Abstract
The inadequate management of agro-waste in intensive agriculture has a severe negative impact on the environment. The valorization of crop residue as a source of crop nutrients is a valid alternative to close the nutrient cycle and reduce the use of external input. In this study, plant material was incorporated into the soil as fresh crop residue, after either composting and vermicomposting processes, to evaluate their effects on tomato yield and nutritional status (petiole sap analysis: NO3 and K+ concentration) over three crop cycles. A control treatment with mineral fertigation and an organic control treatment with goat manure were also included. Enzymatic activity and microbial population in the soil were evaluated. Although no differences between treatments were observed in the first cycle, in the second and third cycles, the yield obtained with the application of organic amendments derived from agro-waste was comparable to the yield obtained with mineral fertilizers. Overall, the sap analysis did not reveal a clear relationship with yield performances. The compost treatment resulted in higher microorganism presence in the soil. Soil dehydrogenase activity (DHA), acid phosphatase activity (ACP), and β-glucosidase activity (β-GLU) were generally more stimulated when organic amendments were used. The study confirms the applicability of soil fertilizers derived from agro-waste as a good alternative to mineral fertilizers.
1. Introduction
Nowadays, the elevated amount of waste generated from human activities is a major concern [1], and its proper management is crucial to preserve human and environmental health.
The horticulture-intensive model of Almeria (SE Spain) generates, each year, around 2 million tons of organic residues. These organic wastes are easily biodegradable, but the main problem is their elevated volume, mostly at the end of the crop cycles, when the non-commercial plant biomass has to be eliminated [2].
Many alternatives to the disposal of agro-waste have been evaluated, with the use of organic matter as a source of crop nutrients being the most competitive solution in an area in which intensive agriculture activity is prevalent [3,4].
The reintegration of agro-waste in the soil of the same agriculture site, fulfils the objective of the European Bio-economy Strategy 2018–2030, and reduces transport costs, the waste volume, and the need for mineral fertilizers [5,6].
The addition of organic amendments enhances soil structure and fertility [7], improving chemical, physical, and biological properties [7,8,9,10]. Soil microorganisms play a key role in agricultural production systems, especially in organic field, since the microbiota intervene in the nutrient cycles, allowing the mineralization and solubilization of organic matter supplied with the organic fertilizers. The microbial community has also an important function in the control of pests and pathogens and in promoting general plant health [11,12,13]. In the last few years the role of agronomic practices on soil microbial community changes has been studied [14,15,16,17]. However, it is difficult to understand the effects of organic fertilizers on soil microbiota [14,17], and better knowledge is needed to improve the management of organic fertilization.
The analysis of soil enzymatic activity is considered a useful tool to assess soil health when comparing different fertilization regimes, as enzymes are very sensitive to any changes on soil conditions [18,19].
Soil enzymes are synthetized mostly by microorganisms in order to degrade the organic matter. Thus, the dynamics of soil enzymes depends on the abundance of the microbial community [18]. Each enzyme catalyzes a particular biochemical reaction [19], and a single enzyme cannot indicate the overall soil biological quality. Dehydrogenase (DHA), acid phosphatase (ACP), and β-glucosidase (β-GLU) are among the enzymes widely used to characterize the soil microbial activity, since they catalyze fundamental biochemical reactions occurring during degradation of organic matter [20,21].
DHA activity is an indicator of the oxidation rate of organic matter and is considered to be a general index of biological activity [21]. In contrast, ACP and β-GLU are related to specific cycles. ACP is a group of enzymes, synthetized by plant roots and microorganisms, which release phosphate groups from organic compounds [21,22]. β-GLU is responsible for the hydrolysis of cellulose synthesised by microorganisms in the presence of adequate substrates [22].
The tomato is one of the most consumed vegetables in the world [23], being Italy and Spain the majors European producers [24]. It is estimated that a mean of 73 t ha−1 of vegetal residues are generated every year in the tomato-intensive crop system in the SE of Spain [3].
Considering the lack of strategies regarding agro-waste management, this study aims to give a practical and local solution to reintroduce into the same production system the waste generated in intensive horticulture.
The viability analysis of the use of agro-waste’s derived fertilizers is an important step in order to reduce dependence on external inputs such as chemical fertilizers. Due to tomato’s economic importance, this study analysed the effects of different fertilization management on a tomato crop. It was hypothesised that: (1) the organic fertilizers derived from vegetal waste can replace the mineral fertilization practices in terms of yield production, ensured by (2) the positive influence of the agro-waste derived amendments on microorganism abundance and enzymatic activity in the soil [15], which increase the solubility and mineralization of nutrients. In this context, three consecutive tomato crop cycles were carried out to evaluate the fertilizing value of vegetal residue, reincorporated in the soil as fresh matter or after composting and vermicomposting processes. The organic fertilization treatments with vegetal waste-derived fertilizers were compared to the mineral fertilization supplied by fertigation and to the organic fertilization with goat manure, a common amendment in the region. The organic treatments were carried out without applying any mineral fertilizers.
2. Materials and Methods
2.1. Field Experiment Design
The experiment was conducted inside a 1300 m2 greenhouse placed in Nijar (Almeria, Spain), during three consecutive tomato crop cycles (TC1, TC2, TC3). The greenhouse had a passive climate control.
Tomato (Solanum lycopersicum L.) cv. ‘Surcal’ (Natursur S.C.A.) grafted on ‘Beaufort’ rootstock (Monsanto) was cultivated in an artificial soil system (“enarenado”), in which 20–30 cm of imported loam soil was placed over the indigenous gravely soil, then 8–12 cm of sandy mulch was set on the top, at 0.66 plants m−2 staking and growing at two stems. A completely randomized experimental design with five treatments and four replicates per treatment with four plants each was used. The five treatments were as follows:
(1) Control treatment in which a mineral fertigation program was applied (FertControl); (2) organic control treatment in which goat manure (organic matter % (OM): 30.2, nitrogen Kjeldahl % (N Kjeldahl): 1.35; electrical conductivity dS m−1 (EC): 7.73; pH: 9.6) was applied at 3 kg m−1 (OrgControl); (3) vegetal residue from a previous tomato crop (OM: 75.5; N Kjeldahl: 2.50; EC: 10.79; pH: 6.9) applied at 4 kg m−1 (CropRes); (4) compost derived from a previous tomato crop vegetal residue (OM: 21.4; N Kjeldahl: 1.20; EC: 12.30; pH: 8.3) at 3 kg m−1 (Comp); and (5) vermicompost derived from a previous tomato crop vegetal residue (OM: 17.2; N Kjeldahl: 1.11; EC: 3.39; pH: 8.3) at 3 kg m−1 (VermiComp). The doses were calculated, taking as reference the common practice in the production area.
The organic amendments were applied and homogenized in the soil of the organic plot on 15 January 2017, before the transplanting of the first tomato crop cycle (TC0), the results of which are not included in this study, and on 15 January 2018 before the transplanting of TC2 (Table 1).

Table 1.
Description of production cycles.
The control treatment did not receive any organic amendment and was fertigated with nutrient mineral solution (mmol L−1): 12.93 NO3, 1.54 NH4, 0.89 PO4, 3.60 K, 1.80 Ca, 1.60 Mg, 13.21 Na, and 11.55 Cl. The pH was adjusted at 6 with nitric acid. Irrigation control was carried out through daily drainage measures ensuring that the EC of the drainage was not greater than 6.0 dS m−1 and a volume of 10–15% of drainage collected in relation to the volume of daily control dripper irrigation.
2.2. Sampling
The yield and nutritional status of plants via petiole sap analysis (N-NO3−, K) were measured to evaluate the effects of different fertilization management strategies on the crop. Moreover, to analyse the effect of organic fertilizers derived from vegetal residues, the soil biotic richness and soil enzyme activity were quantified.
Total fruit yield was quantified for each crop cycle (TC1, TC2, TC3). The yield was quantified with an electric balance with autocalibration and expressed in kg m−2. Petiole sap [NO3− and K+] was determined three times during the second cycle (TC2), at 111, 126, and 141 days after transplanting (DAT) and during the third cycle (TC3), at 101, 118, and 151 DAT. No sap analysis was carried out in TC1. Sap analyses were conducted following methods proposed by [25]. Twenty recently expanded leaves from different plants of each repetition were randomly collected between 9 and 11 am. The petioles were separated from the leaf, placed in a plastic bag inside a refrigerated box, and transported to the laboratory of the University of Almeria. At the laboratory, the petioles were cut into 1 cm sections and then squeezed with a domestic garlic press for sap extraction. The NO3− and K+ concentrations of the sap were analysed with a multi-ion sensor with potentiometry and a selective ion electrode with a modular probe (NT Sensor S.L., Tarragona, Spain). The following microorganisms of the rhizosphere were quantified in TC3 at 160 DAT: the total bacteria (BT), the total fungi (FT), and the ammonifying bacteria (AB). In the previous cycles no microbial analysis of the soil was carried out. To quantify the microbial population, decimal serial dilutions of soil samples were obtained in sterile saline solution (NaCl, 0.9%, p/v), which were seeded (0.1 mL) in culture medium for each microbial group. To quantify total bacteria (BT) and total fungi (FT), APHA plates and rose bengal plates (Panreac Quimica S.L.U., Castellar del Vallès, Barcelona, Spain) were used, respectively. After incubation at 30 °C (48 h BT and 72–120 h HT), colonies were counted and the results were expressed in CFU (colony-forming units) g−1 log10 [26]. To quantify AB, the most probable number technique [27] was used.
Dehydrogenase (DHA), acid phosphatase (ACP) and β-glucosidase (β-GLU) activity were measured. Three rhizosphere soil samples were collected for each treatment at a 10 cm depth. Samplings were carried out during TC1 at 98 DAT, during TC2 at 9 DAT and 49 DAT, between TC2 and TC3 at 161 DAT of TC2, during TC3 at 160 DAT and once finished the TC3 at 194 DAT. DHA was determined calculating the amount of triphenylformazan produced by microorganism after the reduction of triphenyl tetrazole chloride [21]. ACP and β-GLU were measured quantifying the concentration of P-nitrophenol G−1 soil−1 H−1 according to the protocol established by Tabatabai and Bremner [28].
2.3. Statistical Analyses
For statistical analysis, Statgraphics 18 software was used. A multifactorial analysis of variance (ANOVA) was carried out, using Fisher’s comparison test of means, with the statistically least significant difference being expressed as p < 0.05 (LSD).
3. Results and Discussion
3.1. Yield
Table 2 shows the yield results depending on the fertilization management strategy over three tomato cycles. Comparing the crop cycles, the highest total yield was obtained in TC2 in all treatments, due to the favourable climate conditions in the spring–summer growing season.

Table 2.
Total yield of tomato (kg m−2) in three cycles (TC1; TC2; TC3), as a function of fertilization treatment: mineral fertigation (FertControl), goat manure applied at 3 kg m−1 (OrgControl), fresh vegetal residue applied at 4 kg m−1 (CropRes), compost applied at 3 kg m−1 (Comp), and vermicompost applied at 3 kg m−1 (VermiComp).
During TC1, the fertilization treatment did not have statistically significant effects on tomato yield.
In the second and third tomato production cycles (TC2, TC3), differences were found between fertilization management strategies, with the highest yield observed in the FertControl treatment, achieving 7.51 kg·m−2 in TC2 and 5.44 kg·m−2 in TC3, while the OrgControl treatment had the lowest production level with 6.35 and 4.53 kg·m−2 in TC2 and TC3, respectively. In TC2, yields achieved in Comp (7.46 kg·m−2), CropRes (7.23 kg·m−2), and VermiComp (6.88 kg·m−2) were equal to the yield obtained in plots where a mineral fertigation program was applied. Similarly, in TC3, no significant differences were observed between FertControl, VermiComp with 5.28 kg·m−2, and Comp with 5.24 kg·m−2. While CropRes reported lower yield than FertControl.
The influence of organic fertilization management on crop production is a controversial matter since mineral fertilization supplies nutrients readily available for plants, resulting in high growth rates and yields [29]. In contrast, with the application of organic fertilizers, nutrient availability depends on the rate of mineralization of organic matter, which may not fulfil the plant’s nutrient demands [29,30,31]. In this study, the application of agro-waste-derived fertilizers did not negatively affect tomato yield during three consecutive tomato crops, except in TC3 where CropRes plots registered lower yields. Previously, several studies showed no significant differences between the commercial production obtained with mineral or organic fertilization management; some authors reported higher yield with the application of organic fertilizers [32,33,34].
3.2. Sap Analysis
The sap petiole analysis shows the nutritional status of the crop during two growing cycles (TC2, TC3) (Table 3).

Table 3.
N-NO3− and K+ content in fresh petiole sap of tomato for two cycles (TC2; TC3) as a function of fertilization treatment: mineral fertigation (FertControl), goat manure applied at 3 kg m−1 (OrgControl), fresh vegetal residue applied at 4 kg m−1 (CropRes), compost applied at 3 kg m−1 (Comp), and vermicompost) applied at 3 kg m−1 (VermiComp).
In TC2, a general decrease in the concentration of N-NO3− and K+ petiole sap was observed in the second analysis (126 DAT), which may be due to the higher demand of nutrients from sink organs. In TC3, the concentration of K+ and NO3− followed the opposite trend, with a general peak reached at the 118 DAT analysis.
Tomatoes fertilized with VermiComp had the highest N-NO3− petiole sap concentration at 111 DAT in TC2, while in the 126 DAT analysis, the highest concentration was observed in FertControl. No significant differences between treatments in N-NO3− concentration were observed at the last sampling time (141 DAT). During TC3, at 101 DAT, FertControl and Comp treatments had the highest N-NO3− concentration, and at 118 DAT, the highest value was observed in the Comp treatment, followed by OrgControl. At 151 DAT, CropRes had the lowest N-NO3− concentration value. In terms of the means, in both cycles, different fertilization treatments did not result in different N-NO3− concentrations in the petiole sap.
K+ petiole sap content in TC2 followed the trend of NO3−, with the lowest concentration level reached in the second analysis (126 DAT), except in the case of the OrgControl treatment, which registered a K+ content decrease throughout TC2.
In TC2, the highest K+ concentration was obtained with the VermiComp treatment at 111 DAT, and with VermiComp and FertControl at 141 DAT. No significant differences in K+ concentration were observed at 126 DAT.
During TC3, the highest K+ concentration was obtained with the OrgControl treatment at 101 DAT, with the Comp treatment at 118 DAT, and with the CropRes treatment at 151 DAT.
A similar response of K+ petiole sap concentration was observed over different fertilization treatments, with the highest mean value obtained in VermiComp treatment during TC2, only significantly different from OrgControl. In TC3, the highest K+ values were obtained in the OrgControl, CropRes, and Comp treatments, which were only significantly different from FertControl.
According to nutritional analyses carried out during the two production cycles, the N-NO3− concentration in sap were statistically comparable between mineral and organic fertilization management, except at 126 DAT TC2 analysis. For the K+ in sap, the statistically significant differences showed greater concentrations in the treatments with organic amendments in most of the analyses. These results justify that the production achieved in organic treatments was not statistically affected.
In general terms, no consistent differences in the nutritional plants’ status were observed between treatments. In organic fertilization management, the most limiting factor is N availability [29], since organic N needs to be mineralized to be available for plants. In this study, no significant differences were observed in the N-NO3− petiole sap concentration between the FerControl treatment and agro-waste-derived fertilizer treatments, indicating that the release of nutrients from organic matter was sufficient to fulfil the nutrients demands of the plants.
3.3. Microorganisms
Table 4 shows the microorganism concentrations in the rhizosphere during TC3. The highest concentration of total bacteria (BT) was observed in the Comp treatment, while FertControl and OrgControl showed the lowest concentrations. Compared to the other organic treatments, FertControl registered the lowest presence of ammonifying bacteria (AB). Regarding total fungi (FT), FertControl had the highest concentration significantly different from other treatments.

Table 4.
Concentration of total bacteria (BT), total fungi (FT), and ammonifying bacteria (AB) expressed in colony-forming units (CFU) g−1 log10, in the rhizosphere in third cycle (TC3) as a function of fertilization treatment: mineral fertigation (FertControl), goat manure applied at 3 kg m−1 (OrgControl), fresh vegetal residue applied at 4 kg m−1 (CropRes), compost applied at 3 kg m−1 (Comp), and vermicompost applied at 3 kg m−1 (VermiComp).
Overall, the Comp treatment enhanced the bacterial biomass in the rhizosphere, including a high presence of ammonifying bacteria, consistent with previous results that reported an enhancement in microbial biomass after the application of organic amendments [35,36]. The increase of the microbial biomass in Hale et al. [35], which tested compost and biochar in turfgrass plots, was attributed to the increase in bacterial population, but not fungal, which is in line with the results of the present study. The increase of fungal abundance (FT) in soils treated with chemical fertilizers (FertControl) coincide with the results of Fu et al. [36], which found an increase of fungal population in response to long-term continuous monoculture crops, and the results of Zhou and Wu [37], which studied a continuous cucumber cropping system, and after 7 years reported an increase of Fusarium fungi combined with a decrease in cucumber yield. The same trend observed in fungi number, both with the application of chemicals and a long-term continuous cropping system, may be ascribed to the soil sickness which these agricultural practices lead to [37], explaining the frequent occurrence of continuous solarisation and disinfection of the soils.
The incorporation of organic materials into the soil as fertilizers, significantly improved the concentration of AB (more than double) in all organic treatments when compared to mineral fertilization management. This may be ascribed to the higher presence of growing substrate for ammonifying micrograms. However, Luo et al. [17] reported that the influence of organic fertilizers on microbial community, may be mostly ascribed to the microorganisms associated with organic matter, which are incorporated in the soil and interact with native microorganisms. These results justify that the levels of N in sap during the cultivation were adequate in all treatments; the increase of and AB populations in the soil allowed availability of N for plants.
3.4. Enzymatic Activity
Figure 1 shows the evolution of dehydrogenase (DHA), acid phosphatase (ACP) and β-glucosidase (β-GLU) activity during the three tomato cycles. A high enzymatic activity is related to the high presence of active microorganisms in the soil, which is essential for nutrient availability for plants, especially in soil where mineral fertilization is replaced with organic nutrient supply.
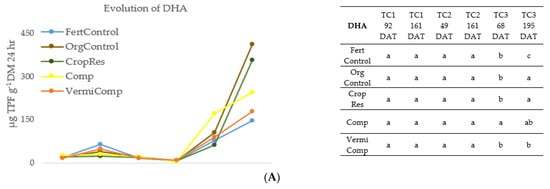
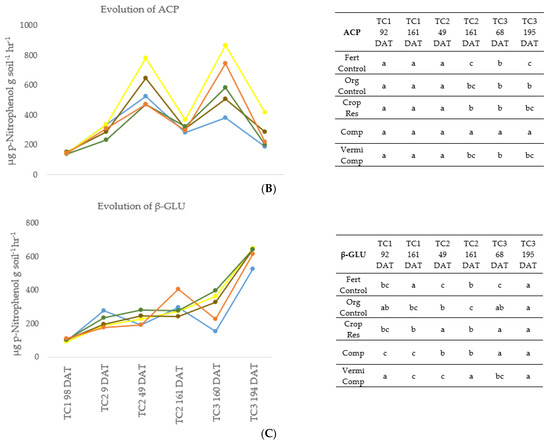
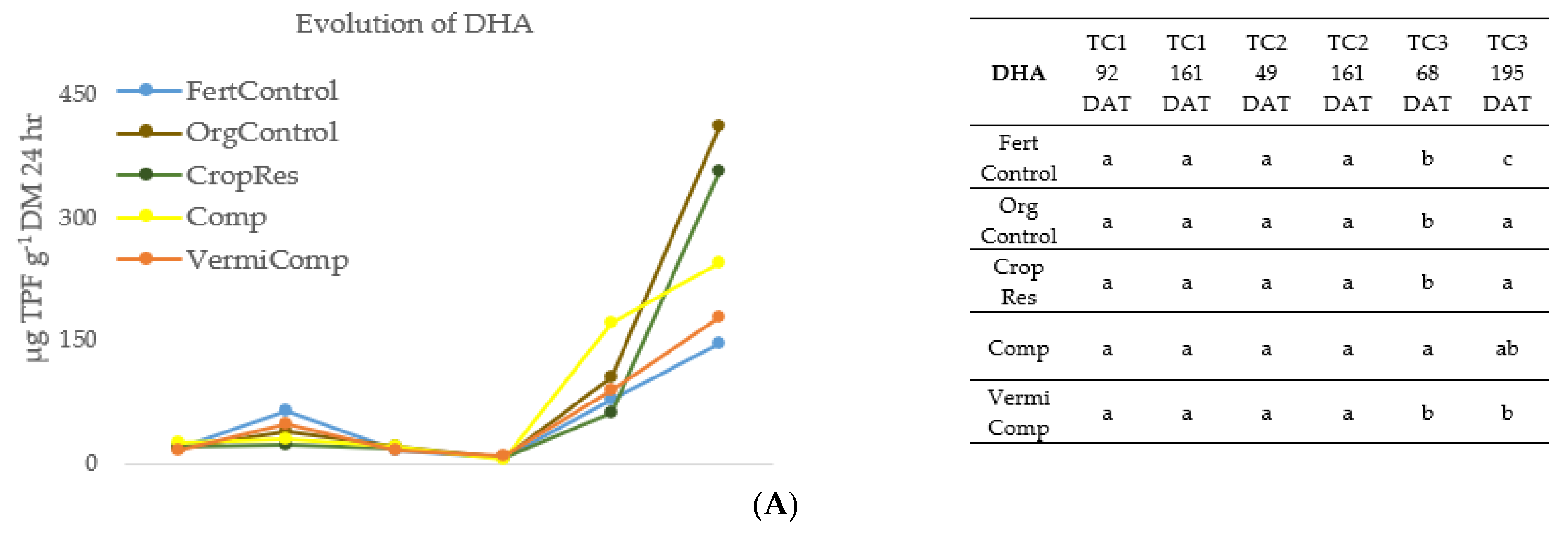
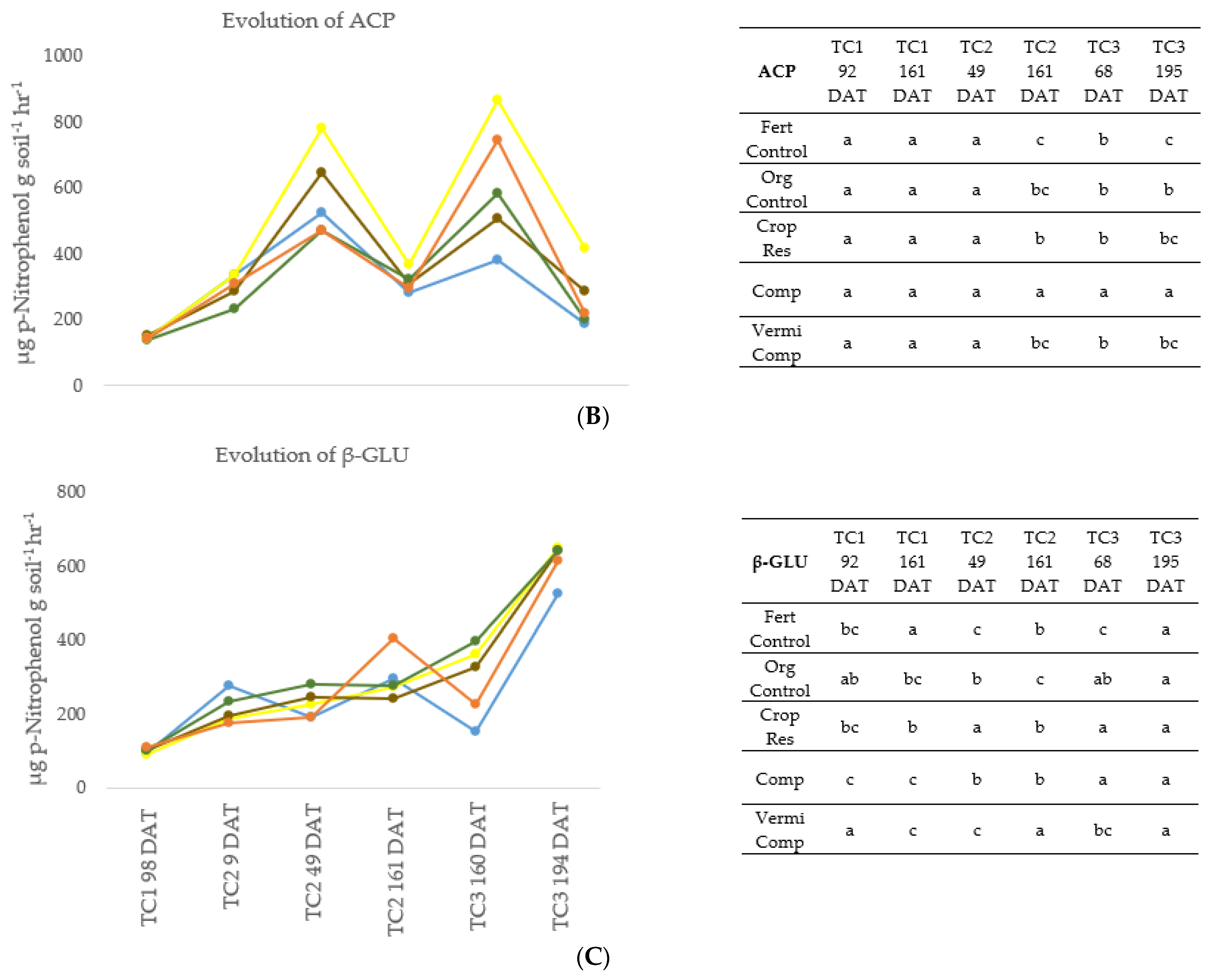
Figure 1.
Evolution of (A) dehydrogenase activity (DHA), (B) acid phosphatase activity (ACP), (C) β-glucosidase activity (β-GLU) in the soil during three crop cycles (TC1, TC2, TC3) as a function of fertilization treatment: mineral fertigation (FertControl), goat manure applied at 3 kg m−1 (OrgControl), fresh vegetal residue applied at 4 kg m−1 (CropRes), compost applied at 3 kg m−1 (Comp), and vermicompost applied at 3 kg m−1 (VermiComp). DAT, Day After Transplanting. Different letters indicate significant differences between treatments at the 95% confidence level (p ≤ 0.05; Fisher’s LSD).
Generally, the enzymatic activity (DHA, ACP, β-GLU) was lower in the FertControl treatment. Otherwise, during TC1 and TC2, the differences between treatments were not consistent. During TC1 and TC2, DHA activity was maintained at a low level, with no significant differences between treatments. DHA is considered a general index of microbial activity [22]. The application of organic amendments before transplanting TC2 did not affect biological activity during the same cycle, which only started to increase during TC3. This could be due to the initial adaptation phase of microorganisms after the application of organic amendments [38]. After the initial phase, soil microbiota reached optimal growth, followed by the enhancement of enzymatic activity.
Regarding ACP, no significant differences between treatments were observed in the first three analyses (TC1 at 98 DAT; TC2 at 9 DAT; TC2 at 49 DAT), while in the last three (TC2 at 161 DAT; TC3 at 160 DAT; TC3 at 194 DAT), the highest ACP activity was observed in plots amended with Comp. It is important to remark that the ACP enzymes are produced both by microorganisms and plant roots. The rapid general decrease in ACP activity observed in TC2 at 161 DAT and in TC3 at 195 DDT was due to the lack of enzymes produced by plants roots, since the analysis was performed between two crop cycles.
β-GLU is synthetized by soil microbiota in the presence of a cellulose substrate, as this enzyme is involved in its decomposition [38]. In the three crop cycles, a general increase in β-GLU activity was observed after the organic amendment application. This increase could be related to the increase of organic matter degradation in a continuous crop cycle [38].
In general, in this study the application of organic amendments derived from agro-waste had a positive effect on soil enzymatic activity, as previously reported [21]. In line with our results, Hernandez et al. [8], evaluating the use of compost alone or combined with inorganic fertilizers in two consecutive lettuce crops, reported that while in the first crop cycle no significant differences between treatments were observed in DHA, ACP and β-GLU activity, in the second lettuce cycle higher enzymatic activity was registered in plots where compost was applied compared to plots managed only with mineral fertilizers.
Enzymatic activity is also a good tool to study the potential availability of nutrients for plants since the enzymes are involved in the mineralization of organic matter and enhance soil quality [21]. The results of this study indicate that the continuous application of agro-waste-derived amendments has a positive effect on microbiota activity, which consequently avoids nutrient deficiency, and results in high-yield results. Moreover, microorganisms can indirectly affect plant growth by the production of plant growth regulators and hormones [32].
3.5. Pearson Product Moment Correlations among Soil Biological Proprieties
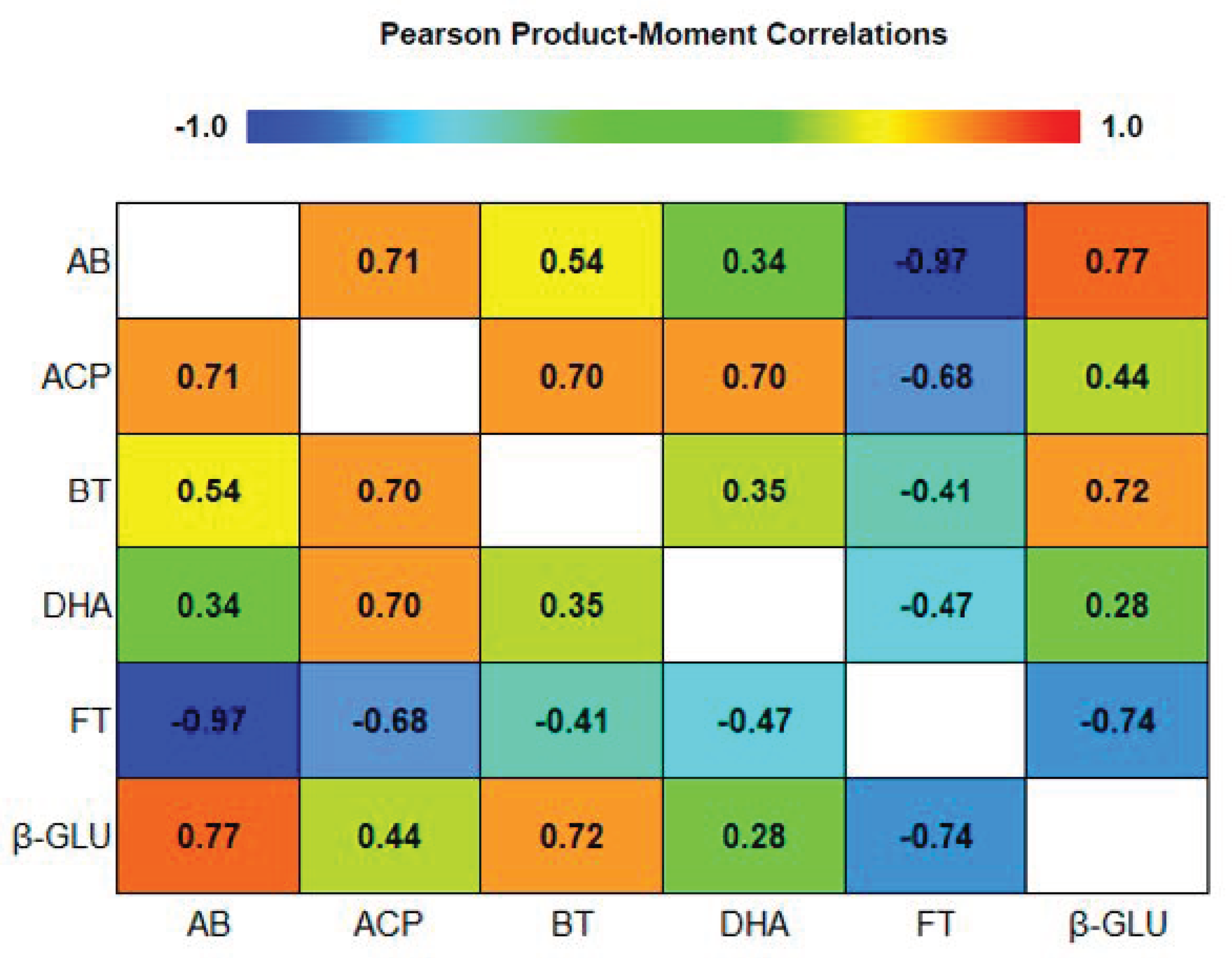
To study the relation between soil microbiota and enzymatic activity a Pearson product–moment correlation analysis was carried out (Figure 2).
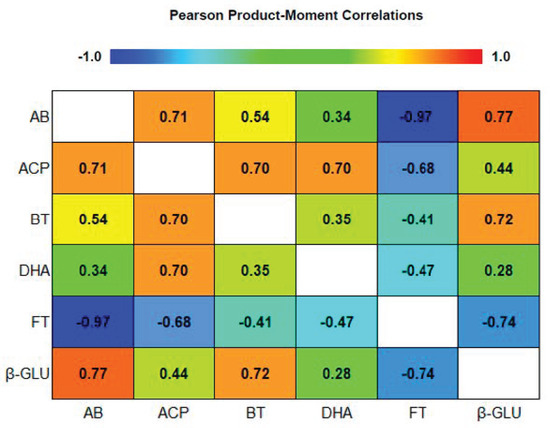
Figure 2.
Pearson product-moment correlation between biological proprieties in third cycle (TC3). DHA, dehydrogenase; ACP, acid phosphatase, β-GLU, β-glucosidase, total bacteria (BT), total fungi (FT), and ammonifying bacteria (AB).
Enzymatic activity is considered an indicator of soil quality and it is used to monitor microbial activity [37]. The negative correlation between DHA, ACP and β-GLU with FT (−0.4716; −0.6758; −0.7396) may be due to the negative effects of fungal community on the beneficial microbial activity. The highest FT population was found in plots managed with a fertigation plan and where no organic amendments were added.
The negative relation between soil enzymatic activity and FT may suggest that the community structure of this fungal population plays a negative role on soil health, as previously reported by Fu et al. [36] and Zhou and Wu [37] when evaluating continued monoculture. Moreover, the results showed a negative correlation between AB and FT (−0.9675), simultaneously the enzymatic activity is increased by bacteria population. The DHA, ACP and β-GLU activity are positively correlated with the microorganisms that facilitate the availability of nutrients: ACP with the AB (0.7121) and BT (0.7002), and β-GLU with AB (0.7736) and BT (0.7194). Simultaneously, the organically treated soils in the experiment showed an increase of DHA, ACP, β-GLU activity, indicating that the organic amendments derived from vegetal residues, provide a specific substrate for the enzymes which play a key role in the decomposition of soil organic matter [8].
4. Conclusions
Our study confirms that compost and vermicompost derived from agro-waste could replace mineral fertilization management, since no significant differences were obtained in yield during three tomato cycles.
The results showed that the total abundance of bacteria, mainly the abundance of ammonifying bacteria (AB), and the enzymatic activity of the soil increased with the application of organic fertilizers. Changes in the microbial abundance of the soil are observed in the short-term, and positively correlated with the enzymatic activity [38].
Chemical fertilizers maintain the short-term productivity of agroecosystems, while their indiscriminate use decreases the abundance of growth-promoting microbial populations such as ammonifying bacteria, and increases the population of total fungi, which may have a negative effect on plant growth and crop health [37]. The most likely reasons for the above results are that the continued cultivation without contributions of organic amendments produces changes in the microbial population of the soil that manifests itself in a significant increase in the abundance of total fungi, and this can lead to soil sickness and problems for subsequent crops [36,37]. On the contrary the application of organic fertilizers allows the restoration of soil microbiota, which mineralizes and solubilizes nutrients, avoiding plant’s nutritional deficiencies (N-NO3− and K+ concentration in sap were statistically comparable to mineral fertilization) and production losses. Among the different alternatives to valorize agricultural residue in an intensive horticulture site, the results suggest that the use of vegetable residue as a source of nutrients is a valid strategy to limit chemical fertilizer use since it helps to improve biological soil health without compromising performance. Compost or vermicompost of vegetable residues can satisfactorily be incorporated in the soil. Moreover, it has been studied that fertilization with agro-waste-derived materials improves tomato quality, resulting in a high content of lycopene, ascorbic acid and phenols [4]. In view of agro-waste valorization, future research should investigate strategies to enrich compost or vermicompost, derived from vegetal residues, with microorganisms in order to improve the fertilizer efficiency. Moreover, considering that fertigation is a common practice in horticulture systems, better knowledge is required on the potentiality of aqueous compost/vermicompost extracts used as alternatives to conventional mineral solutions.
This study should encourage the horticulture sector to implement a circular strategy in nutritional crop management, reducing the need for external inputs.
Author Contributions
Conceptualization, I.C.-M. and M.d.C.S.-S.; Data curation, I.C.-M.; D.F.; Formal analysis, I.C.-M., D.F., F.B. and M.d.C.S.-S.; Investigation, I.C.-M., F.B. and M.d.C.S.-S.; Methodology, M.d.C.S.-S. and F.O.; Project administration, M.d.C.S.-S.; Supervision, M.d.C.S.-S. and F.O.; Writing—original draft, I.C.-M., D.F., F.B. and M.d.C.S.-S.; Writing—review and editing, I.C.-M., F.B. and M.d.C.S.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Demirbas, A. Waste management, waste resource facilities and waste conversion processes. Energy Convers. Manag. 2011, 52, 1280–1287. [Google Scholar] [CrossRef]
- Aznar-Sánchez, J.A.; Velasco-Muñoz, J.F.; García-Arca, D.; López-Felices, B. Identification of opportunities for applying the circular economy to intensive agriculture in Almería (South-East Spain). Agronomy 2020, 10, 1499. [Google Scholar] [CrossRef]
- Lopez, M.J.; Masaguer, A.; Paredes, C.; Perez, L.; Muñoz, M.; Salas, M.C.; Hernandez, R. De resíduos a recursos. El camino hacia la Sostenibilidad. Red Española Compost. 2015, 91–121. [Google Scholar]
- Carricondo-Martínez, I.; Berti, F.; Salas-Sanjuán, M.C. Different Organic Fertilization Systems Modify Tomato Quality: An Opportunity for Circular Fertilization in Intensive Horticulture. Agronomy 2022, 12, 174. [Google Scholar] [CrossRef]
- Janssen, B.H.; Oenema, O. Global economics of nutrient cycling. Turk. J. Agric. For. 2008, 32, 165–176. [Google Scholar]
- Cambier, P.; Pot, V.; Mercier, V.; Michaud, A.; Benoit, P.; Revallier, A.; Houot, S. Impact of long-term organic residue recycling in agriculture on soil solution composition and trace metal leaching in soils. Sci. Total Environ. 2014, 499, 560–573. [Google Scholar] [CrossRef]
- Williams, H.; Colombi, T.; Keller, T. The influence of soil management on soil health: An on-farm study in southern Sweden. Geoderma 2020, 360, 114010. [Google Scholar] [CrossRef]
- Hernández, T.; Chocano, C.; Moreno, J.L.; García, C. Use of compost as an alternative to conventional inorganic fertilizers in intensive lettuce (Lactuca sativa L.) crops—Effects on soil and plant. Soil Tillage Res. 2016, 160, 14–22. [Google Scholar] [CrossRef]
- Carrera, L.M.; Buyer, J.S.; Vinyard, B.; Abdul-Baki, A.A.; Sikora, L.J.; Teasdale, J.R. Effects of cover crops, compost, and manure amendments on soil microbial community structure in tomato production systems. Appl. Soil Ecol. 2007, 37, 247–255. [Google Scholar] [CrossRef]
- Liang, B.; Zhao, W.; Yang, X.; Zhou, J. Fate of nitrogen-15 as influenced by soil and nutrient management history in a 19-year wheat–maize experiment. Field Crops Res. 2013, 144, 126–134. [Google Scholar] [CrossRef]
- Pant, A.P.; Radovich, T.J.; Hue, N.V.; Talcott, S.T.; Krenek, K.A. Vermicompost extracts influence growth, mineral nutrients, phytonutrients and antioxidant activity in pak choi (Brassica rapa cv. Bonsai, Chinensis group) grown under vermicompost and chemical fertilizer. J. Sci. Food Agric. 2009, 89, 2383–2392. [Google Scholar] [CrossRef]
- Grobelak, A.; Napora, A.; Kacprzak, M. Using plant growth-promoting rhizobacteria (PGPR) to improve plant growth. Ecol. Eng. 2015, 84, 22–28. [Google Scholar] [CrossRef]
- Wang, L.; Kaur, M.; Zhang, P.; Li, J.; Xu, M. Effect of Different Agricultural Farming Practices on Microbial Biomass and Enzyme Activities of Celery Growing Field Soil. Int. J. Environ. Res. Public Health 2021, 18, 12862. [Google Scholar] [CrossRef]
- Bhunia, S.; Bhowmik, A.; Mallick, R.; Mukherjee, J. Agronomic Efficiency of Animal-Derived Organic Fertilizers and Their Effects on Biology and Fertility of Soil: A Review. Agronomy 2021, 11, 823. [Google Scholar] [CrossRef]
- Treonis, A.M.; Austin, E.E.; Buyer, J.S.; Maul, J.E.; Spicer, L.; Zasada, I.A. Effects of organic amendment and tillage on soil microorganisms and microfauna. Appl. Soil Ecol. 2010, 46, 103–110. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y.; Ma, T.; Raza, W.; Li, J.; Howland, J.G.; Huang, Q.; Shen, Q. Impacts of inorganic and organic fertilization treatments on bacterial and fungal communities in a paddy soil. Appl. Soil Ecol. 2017, 112, 42–50. [Google Scholar] [CrossRef]
- Luo, Y.; van Veelen, H.P.J.; Chen, S.; Sechi, V.; ter Heijne, A.; Veeken, A.; Buisman, C.J.N.; Bezemer, T.M. Effects of sterilization and maturity of compost on soil bacterial and fungal communities and wheat growth. Geoderma 2022, 409, 115598. [Google Scholar] [CrossRef]
- Chen, Y.P.; Tsai, C.F.; Rekha, P.D.; Ghate, S.D.; Huang, H.Y.; Hsu, Y.H.; Liaw, L.L.; Young, C.C. Agricultural management practices influence the soil enzyme activity and bacterial community structure in tea plantations. Bot. Stud. 2021, 62, 8. [Google Scholar] [CrossRef]
- Adetunji, A.T.; Lewu, F.B.; Mulidzi, R.; Ncube, B. The biological activities of β-glucosidase, phosphatase and urease as soil quality indicators: A review. J. Soil Sci. Plant Nutr. 2017, 17, 794–807. [Google Scholar] [CrossRef] [Green Version]
- Cordovil, C.M.D.S.; de Varennes, A.; Pinto, R.M.D.S.; Alves, T.F.; Mendes, P.; Sampaio, S.C. Decomposition rate and enzymatic activity of composted municipal waste and poultry manure in the soil in a biofuel crops field. J. Sci. Food Agric. 2017, 97, 2245–2255. [Google Scholar] [CrossRef]
- Ruiz, J.L.; Salas, M.C. Evaluation of organic substrates and microorganisms as bio-fertilization tool in container crop production. Agronomy 2019, 9, 705. [Google Scholar] [CrossRef] [Green Version]
- Vargas-García, M.C.; Suárez-Estrella, F.; López, M.J.; Moreno, J. Microbial population dynamics and enzyme activities in composting processes with different starting materials. Waste Manag. 2010, 30, 771–778. [Google Scholar] [CrossRef]
- Bergougnoux, V. The history of tomato: From domestication to biopharming. Biotechnol. Adv. 2014, 32, 70–189. [Google Scholar] [CrossRef]
- Faostat. Available online: http://www.fao.org/faostat/es (accessed on 13 December 2021).
- Cadahia, C. La savia como índice de fertilización. Cultivos agroenergéticos, hortícolas, frutales y ornamentales. Mundi-Prensa Madr. 2008, 35–68. [Google Scholar]
- Wilson, P.W.; Knight, S.C. Experiments in Bacterial Physiology; Burgess: Minneapolis, MN, USA, 1952. [Google Scholar]
- Herigstad, B.; Hamilton, M.; Heersink, J. How to optimize the drop plate method for enumerating bacteria. J. Microbiol. Methods 2001, 44, 121–129. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 4, 301–307. [Google Scholar] [CrossRef]
- Sánchez, N.A.; Sánchez, J.A.; Salas, M.C.; Arantzazu, M.B.; Delgado, M.J. Medium-term influence of organic fertilization on the quality and yield of a celery crop. Agronomy 2020, 10, 1418. [Google Scholar]
- Bilalis, D.; Krokida, M.; Roussis, I.; Papastylianou, P.; Travlos, I.; Cheimona, N.; Dede, A. Effects of organic and inorganic fertilization on yield and quality of processing tomato (Lycopersicon esculentum Mill.). Folia Hort. 2018, 30, 321–332. [Google Scholar] [CrossRef] [Green Version]
- Riahi, A.; Hdider, C.; Sanaa, M.; Tarchoun, N.; Kheder, M.B.; Guezal, I. Effect of conventional and organic productions systems on the yield and quality of field tomato cultivars grown in Tunisia. J. Sci. Food Agric. 2009, 89, 2275–2282. [Google Scholar] [CrossRef]
- Arancon, N.Q.; Edwards, C.A.; Bierman, P.; Welch, C.; Metzger, J.D. Influences of vermicomposts on field strawberries: Effects on growth and yields. Bioresour. Technol. 2004, 93, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Zhao, F.; Zhang, G.; Zhang, Y.; Yang, L. Vermicompost improves tomato yield and quality and the biochemical properties of soils with different tomato planting history in a greenhouse study. Front. Plant Sci. 2017, 8, 1978. [Google Scholar] [CrossRef] [Green Version]
- Murmu, K.; Ghosh, B.C.; Swain, D.K. Yield and quality of tomato grown under organic and conventional nutrient management. Arch. Agron. Soil Sci. 2013, 59, 1311–1321. [Google Scholar] [CrossRef]
- Hale, L.; Curtis, D.; Azeem, M.; Montgomery, J.; Crowley, D.E.; McGiffen, M.E., Jr. Influence of compost and biochar on soil biological properties under turfgrass supplied deficit irrigation. Appl. Soil Ecol. 2021, 168, 104134. [Google Scholar] [CrossRef]
- Fu, H.; Zhang, G.; Zhang, F.; Sun, Z.; Geng, G.; Li, T. Effects of continuous tomato monoculture on soil microbial properties and enzyme activities in a solar greenhouse. Sustainability 2017, 9, 317. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.G.; Wu, F.Z. Changes in soil chemical characters and enzyme activities during continuous monocropping of cucumber (Cucumis sativus). Pak. J. Bot. 2015, 47, 691–697. [Google Scholar]
- Guerra, P.A.M.; Sanjúan, M.C.S.; López, M.J. Evaluation of physicochemical properties and enzymatic activity of organic substrates during four crop cycles in soilless containers. Food Sci. Nutr. 2018, 6, 2066–2078. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).