The Influence of Different Nitrogen Fertilizer Rates, Urease Inhibitors and Biological Preparations on Maize Grain Yield and Yield Structure Elements
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Location and Arrangement of the Experiment
- 1.
- (N100) 238 L ha−1 KAS-32 (solution of urea CO(NH2)2 and ammonium nitrate NH4NO3) was applied to the soil surface immediately after sowing;
- 2.
- (N140) 333.2 L ha−1 KAS-32 (solution of urea CO(NH2)2 and ammonium nitrate NH4NO3) was applied to the soil surface immediately after sowing;
- 3.
- (N180) 428.4 L ha−1 KAS-32 (solution of urea CO(NH2)2 and ammonium nitrate NH4NO3) was applied to the soil surface immediately after sowing.
- 1.
- (Without UI and BP) urease inhibitors and biological preparations not used;
- 2.
- (UI ATS) urease inhibitor—ammonium thiosulfate [ATS, (NH4)2S2O3 12-0-0-26S] (10% irrigated with KAS-32: in fields fertilized with N100–23.8 L ha−1; in fields fertilized with N140–33.3 L ha−1; in fields fertilized with N180–42.8 L ha−1;
- 3.
- (UI URN) urease inhibitor—N-butyl-thiophosphoric triamide (NBPT) 188 g L−1 and N-propyl-thiophosphoric triamide (NPPT) 87 g L−1 (1.0 L ha−1 irrigated with KAS-32);
- 4.
- (BP HUM) biological preparation—15% suspension of humic and fulvic acids, pH 4–5 (1.0 L ha−1 irrigated together with KAS-32);
- 5.
- (BP FIT) biological preparation—20% suspension of Ascophyllum nodosum (0.6 L ha−1 sprayed on maize at stage BBCH 26).
2.2. Statistical Analysis
2.3. Weather Conditions
3. Results
3.1. The Influence of the Studied Factors on Maize Grain Yield
3.2. The Influence of the Studied Factors on the 1000-Grain Weight of Maize
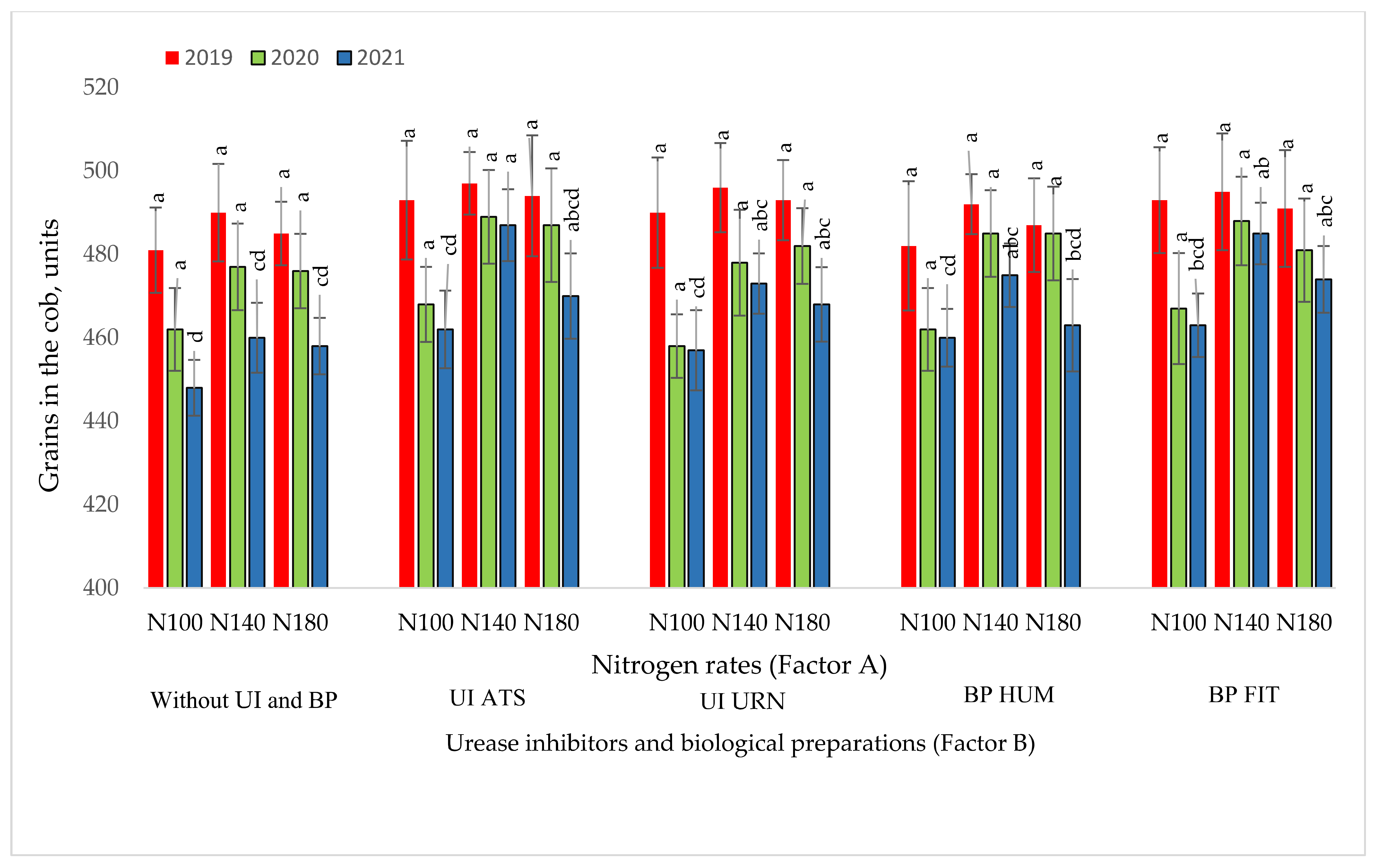
3.3. The Influence of the Studied Factors on the Number of Maize Grains in the Cob
3.4. Correlation-Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Amnuaylojaroen, T.; Chanvichit, P.; Janta, R.; Surapipith, V. Projection of Rice and Maize Productions in Northern Thailand under Climate Change Scenario RCP8.5. Agriculture 2021, 11, 23. [Google Scholar] [CrossRef]
- Neumann, K.; Verburg, P.H.; Stehfest, E.; Müller, C. The yield gap of global grain production: A spatial analysis. Agric. Syst. 2010, 103, 316–326. [Google Scholar] [CrossRef]
- Paponov, I.; Sambo, P.; Erley, G.S.A.; Presterl, T.; Geiger, H.H.; Engels, C. Grain yield and kernel weight of two maize genotypes differing in nitrogen use efficiency at various levels of nitrogen and carbohydrate availability during flowering and grain filling. Plant Soil 2005, 272, 111–123. [Google Scholar] [CrossRef]
- Baohua, L.; Xinping, C.; Qingfeng, M.; Haishun, Y.; Justin, V.W. Estimating maize yield potential and yield gap with agro-climatic zones in China-Distinguish irrigated and rainfed conditions. Agric. For. Meteorol. 2017, 239, 108–117. [Google Scholar] [CrossRef]
- Bänziger, M.; Edmeades, G.; Lafitte, H. Physiological mechanisms contributing to the increased N stress tolerance of tropical maize selected for drought tolerance. Field Crop. Res. 2002, 75, 223–233. [Google Scholar] [CrossRef]
- Milander, J.J. Maize Yield and Components as Influenced by Environment and Agronomic Management. Master’s Thesis, University of Nebraska, Lincoln, NE, USA, 2015; p. 86. [Google Scholar]
- Araus, J.L.; Slafer, G.A.; Royo, C.; Serret, M.D. Breeding for Yield Potential and Stress Adaptation in Cereals. Crit. Rev. Plant Sci. 2008, 27, 377–412. [Google Scholar] [CrossRef]
- Balawejder, M.; Szostek, M.; Gorzelany, J.; Antos, P.; Witek, G.; Matłok, N. A Study on the Potential Fertilization Effects of Microgranule Fertilizer Based on the Protein and Calcined Bones in Maize Cultivation. Sustainability 2020, 12, 1343. [Google Scholar] [CrossRef]
- Bender, R.R.; Haegele, J.W.; Rufo, M.L.; Below, F.E. Nutrient Uptake, Partitioning, and Remobilization in Modern, Transgenic Insect-Protected Maize Hybrids. Agron. J. 2013, 105, 161–170. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2012. [Google Scholar]
- Urioste, A.; Hevia, G.; Hepper, E.; Anton, L.; Bono, A.; Buschiazzo, D. Cultivation effects on the distribution of organic carbon, total nitrogen and phosphorus in soils of the semiarid region of Argentinian Pampas. Geoderma 2006, 136, 621–630. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, D.; Hu, K.; Willett, I.R.; Langford, J. Policy incentives for reducing nitrate leaching from intensive agriculture in desert oases of Alxa, Inner Mongolia, China. Agric. Water Manag. 2009, 96, 1114–1119. [Google Scholar] [CrossRef]
- Zhou, Z.; Andersen, M.N.; Plauborg, F. Radiation interception and radiation use efficiency of potato affected by different N fertigation and irrigation regimes. Eur. J. Agron. 2016, 81, 129–137. [Google Scholar] [CrossRef]
- Heffer, P.; Prud’homme, M. Fertilizer Outlook 2017–2021. In Proceedings of the 85th IFA Annual Conference, Marrakech, Morocco, 21–23 May 2017. [Google Scholar]
- Gao, Q.; Li, C.; Feng, G.; Wang, J.; Cui, Z.; Chen, X.; Zhang, F. Understanding Yield Response to Nitrogen to Achieve High Yield and High Nitrogen Use Efficiency in Rainfed Corn. Agron. J. 2012, 104, 165–168. [Google Scholar] [CrossRef]
- Asibi, A.E.; Chai, Q.; Coulter, J.A. Mechanisms of Nitrogen Use in Maize. Agronomy 2019, 9, 775. [Google Scholar] [CrossRef]
- Andraski, T.W.; Bundy, L.G.; Brye, K.R. Crop Management and Corn Nitrogen Rate Effects on Nitrate Leaching. J. Environ. Qual. 2000, 29, 1095–1103. [Google Scholar] [CrossRef]
- Szulc, P.; Bocianowski, J.; Rybus, M. Response of nitrogen nutritional indices of maize leaves to different mineral-organic fertilization. Maydica 2012, 57, 260–265. Available online: https://journals-crea.4science.it/index.php/maydica/article/view/887/768 (accessed on 24 November 2021).
- Zeidan, M.S.; Amany, A.; Bahr El-Kramany, M.F. Effect of N fertilizer and plant density on yield and quality of maize in sandy soil. Res. J. Biol. Sci. 2006, 2, 156–161. [Google Scholar]
- Snyder, C.; Bruulsema, T.; Jensen, T.; Fixen, P. Review of greenhouse gas emissions from crop production systems and fertilizer management effects. Agric. Ecosyst. Environ. 2009, 133, 247–266. [Google Scholar] [CrossRef]
- Cassman, K.G.; Dobermann, A.; Walters, D.T. Agroecosystems, nitrogen-use efficiency, and nitrogen management. AMBIO J. Hum. Environ. 2002, 31, 132–140. [Google Scholar] [CrossRef]
- Sawyer, J.; Nafziger, E.; Randall, G.; Bundy, L.; Rehm, G.; Joern, B. Concepts and Rationale for Regional Nitrogen Rate Guidelines for Corn; Iowa State University-University Extension: Ames, IA, USA, 2006; pp. 6–24. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Parkin, T.B. Enhanced Efficiency Fertilizers: Effect on Agronomic Performance of Corn in Iowa. Agron. J. 2014, 106, 771–780. [Google Scholar] [CrossRef]
- Majidian, M.; Ghalavand, A.; Karimian, N.; Haghighi, A.K. Effects of water stress, nitrogen fertilizer and organic fertilizer in various farming systems in different growth stages on physiological characteristics, physical characteristics, quality and chlorophyll content of maize single cross hybrid 704. Iran. Crop Sci. J. 2006, 10, 303–330. [Google Scholar] [CrossRef]
- Adhikary, B.H.; Baral, B.R.; Shrestha, J.; Adhikary, R. Genotypes and fertilization influence on grain yield of winter maize. Int. J. Agri. Sci. 2015, 5, 844–848. [Google Scholar]
- Saruhan, V.; Kusvuran, A.; Babat, S. The effect of different humic acid fertilization on yield and yield components performances of common millet (Panicum miliaceum L.). Sci. Res. Essay 2011, 6, 663–669. [Google Scholar]
- Garcia-Mina, J.M.; Antolín, M.C.; Sánchez-Díaz, M. Metal-humic complexes and plant micronutrient uptake: A study based on different plant species cultivated in diverse soil types. Plant Soil 2004, 258, 57–68. [Google Scholar] [CrossRef]
- Nardi, S.; Ertani, A.; Ornella, F. Soil-root cross-talking: The role of humic substances. J. Plant. Nutr. Soil Sci. 2017, 180, 5–13. [Google Scholar] [CrossRef]
- Olivares, F.L.; Busato, J.G.; De Paula, A.M.; Lima, L.D.S.; Aguiar, N.O.; Canellas, L.P. Plant growth promoting bacteria and humic substances: Crop promotion and mechanisms of action. Chem. Biol. Technol. Agric. 2017, 4, 30. [Google Scholar] [CrossRef]
- Salehi-Lisar, S.Y.; Bakhshayeshan-Agdam, H. Agronomic Crop Responses and Tolerance to Drought Stress. In Agronomic Crops; Hasanuzzaman, M., Ed.; Springer: Singapore, 2020; pp. 63–91. [Google Scholar]
- Kinoshita, T.; Seki, M. Epigenetic Memory for Stress Response and Adaptation in Plants. Plant Cell Physiol. 2014, 55, 1859–1863. [Google Scholar] [CrossRef]
- Canellas, L.P.; Canellas, N.O.A.; Irineu, L.E.S.D.S.; Olivares, F.L.; Piccolo, A. Plant chemical priming by humic acids. Chem. Biol. Technol. Agric. 2020, 7, 1–17. [Google Scholar] [CrossRef]
- Caradonia, F.; Caradonia, V.; Battaglia, L.; Righi, G.; Pascali, A. La Torre Plant biostimulant regulatory framework: Prospects in Europe and current situation at international level. J. Plant Growth Regul. 2019, 38, 438–448. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Del Buono, D.; Regni, L.; Del Pino, A.M.; Bartucca, M.L.; Palmerini, C.A.; Proietti, P. Effects of megafol on the olive cultivar ‘Arbequina’ grown under severe saline stress in terms of physiological traits, oxidative stress, antioxidant defenses, and cyto-solic Ca2+. Front. Plant Sci. 2021, 11, 2188. [Google Scholar] [CrossRef]
- Goñi, O.; Fort, A.; Quille, P.; McKeown, P.C.; Spillane, C.; O’Connell, S. Comparative Transcriptome Analysis of Two Ascophyllum nodosum Extract Biostimulants: Same Seaweed but Different. J. Agric. Food Chem. 2016, 64, 2980–2989. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.D.; Hien, H.M.; Son, P.N. Seaweeds from Vietnam used for functional food, medicine and biofertilizer. J. Appl. Phycol. 2007, 19, 817–826. [Google Scholar] [CrossRef]
- Ugarte, R.A.; Sharp, G.; Moore, B. In Changes in the brown seaweed Ascophyllum nodosum (L.) Le Jol. plant morphology and biomass produced by cutter rake harvests in southern New Brunswick, Canada. In Eighteenth International Seaweed Symposium Developments in Applied Phycology; Anderson , R., Brodie, J., Onsøyen, E., Critchley, A.T., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 125–133. [Google Scholar]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed Extracts as Biostimulants of Plant Growth and Development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Editorial: Biostimulants in agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Panfili, I.; Bartucca, M.L.; Marrollo, G.; Povero, G.; Del Buono, D. Application of a Plant Biostimulant To Improve Maize (Zea mays) Tolerance to Metolachlor. J. Agric. Food Chem. 2019, 67, 12164–12171. [Google Scholar] [CrossRef]
- Puglia, D.; Pezzolla, D.; Gigliotti, G.; Torre, L.; Bartucca, M.; Del Buono, D. The Opportunity of Valorizing Agricultural Waste, Through Its Conversion into Biostimulants, Biofertilizers, and Biopolymers. Sustainability 2021, 13, 2710. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, update 2015 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Report No. 106; FAO: Rome, Italy, 2015; Available online: https://www.fao.org/3/i3794en/I3794EN.pdf (accessed on 12 September 2021).
- Velička, R.; Raudonius, S.; Marcinkevičienė, A.; Trečiokas, K. In Planning and conducting field tests. J. Agron. Crop Sci. 2004, 110, 64. [Google Scholar]
- Raudonius, S. Application of statistics in plant and crop research: Important issues Statistikos taikymas augalų ir pasėlių tyrimuose: Svarbiausi aspektai. Zemdirb Agric. 2017, 104, 377–382. [Google Scholar] [CrossRef]
- Pikul, J.L.; Hammack, L.; Riedell, W.E. Corn Yield, Nitrogen Use, and Corn Rootworm Infestation of Rotations in the Northern Corn Belt. Agron. J. 2005, 97, 854–863. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, P.; Tong, Y.; Norse, D.; Lu, Y.; Powlson, D. Overcoming nitrogen fertilizer over-use through technical and advisory approaches: A case study from Shaanxi Province, northwest China. Agric. Ecosyst. Environ. 2015, 209, 89–99. [Google Scholar] [CrossRef]
- Sanz-Cobena, A.; Sanchez-Martin, L.; Garcia, A.V.; Vallejo, A. Gaseous emissions of N2O and NO and NO3− leaching from urea applied with urease and nitrification inhibitors to a maize (Zea mays) crop. Agric. Ecosyst. Environ. 2012, 149, 64–73. [Google Scholar] [CrossRef]
- He, T.; Liu, D.; Yuan, J.; Luo, J.; Lindsey, S.; Bolan, N.; Ding, W. Effects of application of inhibitors and biochar to fertilizer on gaseous nitrogen emissions from an intensively managed wheat field. Sci. Total Environ. 2018, 628–629, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef]
- Erisman, J.; Bleeker, A.; Galloway, J.; Sutton, M. Reduced nitrogen in ecology and the environment. Environ. Pollut. 2007, 150, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Bogucka, B.; Szempliński, W.; Wróbel, E. Effect of nitrogen fertilization on the yield of grain maize grown under climate conditions of north-eastern Poland. Acta Sci. Pol. Agric. 2008, 7, 21–30. Available online: http://old-agricultura.acta.utp.edu.pl/uploads/pliki/000010200800007000030002100030.pdf (accessed on 10 October 2021).
- Gołebiewska, M.; Wróbel, E. The Effect of Nitrogen Fertilization on Yielding of Maize. Instytut Hodowli I Aklimatyzacji Ro´slin: Radzików, Poland. Biul. IHAR 2009, 251, 121–136. [Google Scholar]
- Pizolato Neto, A.; Camargos, A.E.; Valeriano, T.B.; Sgobi, M.A.; Santana, M.J. Doses de nitrogênio para cultivares de milho irrigado. Nucleus 2016, 13, 87–96. [Google Scholar] [CrossRef]
- Silva, P.C.; Costa, R.A.; Martins, Y.A.; Moura Alves, P.; Barbosa, K.F. Use of Different Doses of Polymerized Urea in Corn crop. Academic Day of UEG Campus Santa Helena de Goiás. 2016. Available online: https://www.anais.ueg.br/index.php/jaueg/article/view/6321/3974 (accessed on 21 February 2022).
- Wang, H.; Köbke, S.; Dittert, K. Use of urease and nitrification inhibitors to reduce gaseous nitrogen emissions from fertilizers containing ammonium nitrate and urea. Glob. Ecol. Conserv. 2020, 22, e00933. [Google Scholar] [CrossRef]
- Drury, C.F.; Yang, X.; Reynolds, W.D.; Calder, W.; Oloya, T.O.; Woodley, A. Combining Urease and Nitrification Inhibitors with Incorporation Reduces Ammonia and Nitrous Oxide Emissions and Increases Corn Yields. J. Environ. Qual. 2017, 46, 939–949. [Google Scholar] [CrossRef]
- Zaman, M.; Nguyen, M.L.; Blennerhassett, J.D.; Quin, B.F. Reducing NH3, N2O and NO3-N losses from a pasture soil with urease or nitrification inhibitors and elemental S-amended nitrogenous fertilizers. Biol. Fertil. Soils. 2008, 44, 693–705. [Google Scholar] [CrossRef]
- Majumdar, D.; Pathak, H.; Kumar, S.; Jain, M. Nitrous oxide emission from a sandy loam Inceptisol under irrigated wheat in India as influenced by different nitrification inhibitors. Agric. Ecosyst. Environ. 2002, 91, 283–293. [Google Scholar] [CrossRef]
- Naveed, M.; Khalid, M.; Jones, D.L.; Ahmad, R.; Zahir, Z.A. Relative efficacy of Pseudomonas spp., containing ACC-deaminase for improving growth and yield of maize (Zea mays L.) in the presence of organic fertilizer. Pak. J. Bot. 2008, 40, 1243–1251. [Google Scholar]
- Nevens, F.; Reheul, D. The application of vegetable, fruit and garden waste (VFG) compost in addition to cattle slurry in a silage maize monoculture: Nitrogen availability and use. Eur. J. Agron. 2003, 19, 189–203. [Google Scholar] [CrossRef]
- Latique, S.; Chernane, H.; Mansori, M.; Kaoua, E. Seaweed liquid fertilizer effect on physiological and biochemical parameters of bean plant (Phaesolus vulgaris var Paulista) under hydroponic system. Eur. Sci. J. 2013, 9, 174–193. [Google Scholar] [CrossRef]
- Alam, M.Z.; Braun, G.; Norrie, J.; Hodges, D.M. Ascophyllum extract application can promote plant growth and root yield in carrot associated with increased root-zone soil microbial activity. Can. J. Plant Sci. 2014, 94, 337–348. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Huang, Z.; Su, G.; Li, X.; Sun, Z.; Qin, Y. Impact of short-term application of seaweed fertilizer on bacterial diversity and community structure, soil nitrogen contents, and plant growth in maize rhizosphere soil. Folia Microbiol. 2020, 65, 591–603. [Google Scholar] [CrossRef]
- Khaliq, T.; Mahmood, T.; Kamal, J.; Masood, A. Effectiveness of farmyard manure, poultry manure and nitrogen for corn (Zea mays L.) productivity. Int. J. Agri. Biol. 2004, 6, 260–263. [Google Scholar]
- Sharar, M.; Ayub, M.; Nadeem, M.; Ahmad, N. Effect of Different Rates of Nitrogen and Phosphorus on Growth and Grain Yield of Maize (Zea mays L.). Asian J. Plant Sci. 2003, 2, 347–349. [Google Scholar] [CrossRef][Green Version]
- Michalski, T.; Bartos-Sychała, M.; Maciejewski, T.; Jarosz, A. Effect of biostymulator Asahi SL on cropping of maize grown for grain. In Monographs Series: Biostimulators in Modern Agriculture. Field Crops; Dąbrowski, Z.T., Ed.; The Editorial House Wieś Jutra: Warsaw, Poland, 2008; pp. 66–76. [Google Scholar]
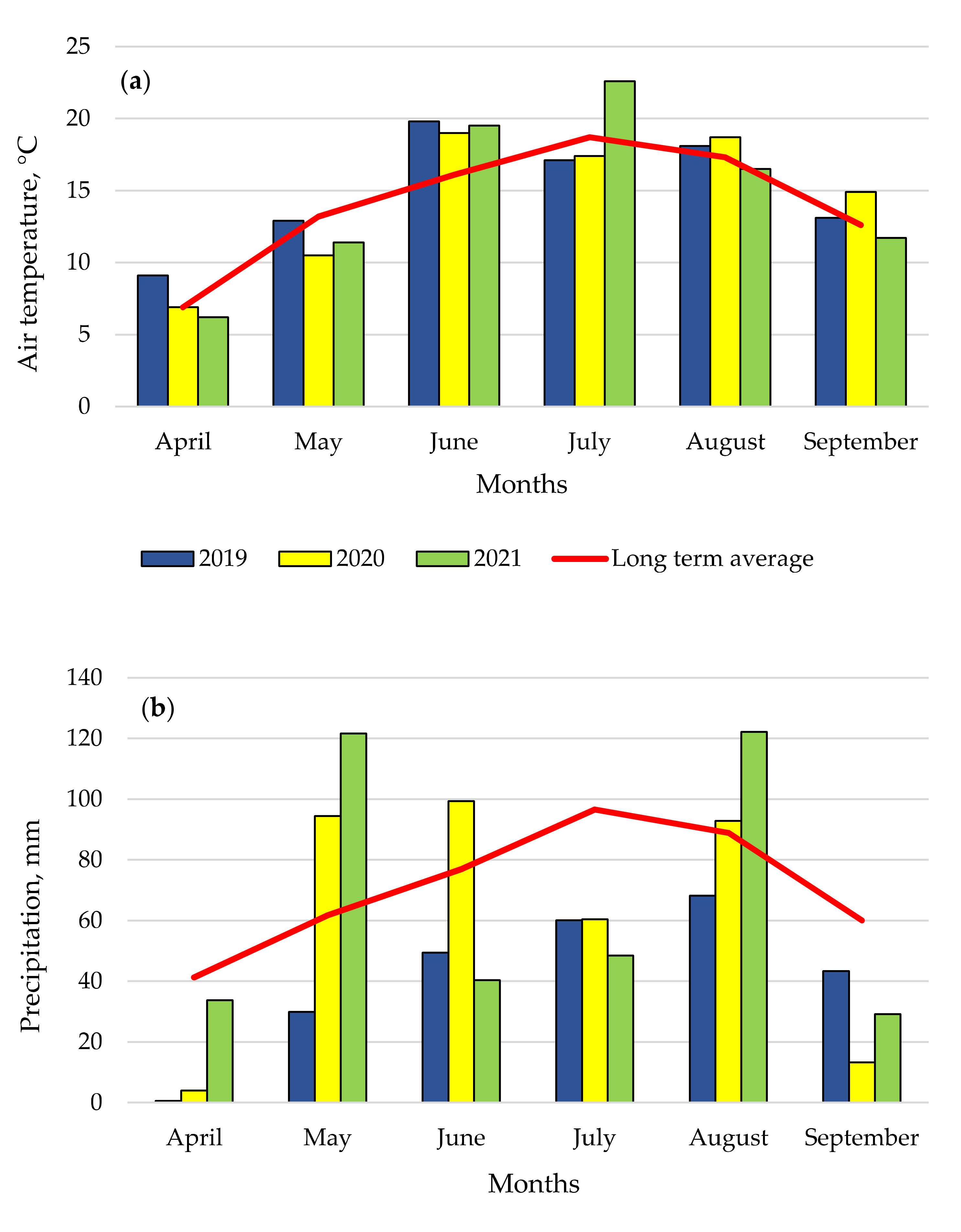
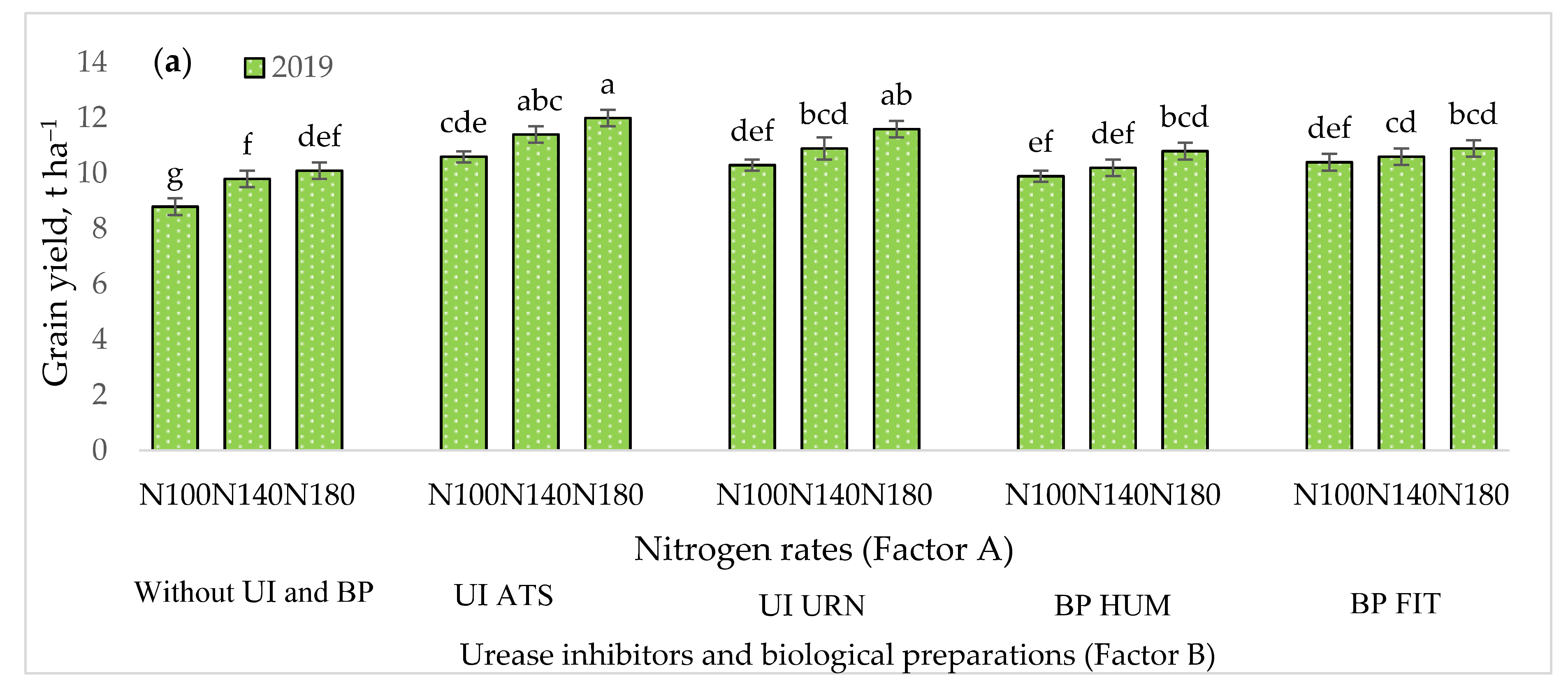
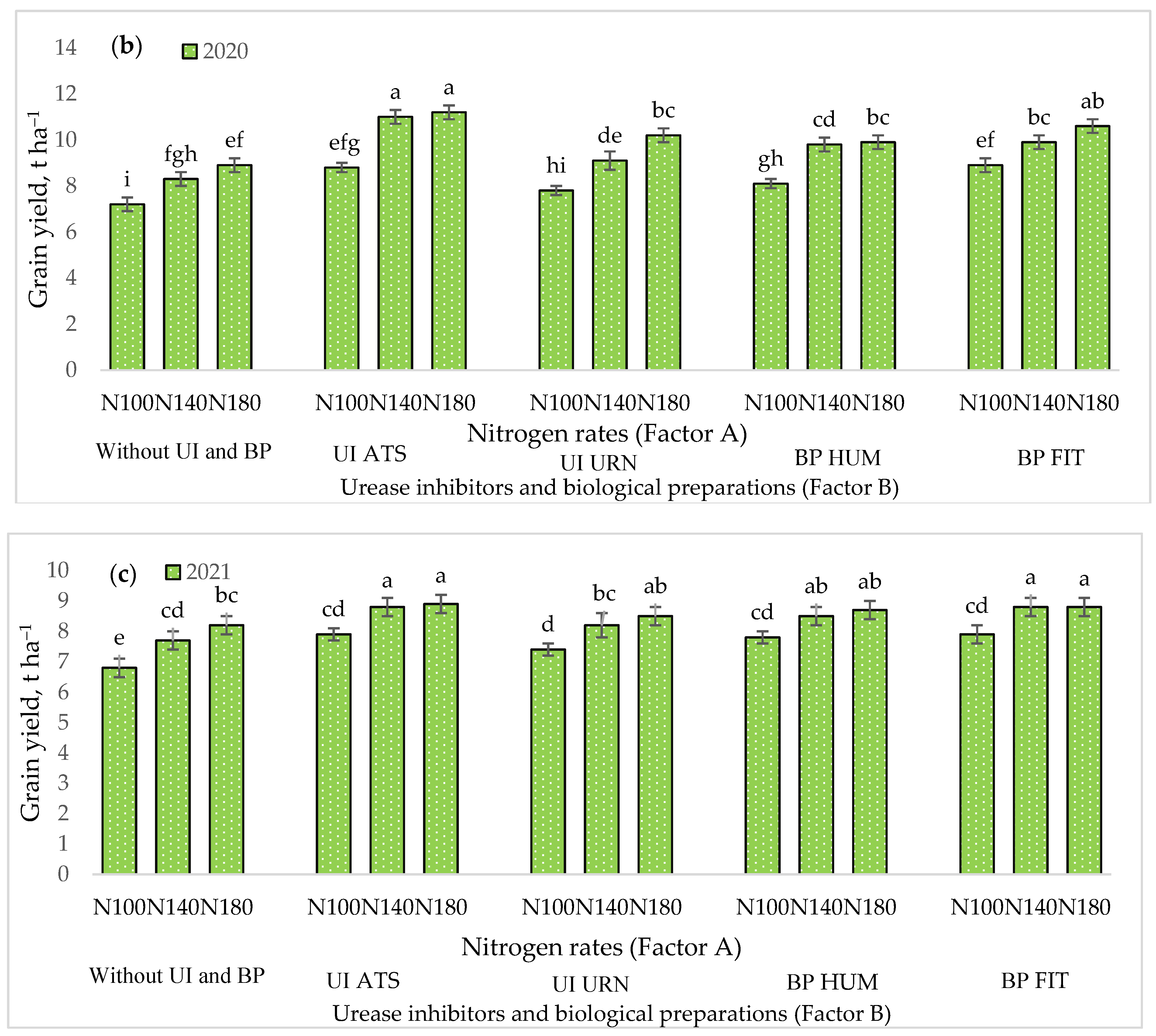
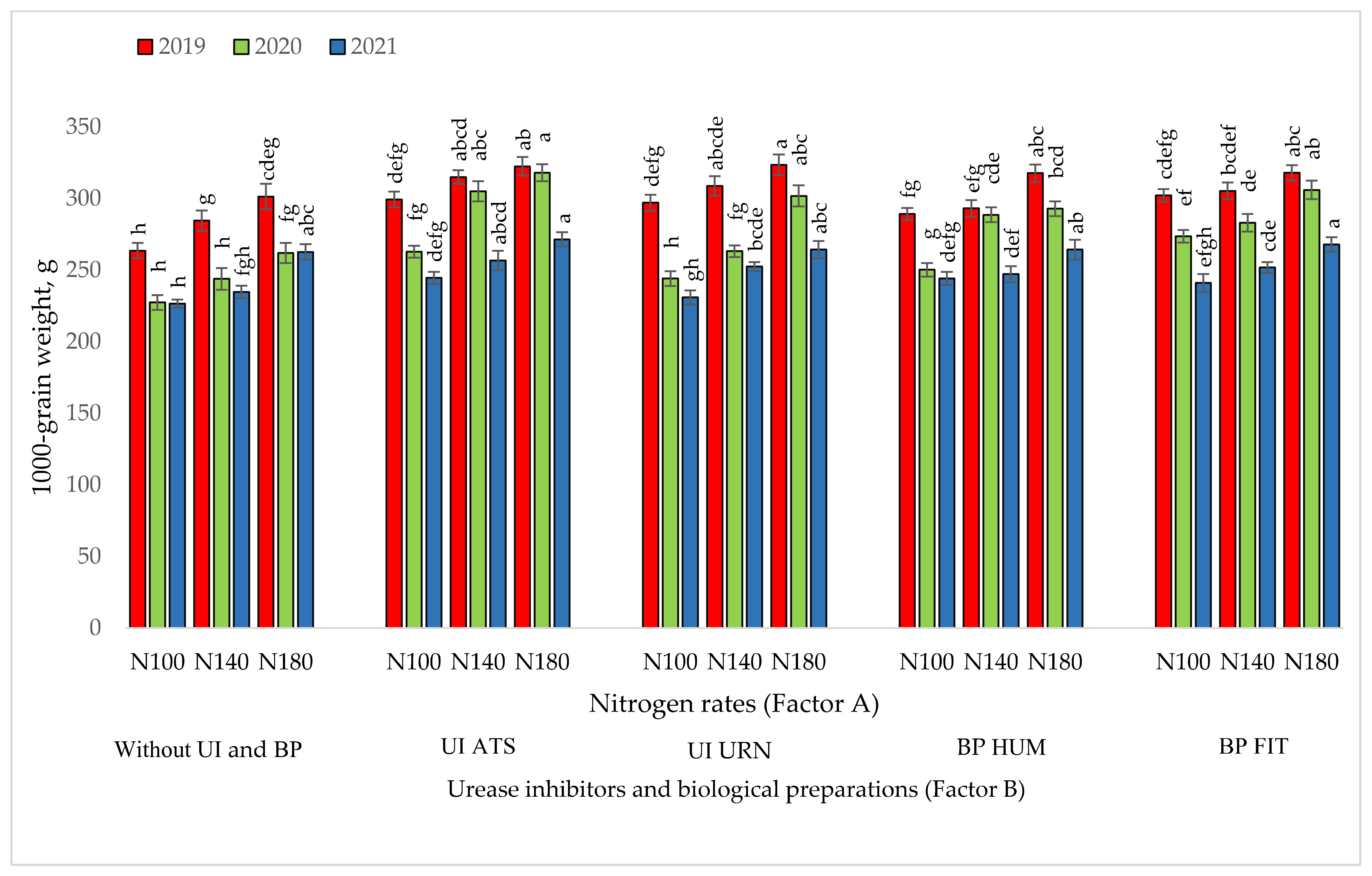
| Dependent Variables y | UI ir BP (Factor B) | Year | Regression Equation | r | r2 | p |
|---|---|---|---|---|---|---|
| y1 Grain yield, t ha−1 | Without UI and BP | 2019 | y= 7.13 + 0.02x | 0.80 | 0.65 | p < 0.01 |
| 2020 | y = 5.12 + 0.02x | 0.89 | 0.79 | p < 0.01 | ||
| 2021 | y = 5.12 + 0.02x | 0.90 | 0.82 | p < 0.01 | ||
| UI ATS | 2019 | y = 8.91 + 0.02x | 0.84 | 0.71 | p < 0.01 | |
| 2020 | y = 6.18 + 0.03x | 0.84 | 0.70 | p < 0.01 | ||
| 2021 | y = 6.83 + 0.01x | 0.76 | 0.57 | p < 0,01 | ||
| UI URN | 2019 | y = 8.67 + 0.02x | 0.77 | 0.59 | p < 0.05 | |
| 2020 | y = 4.69 + 0.03x | 0.94 | 0.89 | p < 0.01 | ||
| 2021 | y = 6.06 + 0.01x | 0.84 | 0.71 | p < 0.01 | ||
| BP HUM | 2019 | y = 8.78 + 0.01x | 0.69 | 0.48 | p < 0.05 | |
| 2020 | y = 6.23 + 0.02x | 0.83 | 0.69 | p < 0.01 | ||
| 2021 | y = 6.63 + 0.01x | 0.84 | 0.71 | p < 0.01 | ||
| BP FIT | 2019 | - | - | - | p > 0.05 | |
| 2020 | y = 6.90 + 0.02x | 0.86 | 0.74 | p < 0.01 | ||
| 2021 | y = 6.84 + 0.01x | 0.77 | 0.59 | p < 0.05 | ||
| y2 1000-grains weight, g | Without UI and BP | 2019 | y = 217.2 + 0.47x | 0.83 | 0.69 | p < 0.01 |
| 2020 | y = 184.1 + 0.43x | 0.83 | 0.69 | p < 0.01 | ||
| 2021 | y = 178.1 + 0.45x | 0.89 | 0.79 | p < 0.01 | ||
| UI ATS | 2019 | y = 271.7 + 0.29x | 0.76 | 0.57 | p < 0.05 | |
| 2020 | y = 198.5 + 0.69x | 0.90 | 0.82 | p < 0.01 | ||
| 2021 | y = 210.5 + 0.34x | 0.82 | 0.68 | p < 0.01 | ||
| UI URN | 2019 | y = 263.5 + 0.33x | 0.76 | 0.57 | p < 0.05 | |
| 2020 | y = 168.7 + 0.72x | 0.93 | 0.87 | p < 0.01 | ||
| 2021 | y = 190.8 + 0.42x | 0.88 | 0.78 | p < 0.01 | ||
| BP HUM | 2019 | y = 250.1 + 0.36x | 0.79 | 0.62 | p < 0.05 | |
| 2020 | y = 202.5 + 0.53x | 0.85 | 0.73 | p < 0.01 | ||
| 2021 | y = 213.3 + 0.28x | 0.72 | 0.51 | p < 0.05 | ||
| BP FIT | 2019 | - | - | - | p > 0.05 | |
| 2020 | y = 230.7 + 0.413x | 0.70 | 0.84 | p < 0.01 | ||
| 2021 | y = 206.5 + 0.33x | 0.83 | 0.70 | p < 0.01 | ||
| y3 Quantity of grain in the cob, units | Without UI and BP UI ATS; UI URN; BP HUM; BP FIT | 2019 2020 2021 | - | - | - | p > 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drulis, P.; Kriaučiūnienė, Z.; Liakas, V. The Influence of Different Nitrogen Fertilizer Rates, Urease Inhibitors and Biological Preparations on Maize Grain Yield and Yield Structure Elements. Agronomy 2022, 12, 741. https://doi.org/10.3390/agronomy12030741
Drulis P, Kriaučiūnienė Z, Liakas V. The Influence of Different Nitrogen Fertilizer Rates, Urease Inhibitors and Biological Preparations on Maize Grain Yield and Yield Structure Elements. Agronomy. 2022; 12(3):741. https://doi.org/10.3390/agronomy12030741
Chicago/Turabian StyleDrulis, Povilas, Zita Kriaučiūnienė, and Vytautas Liakas. 2022. "The Influence of Different Nitrogen Fertilizer Rates, Urease Inhibitors and Biological Preparations on Maize Grain Yield and Yield Structure Elements" Agronomy 12, no. 3: 741. https://doi.org/10.3390/agronomy12030741
APA StyleDrulis, P., Kriaučiūnienė, Z., & Liakas, V. (2022). The Influence of Different Nitrogen Fertilizer Rates, Urease Inhibitors and Biological Preparations on Maize Grain Yield and Yield Structure Elements. Agronomy, 12(3), 741. https://doi.org/10.3390/agronomy12030741






