–1882. [Google Scholar] [CrossRef] [Green Version]
- Mazzarino, M.J.; Satti, P.; Moyano, S.; Laos, F. Compost de Biosólidos: Efecto del tamizado sobre la inmovilización de nitrógeno del suelo. Cienc. Del Suelo 2004, 22, 19–26. (In Spanish) [Google Scholar]
- Cardoso, C.; López, F.M.; Orden, L.; Rodríguez, R.A.; Díaz, J. Phosphorous availability of seven organic manures in a sandy soil. Acta Hortic. 2016, 1146, 149–155. [Google Scholar] [CrossRef]
- Bernal, M.; Roig, A. Nitrogen transformations in calcareous soils amended with pig slurry under aerobic incubation. J. Agric. Sci. 1993, 120, 89–97. [Google Scholar] [CrossRef]
- Cayuela, M.L.; Sinicco, T.; Mondini, C. Mineralization dynamics and biochemical properties during initial decomposition of plant and animal residues in soil. Appl. Soil Ecol. 2009, 41, 118–127. [Google Scholar] [CrossRef]
- Hadas, A.; Portnoy, R. Rates of decomposition in soil and release of available nitrogen from cattle manure and municipal waste composts. Compost. Sci. Util. 1997, 5, 48–54. [Google Scholar] [CrossRef]
- Eghball, B.; Wienhold, B.J.; Gilley, J.E.; Eigenberg, R.A. Mineralization of manure nutrients. J. Soil Water Conserv. 2002, 57, 470–473. [Google Scholar]
- Sánchez, F.I.; Delgado, J.L.R. Efecto de la aplicación del compost sobre las propiedades físicas y químicas del suelo. In Compostaje; Moreno Casco, J., Moral Herrero, R., Eds.; Mundi Prensa: Madrid, Spain, 2008; pp. 307–327. (In Spanish) [Google Scholar]
- Cardoso, C.; Laurent, G.; Rodríguez, R.A.; Miglierina, A.M.; Minoldo, G.; Dagna, N.; Orden, L. Potentially crop-N supply from different organic amendments to a soil from the low valley of the Río Negro province. Argentine. Acta Hortic. 2013, 1076, 193–198. [Google Scholar] [CrossRef]
- Griffin, T.; Honeycutt, C.W.; He, Z. Effects of temperature. soil water status. and soil type on swine slurry nitrogen transformations. Biol. Fertil. Soils 2002, 36, 442–446. [Google Scholar]
- Shah, G.M.; Rashid, M.I.; Shah, G.A.; Groot, J.C.J.; Lantinga, E.A. Mineralization and herbage recovery of animal manure nitrogen after application to various soil types. Plant Soil 2013, 365, 69–79. [Google Scholar] [CrossRef] [Green Version]
- Mubarak, A.R.; Gali, E.A.; Mohamed, A.G.; Steffens, D.; Awadelkarim, A.H. Nitrogen mineralization from five manures as influenced by chemical composition and soil type. Soil Sci. Plant Anal. 2010, 41, 1903–1920. [Google Scholar] [CrossRef]
- Thomsen, I.K.; Schjønning, P.; Christensen, B.T. Mineralisation of 15N-labelled sheep manure in soils of different texture and water contents. Biol. Fert. Soils 2003, 37, 295–301. [Google Scholar] [CrossRef]
- Hadas, A.; Portnoy, R. Nitrogen and carbon mineralization rates of composted manures incubated in soil. J. Environ. Qual. 1994, 23, 1184–1189. [Google Scholar] [CrossRef]
- Dao, T.H.; Schwartz, R.C. Effects of manure management on phosphorus biotransformations and losses during animal production. In Phosphorus in Action; Bünemann, E.K., Oberson, A., Frossard, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 407–429. [Google Scholar]
- Zvomuya, F.; Helgason, B.L.; Larney, F.J.; Janzen, H.H.; Akinremi, O.O.; Olson, B.M. Predicting phosphorus availability from soil-applied composted and non-composted cattle feedlot manure. J. Environ. Qual. 2006, 35, 928–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helgason, B.L.; Larney, F.J.; Janzen, H.H. Estimating carbon retention in soils amended with composted beef cattle manure. Can. J. Soil Sci. 2005, 85, 39–43. [Google Scholar] [CrossRef]
- Aber, J.D.; Melillo, J.M. Terrestrial Ecosystems; Saunders College Pub.: Philadelphia, PA, USA, 1999; p. 430. [Google Scholar]
- Landi, A.; Mermut, A.R.; Anderson, D.W. Origin and rate of pedogenic carbonate accumulation in Saskatchewan soils, Canada. Geoderma 2003, 117, 143–156. [Google Scholar] [CrossRef]
- Serna-Pérez, A.; Monger, H.C.; Herrick, J.E.; Murray, L. Carbon dioxide emissions from exhumed petrocalcic horizons. Soil Sci. Soc. Am. J. 2006, 70, 795–805. [Google Scholar] [CrossRef] [Green Version]
- Thomsen, I.; Olesen, J.C. N mineralization of composted and anaerobically stored ruminant manure in differently textured soils. J. Agric. Sci. 2000, 135, 151–159. [Google Scholar] [CrossRef]
- Murwira, H.K.; Kirchmann, H.; Swift, M.J. The effect of moisture on the decomposition rate of cattle manure. Plant Soil 1990, 122, 197–199. [Google Scholar] [CrossRef]
- De Nobili, M.; Contin, M.; Mondini, C.; Brookes, P.C. Soil microbial biomass is triggered into activity by trace amounts of substrate. Soil Biol. Biochem. 2001, 33, 1163–1170. [Google Scholar] [CrossRef]
- Antil, R.S.; Bar-Tal, A.; Fine, P.; Hadas, A. Predicting nitrogen and carbon mineralization of composted manure and sewage sludge in soil. Compost. Sci. Util. 2011, 19, 33–43. [Google Scholar] [CrossRef]



| Soil | pH | EC (dS m−1) | Cot (g kg−1) | Not (g kg−1) | Cot/Not | Pe (mg kg−1) | K (mg kg−1) | Ca (mg kg−1) | Mg (mg kg−1) |
|---|---|---|---|---|---|---|---|---|---|
| S1 | 8.4 | 0.14 | 14.1 | 1.4 | 10 | 13 | 86 | 130 | 177 |
| S2 | 6.5 | 0.13 | 18.0 | 1.5 | 12 | 8 | 102 | 67 | 100 |
| CRUB | CSP | ||
|---|---|---|---|
| Cot | (g kg−1) | 96.4 | 136.1 |
| pH | 6.59 | 7.02 | |
| EC | (dS m−1) | 0.06 | 1.17 |
| Not | (mg kg−1) | 6300 | 13900 |
| NO3−-N | (mg kg−1) | 28.5 | 573 |
| NH4+-N | (mg kg−1) | 75.7 | 88.5 |
| N | (mg kg−1) | 6328.5 | 14473 |
| No | (mg kg−1) | 6196 | 13811.5 |
| Ni | (mg kg−1) | 104 | 661.5 |
| Pt | (mg kg−1) | 4620 | 14723 |
| K | (mg kg−1) | 64 | 293 |
| Ca | (mg kg−1) | 173 | 255 |
| Mg | (mg kg−1) | 306 | 1204 |
| Cot/N | 15.23 | 9.40 | |
| Cot/No | 15.55 | 9.85 | |
| Ni/N | 0.01 | 0.04 | |
| N/Pt | 1.37 | 0.98 | |
| As | (mg kg−1) | 5.13 | 1.65 |
| Cd | (mg kg−1) | <0.1 | <0.1 |
| Cr | (mg kg−1) | 0.68 | 4.60 |
| Cu | (mg kg−1) | 11.5 | 52.60 |
| Hg | (mg kg−1) | <0.05 | <0.05 |
| Mo | (mg kg−1) | 0.96 | 2.20 |
| Ni | (mg kg−1) | 1.70 | 7.20 |
| Pb | (mg kg−1) | 0.27 | 1.70 |
| Se | (mg kg−1) | <0.1 | < 0.1 |
| Zn | (mg kg−1) | 31 | 305 |
| Days | S | CSP | CRUB | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 87 ± 14 | a | A | 122 ± 2 | a | A | 88 ± 0 | a | A |
| 3 | 148 ± 7 | a | BC | 173 ± 1 | b | C | 165 ± 7 | ab | BC |
| 7 | 124 ± 10 | a | B | 126 ± 6 | a | B | 162 ± 7 | b | BC |
| 21 | 170 ± 9 | a | C | 208 ± 4 | a | D | 193 ± 29 | a | BC |
| 35 | 181 ± 29 | a | BC | 275 ± 17 | b | E | 167 ± 29 | a | AB |
| 49 | 144 ± 10 | a | C | 159 ± 6 | a | C | 166 ± 23 | a | CD |
| 70 | 266 ± 18 | a | D | 289 ± 2 | a | F | 238 ± 41 | a | DE |
| 91 | 246 ± 14 | a | D | 268 ± 5 | a | F | 243 ± 36 | a | E |
| 119 | 314 ± 14 | a | E | 324 ± 16 | a | G | 285 ± 36 | a | E |
| Days | S | CSP | CRUB | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 119 ± 5 | a | A | 135 ± 5 | a | A | 129 ± 12 | a | A |
| 3 | 114 ± 11 | a | A | 126 ± 4 | a | A | 126 ± 19 | a | A |
| 7 | 137 ± 9 | a | B | 155 ± 2 | a | B | 129 ± 9 | a | A |
| 21 | 194 ± 4 | a | DE | 230 ± 6 | b | C | 184 ± 14 | a | B |
| 35 | 153 ± 13 | a | BC | 130 ± 19 | a | AB | 101 ± 22 | a | A |
| 49 | 163 ± 6 | a | CD | 199 ± 5 | a | C | 176 ± 25 | a | B |
| 70 | 220 ± 13 | a | F | 238 ± 32 | a | CD | 203 ± 33 | a | B |
| 91 | 186 ± 12 | a | EF | 227 ± 3 | b | D | 216 ± 8 | b | C |
| 119 | 233 ± 22 | a | G | 273 ± 34 | a | E | 233 ± 12 | a | C |
| Treatment | Soil | Psoil | Papplied | Pmedium | Pmaxim | Peft | N/P | |||
|---|---|---|---|---|---|---|---|---|---|---|
| ppm | ppm | ppm | ppm | % | % | |||||
| CSP | S1 | 13.93 | 69.5 | 35.4 | 39.0 | 36.1 | 0.9 | |||
| CRUB | 64.8 | 26.4 | 30.5 | 26.6 | 1.4 | |||||
| CSP | S2 | 16.07 | 47.8 | 38.3 | 41.7 | 53.6 | 0.9 | |||
| CRUB | 42.0 | 27.5 | 30.4 | 34.1 | 1.4 |
| Parameter | Soil | CSP | CRUB |
|---|---|---|---|
| Co | S1 | 148.92 ± 4.145 | 149.07 ± 3.262 |
| ko | 0.01167 ± 0.000537 | 0.01250 ± 0.000467 | |
| Co | S2 | 129.80 ± 6.665 | 122.23 ± 5.478 |
| ko | 0.00965 ± 0.000758 | 0.01031 ± 0.000725 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orden, L.; Iocoli, G.A.; Bustamante, M.Á.; Moral, R.; Rodríguez, R.A. Nutrient Release Dynamics in Argentinean Pampean Soils Amended with Composts under Laboratory Conditions. Agronomy 2022, 12, 795. https://doi.org/10.3390/agronomy12040795
Orden L, Iocoli GA, Bustamante MÁ, Moral R, Rodríguez RA. Nutrient Release Dynamics in Argentinean Pampean Soils Amended with Composts under Laboratory Conditions. Agronomy. 2022; 12(4):795. https://doi.org/10.3390/agronomy12040795
Chicago/Turabian StyleOrden, Luciano, Gastón A. Iocoli, María Ángeles Bustamante, Raúl Moral, and Roberto A. Rodríguez. 2022. "Nutrient Release Dynamics in Argentinean Pampean Soils Amended with Composts under Laboratory Conditions" Agronomy 12, no. 4: 795. https://doi.org/10.3390/agronomy12040795
APA StyleOrden, L., Iocoli, G. A., Bustamante, M. Á., Moral, R., & Rodríguez, R. A. (2022). Nutrient Release Dynamics in Argentinean Pampean Soils Amended with Composts under Laboratory Conditions. Agronomy, 12(4), 795. https://doi.org/10.3390/agronomy12040795







