Abstract
Understanding the existing nematode biodiversity is of significant concern because nematodes may divert nutrients from plants and use them for their own development and reproduction. The presence and diagnostics of Filenchus species occurring in southern Alberta have not been addressed in previous studies. Herein, we provide a comprehensive characterization of adult females of four known Filenchus species (F. cylindricus, F. hazenensis, F. sheri, and F. thornei) recovered from cultivated fields in southern Alberta. Three of the species are new records in Canada, while one is a native species that was previously described from the Canadian high arctic area. These organisms are mild parasitic species; we describe them here to enhance the visibility of soil nematodes and facilitate accurate species identification. The diagnostic resolution within Filenchus is low, because many species are described without adequate consideration of intra-specific variation. The species descriptions and molecular data obtained during the present study will reduce the confusion in examining the existing lineages among Filenchus species and will aid in improving phylogenetic resolution. Our results suggest that the known diversity of Canadian nemato-fauna has increased. However, more research is needed to further identify other genera and species of phytoparasitic nematodes that may occur in grasses, weeds, and wild plants present in cultivated areas. Moreover, the molecular characterization of these species from Canada, in comparison to a reference dataset (NCBI) of Tylenchidae nematodes, provides insight into the biogeography of nematodes.
1. Introduction
The cosmopolitan genus Filenchus was first described by Andrássy in 1954 [1]. Since then, the characterization of the genus has developed, leading to several taxonomic revisions, such as the formulation of various genera (e.g., Ottolenchus Hussain and Khan [2]; Dactylotylenchus Wu [3]; Lambertia Brzeski [4]; Duosulcius Siddiqi [5]; Zanenchus Siddiqi [5]), and subsequent genus synonymization by different nematologists based on various morphological characteristics, as discussed by Raski and Geraert [6]. Currently, the taxonomic classifications provided by Siddiqi [7] and Geraert [8] are well-accepted and used in species delimitation. In both classification systems, Filenchus is a well-established genus, containing the highest number of species in the subfamily Tylenchinae. Despite their abundance, the feeding habits of Filenchus species are poorly understood. These nematodes are often regarded as plant epidermal cell/root hair feeders [9] or fungivores [10,11]. To date, Filenchus species are known to feed on various plant pathogenic (Rhizoctonia solani, Fusarium oxysporum, and Pythium ultimum), saprophytic, and mushroom-producing fungi [12,13,14].
Alberta is renowned for the quality and high marketable yields of its agricultural produce [15]. For sustainable production, continual and special pest monitoring programs are in place to examine the density and diversity of pest species in Albertan agricultural soils. In our recent nematode inventory survey, we recovered four Filenchus spp. from the cultivated areas of southern Alberta. The genus Filenchus is not common in Canada and only five species have been described from the country [3,8,16,17]. The scarcity of this genus led us to characterize these populations in detail and ascertain the species status for each recovered species. By preliminary microscopic examination, we found that these species have four lateral lines, delicate stylets, and long filiform tails. We also collected the molecular and morphometrical data from these species (integrative taxonomy) and compared them with related Filenchus species to find that the recovered nematodes belong to F. cylindricus (Thorne and Malek) Niblack and Bernard [18,19], F. hazenensis (Wu) Andrássy [16,20], F. sheri (Khan and Khan) Siddiqi [21,22], and F. thornei (Andrássy) Andrássy [1,23]. Among these species, F. hazenensis is a Canadian native species reported from high arctic areas, whereas we record the other recovered Filenchus species for the first time in Canada.
The discovery of these Filenchus species has expanded the geographic range of this genus. Therefore, we carried out our study to completely characterize the recovered Filenchus species and examine their phylogenetic relationships with other Tylenchidae species. The results we report herein form a valuable database of Filenchus species occurring in southern Alberta and will facilitate accurate species identification.
2. Materials and Methods
2.1. Nematode Isolation and Morphological Studies
To examine and better understand soil-inhabiting nematodes, we conducted a survey near the north of Taber and Bow Island areas of southern Alberta, Canada. Approximately 80 soil and root samples were collected and stored at 4 °C at the University of Lethbridge (Alberta, Canada) until processing. Nematodes were extracted from soil samples using the modified Cobb’s sieving and flotation-centrifugation method [24]. Individual Filenchus taxa were collected from the mixture of soil nematodes and mounted on slides for observation and preservation. For preliminary examination, fresh specimens of each species were transferred to a drop of distilled water, heat relaxed, and observed under a Zeiss Axioskope 40 microscope. For morphometrical studies, the nematodes were fixed, and permanent slides were prepared as described by Seinhorst [25] and De Grisse [26]. The permanent slides of each species are currently stored in the Department of Biological Sciences, University of Lethbridge. Images of each specimen were acquired using a Zeiss Axioskope 40 microscope equipped with a Zeiss Axiocam 208 camera (Carl Zeiss, Jena, Germany). Measurements from the images were performed using ZEN blue 3.1 imaging software (Carl Zeiss).
2.2. DNA Extraction, PCR, and Sequencing
After microscopic examination, each taxon was processed for DNA analysis. The single adult nematode of each species was transferred to a 0.2 mL PCR tube, and the DNA was extracted as described in Maria et al. [27]. Three sets of DNA primers (Integrated DNA Technologies, Coralville, IA, USA) were used to amplify the 18S, 28S, and ITS ribosomal RNA (rRNA) genes. The partial 18S rRNA gene sequence was amplified with the 1813F and 2646R primers [28]. The 28S rRNA gene was amplified using the D2A and D3B primers [29], and the ITS gene was amplified using the F194 [30] and AB28-R primers [31]. For the 18S, 28S, and ITS genes, the PCR conditions were as described earlier [28,29,30]. Amplified PCR products were resolved by electrophoresis in 1% agarose gels and visualized by staining with GelRed (Biotium, Fremont, CA, USA). PCR products containing amplified DNA fragments of interest were sent to Azenta Life Sciences for DNA sequencing (South Plainfield, NJ, USA).
2.3. Phylogenetic Analyses
The DNA sequences of the 18S rRNA, 28S rRNA and ITS1 rRNA genes were obtained for each Filenchus species. Newly obtained sequences and additional Tylenchidae taxa DNA sequences present in GenBank were used for phylogenetic analysis. The selection of outgroup taxa for each dataset was based on previously published studies [32,33,34,35]. Multiple nucleotide sequence alignments for the different genes were performed using the heuristics progressive method FFT-NS-2 algorithm of MAFFT v.7.450 [36]. The BioEdit v7.2.5 program [37] was used for sequence alignment visualization. For alignment editing, Gblocks v0.91b [38] was used on the Castresana Laboratory server (available online: http://molevol.cmima.csic.es/castresana/Gblocks_server.html (accessed on 30 December 2021)) with options for a less stringent selection (minimum number of sequences for a conserved or a flanking position: 50% of the number of sequences +1; maximum number of contiguous nonconserved positions: 8; minimum length of a block: 5; allowed gap positions: with half). Phylogenetic analyses were performed using Bayesian inference (BI) in MrBayes v3.1.2. The best-fit model of DNA evolution was achieved using JModelTest v2.1.7 [39] with the Akaike Information Criterion (AIC). Accordingly, the selected models were: (1) the general time-reversible model with invariable sites and a gamma-shaped distribution (GTR + I + G) for partial 18S, (2) the Tamura and Nei model with invariable sites and a gamma-shaped distribution (TrN + I + G) for the D2–D3 segments of the 28S rRNA, and (3) GTR + G for the ITS. The best-fit model, base frequency, proportion of invariable sites, gamma distribution shape parameters, and substitution rates in the AIC were then used in MrBayes for the phylogenetic analyses, which ran with four chains for 4 × 106 generations in all datasets. A combined analysis of the three ribosomal genes was not undertaken, due to several sequences not being available for all species. The sampling for Markov chains was carried out at intervals of 100 generations. For each analysis, two runs were conducted. After discarding 30% of the samples for burn-in and evaluating convergence, the remaining samples were retained for more in-depth analyses. The topologies were used to generate a 50% majority-rule consensus tree. On each appropriate clade, posterior probabilities (PP) were calculated. FigTree software v1.42 [40] was used for the visualization of the phylogenetic trees from all analyses.
3. Results
3.1. Description of Filenchus cylindricus
Female: Body cylindrical slightly ventrally arcuate when heat relaxed. Cuticle finely annulated with 4 lateral lines. Lip region conical, anteriorly flattened, 6.0–7.0 µm wide and 3.0–3.5 µm high, continuous with body contour. Stylet straight, strong, with rounded knobs. Median bulb oval with refractive valve plates, situated at ca 40–43% of the pharyngeal length. Isthmus slender, encircled with nerve ring gradually expanding into a small pyriform basal pharyngeal bulb. Excretory pore at the anterior end of the basal pharyngeal bulb. Reproductive system mono-prodelphic, composed of an outstretched ovary with oocytes mostly in a single row; vulva smooth, vagina straight; spermatheca elongated and irregular shaped, filled with sperm; post-vulval uterine sac (PUS) shorter than the vulval body diameter. Anus a minute pore. Tail elongated, filiform, ending in a finely attenuated tip (Figure 1, Table 1).
Male: Not found.
Juveniles: Present but not studied.
Remarks: This species was described by Thorne and Malek [19] from the prairie sod adjacent to a wheat field in South Dakota, USA. The same species was found in cultivated maize fields in Iowa [41] and Colorado [6], indicating that F. cylindricus is a common species in North America. Multiple populations of this species were reported from Sudan [42] and Romania [43] in the rhizosphere of Poinciana, lemon, guava, maize, potato, onion, garlic, and parsley.
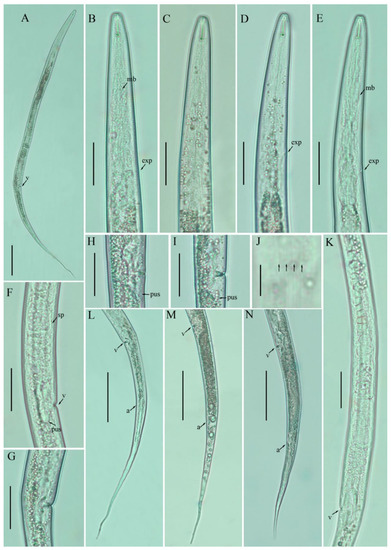
Figure 1.
Photomicrographs of female Filenchus cylindricus (Thorne and Malek) Niblack and Bernard [18,19]. (A) Entire body; (B–E) pharyngeal regions; (F–I) vulval regions; (J) lateral field lines; (K) gonad; (L–N) posterior body to tail terminus. Scale bars: (A) 50 µm; (C–I,K–N) 20 µm; (J) 5 µm. Arrowheads: (a) anus; (exp) excretory pore; (mb) median bulb; (PUS) post-uterine sac; (sp) spermatheca; (v) vulva.

Table 1.
Morphometrics of female Filenchus cylindricus (Thorne and Malek) Niblack and Bernard [18,19] examined in this study and from the original and subsequently published descriptions. All measurements are in µm and in the form: mean ± standard deviation and/or range.
Table 1.
Morphometrics of female Filenchus cylindricus (Thorne and Malek) Niblack and Bernard [18,19] examined in this study and from the original and subsequently published descriptions. All measurements are in µm and in the form: mean ± standard deviation and/or range.
| Characteristics | This Study | Thorne and Malek [19] | Elmiligy [41] | Raski and Geraert [6] | Zeidan and Geraert [42] | Dobrin and Geraert [43] |
|---|---|---|---|---|---|---|
| Locality | Alberta, Canada | South Dakota, USA | Iowa, USA | Colorado, USA | Sudan | Romania |
| n | 13 | 1 | 10 | 8 | 8 *1 | 6 *1 |
| Body length | 642.4 ± 42.4 (557.0–711.0) | 1000 | 896 (750–990) | 1060 (970–1150) | 700–870 | 600–710 |
| a | 36.6 ± 2.5 (34.0–43.0) | 40 | 39 (30–45) | 39 (34–43) | 33–41 | 28.1–39.4 |
| b | 6.5 ± 0.4 (6.0–7.2) | 6.6 | 6.4 (6.0–7.8) | 6.7 (6.1–7.6) | 5.7–6.9 | 4.6–6.5 |
| c | 4.8 ± 0.4 (4.4–5.5) | 6.5 | 5.2 (4.7–5.7) | 5.0 (4.7–5.2) | 4.6–5.6 | 4.0–4.7 |
| c′ | 13.0 ± 1.4 (11.1–15.2) | – | 11.2 (10.2–17.0) | 11.6 (9.8–13.2) | 9.3–11.9 | 10–15.6 |
| MB | 41.2 ± 1.3 (40.0–43.6) | – | 42 | 43.8 (42–46) | 42–44 | 44–54 |
| V | 62.0 ± 1.2 (59.2–64.4) | 64 | 60 (55–64) | 60 (57–62) | 59–65 | 53–61.6 |
| Lip height | 3.1 ± 0.1 (3.0–3.5) | – | 1.7–2.0 | 3.5 | – | – |
| Lip width | 6.2 ± 0.3 (6.0–7.0) | – | – | 7.5–8.0 | – | – |
| Stylet length | 12.1 ± 0.8 (10.0–13.0) | 13 | 13 (12–14) | 12.4 (12–13) | 12.0–13.0 | 12–14.5 |
| Anterior end to excretory pore | 81.0 ± 3.1 (75.0–86.0) | – | – | – | – | – |
| Pharynx length | 98.2 ± 3.8 (91.0–103.0) | – | – | – | – | – |
| Maximum body width | 17.6 ± 1.8 (15.0–21.0) | – | – | – | – | – |
| Vulva body width | 16.6 ± 1.0 (15.0–18.0) | – | – | – | – | – |
| Post-uterine sac (PUS) length | 11.0 ± 1.6 (9.0–14.0) | – | – | – | – | – |
| Distance from vulva to anus | 110.2 ± 13.1 (98.0–139.0) | – | – | – | – | – |
| Distance from vulva to tail terminus | 245.3 ± 18.7 (210.0–279.0) | – | – | – | – | – |
| Anal body width | 10.5 ± 1.1 (9.0–13.0) | – | – | – | – | – |
| Tail length | 135.1 ± 16.5 (112.0–159.0) | – | 172 (154–194) | 213 (189–242) | 140–178 | – |
*1 composite value of two populations. Abbreviations: n, number of specimens on which the measurements are based; a, body length/greatest body diameter; b, body length/distance from anterior end to pharyngo-intestinal junction; c, body length/tail length; c′, tail length/tail diameter at anus; MB, distance between the anterior end of the body and the center of the median pharyngeal bulb expressed as a percentage (%) of the pharynx length; V, distance from the body anterior end to the vulva expressed as a percentage (%) of the body length.
In the present study, F. cylindricus was recovered from the rhizosphere of grass growing on the headland of a hard red spring wheat field in southern Alberta, Canada (Table 1). Regarding habitat, the original and Canadian populations of F. cylindricus are similar; both populations were found in grasses growing close to cultivated wheat. The presence of males was not reported in the original description; however, the spermatheca was noted to contain sperm [19]. In the Canadian population, we observed a similar pattern: no males were detected in any examination, but the spermatheca was filled with sperm. Few males were described in the Iowa, USA, and Sudan populations. The morphological characteristics of the Canadian population of F. cylindricus are consistent with the original and subsequent descriptions. Morphometrically, the Canadian population measurement details are within the limits of F. cylindricus, except for the smaller body and tail length. Such small differences may be attributed to intraspecific geographical variability. This is the first integrative identification of this species, and consequently, this population of F. cylindricus is proposed here as a standard and reference population for this species until topotype material becomes available and molecularly characterized.
3.2. Description of Filenchus hazenensis
Female: Body cylindrical slightly ventrally arcuate when heat relaxed. Cuticle finely annulated with 4 lateral lines. Lip region conical to trapezoid, anteriorly flattened, 6.0–7.0 µm wide and 4.0–5.0 µm high, continuous with body contour. Stylet straight, with elongated–rounded knobs. Median bulb oval with refractive valve plates, situated at ca 39–45% of pharyngeal length. Isthmus slender, encircled with nerve ring gradually expanding into a small pyriform basal pharyngeal bulb. Excretory pore at the anterior end of the basal pharyngeal bulb. Reproductive system mono-prodelphic, composed of an outstretched ovary with oocytes mostly in a single row; vulva smooth, vagina straight; spermatheca degenerated, irregular shaped, without sperm; post-vulval uterine sac (PUS) shorter than the vulval body diameter. Anus a minute pore. Tail elongated, filiform, ending in a needle-like terminus (Figure 2, Table 2).
Male: Not found.
Juveniles: Present but not studied.
Remarks: Filenchus hazenensis was initially described from the Lake Hazen area, Nunavut, Canada in the rhizosphere of dry grass [16]. The same species was described from Poland by Brzeski [44]; however, no host association was provided with the Polish population. In the present study, F. hazenensis was recovered from a post-harvest wheat field in southern Alberta, Canada (Table 2). The presence of males was not detected in the original description, whereas the spermatheca was described as “present”. No further description was provided for the Nunavut population [16]. In the Alberta population, we observed a similar arrangement: no males were detected in any examination, and the spermatheca appears irregularly shaped without sperm. Conversely, Brzeski [44] noted the presence of a single male in the Polish population and reported that the spermatheca is offset and filled with sperm. Based on the available data, it is evident that males are present in F. hazenensis, but not commonly occurring in each population. Morphological characteristics of the Alberta population are consistent with the Nunavut and Polish populations of F. hazenensis. Morphometrically, the Canadian population measurement details are within the limits of F. hazenensis, except for a smaller body length. This is the first integrative identification of this species, and consequently, this population of F. hazenensis is proposed here as a standard and reference population for this species until topotype material becomes available and molecularly characterized.
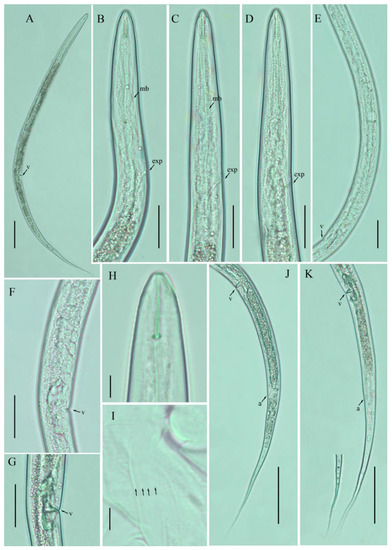
Figure 2.
Photomicrographs of female Filenchus hazenensis (Wu) Andrássy [16,20]. (A) Entire body; (B–D) pharyngeal regions; (E) gonad; (F,G) vulval region; (H) lip region; (I) lateral lines; (J,K) posterior body to tail terminus. Scale bars: (A) 50 µm; (B–G,J–K) 20 µm; (H,I) 5 µm. Arrowheads: (a) anus; (exp) excretory pore; (mb) median bulb; (PUS) post-uterine sac; (v) vulva.

Table 2.
Morphometrics of female Filenchus hazenensis (Wu) Andrássy [16,20] examined in this study and from the original and subsequently published descriptions. All measurements are in µm and in the form: mean ± standard deviation and/or range.
Table 2.
Morphometrics of female Filenchus hazenensis (Wu) Andrássy [16,20] examined in this study and from the original and subsequently published descriptions. All measurements are in µm and in the form: mean ± standard deviation and/or range.
| Characteristics | This Study | Wu [16] | Brzeski [44] |
|---|---|---|---|
| Locality | Alberta, Canada | Nunavut, Canada | Poland |
| n | 17 | 4 | 5 |
| Body length | 655.6 ± 34.4 (547.0–701.0) | 1020–1090 | 877–1003 |
| a | 34.2 ± 3.2 (29.4–39.3) | 38–39 | 36.2 (33–39) |
| b | 6.0 ± 0.3 (5.0–6.3) | 6.6–6.8 | 6.5 (6.1–7.4) |
| c | 4.5 ± 0.2 (4.0–5.0) | 4.4–4.7 | 5.0 (4.6–6.3) |
| c′ | 13.0 ± 1.5 (10.2–15.0) | – | 11.5 (8.4–13.3) |
| MB | 42.0 ± 1.5 (39.5–45.0) | – | 43.6 (42–45) |
| V | 61.4 ± 1.4 (56.7–62.4) | 60–62 | 61.1 (57–60) |
| Lip height | 4.4 ± 0.2 (4.0–5.0) | – | – |
| Lip width | 6.5 ± 0.3 (6.0–7.0) | – | 8–9 |
| Stylet length | 14.0 ± 0.6 (13.0–15.0) | 14.6–15.5 | 14.5 (14–15) |
| Anterior end to excretory pore | 89.0 ± 4.2 (81.0–95.0) | 119–125 | – |
| Pharynx length | 109.4 ± 3.1 (104.0–114.0) | 149–165 | 143 (134–152) |
| Maximum body width | 19.3 ± 1.6 (16.0–22.0) | – | – |
| Vulva body width | 17.1 ± 1.3 (15.0–20.0) | – | – |
| Post-uterine sac (PUS) length | 12.6 ± 1.5 (9.0–15.0) | 15–17 | |
| Distance from vulva to anus | 108.6 ± 6.0 (100.0–119.0) | – | – |
| Distance from vulva to tail terminus | 253.0 ± 9.9 (237.0–275.0) | – | – |
| Anal body width | 11.2 ± 1.1 (10.0–14.0) | – | – |
| Tail length | 144.2 ± 6.4 (133.0–155.0) | 224–232 | 189 (138–219) |
Abbreviations: n, number of specimens on which the measurements are based; a, body length/greatest body diameter; b, body length/distance from anterior end to pharyngo-intestinal junction; c, body length/tail length; c’, tail length/tail diameter at anus; MB, distance between the anterior end of the body and center of the median pharyngeal bulb expressed as a percentage (%) of the pharynx length; V, distance from the body anterior end to the vulva expressed as a percentage (%) of the body length.
3.3. Description of Filenchus sheri
Female: Body cylindrical, slightly ventrally arcuate, when heat relaxed. Cuticle finely annulated with 4 lateral lines; outer lines are clearer than the inner ones. In some specimens, inner lines fused and appear as 3 lined lateral field. Lip region conical, anteriorly flattened, 5.0–6.5 µm wide and 2.0–3.0 µm high, continuous with body contour. Stylet straight, delicate, with rounded knobs. Median bulb oval with refractive valve plates, situated at ca. 34–47% of the pharyngeal length. Isthmus slender, encircled with nerve ring gradually expanding into a small pyriform basal pharyngeal bulb. Excretory pore at the middle of the basal pharyngeal bulb. Reproductive system mono-prodelphic, composed of an outstretched ovary with oocytes mostly in a single row; vulva smooth, vagina straight; spermatheca rounded, partially filled with sperm; post-vulval uterine sac (PUS) shorter than the vulval body diameter. Anus a minute pore. Tail elongated, filiform, ending in a finely pointed terminus (Figure 3, Table 3).
Male: Not found.
Juveniles: Present but not studied.
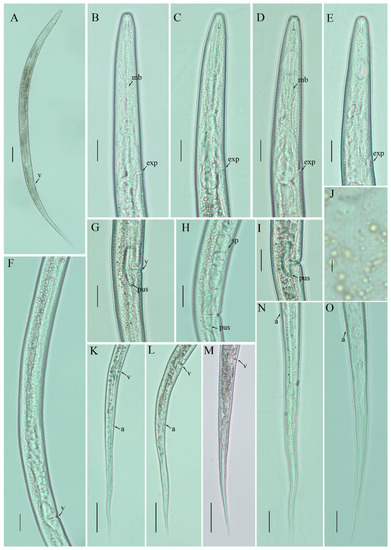
Figure 3.
Photomicrographs of female Filenchus sheri (Khan and Khan) Siddiqi [21,22]. (A) Entire body; (B–E) pharyngeal regions; (F) gonad; (G–I) vulval regions; (J) lateral lines; (K–O) posterior body to tail terminus. Scale bars: (A) 50 µm; (B–I,K–O) 20 µm; (J) 5 µm. Arrowheads: (a) anus; (exp) excretory pore; (mb) median bulb; (PUS) post-uterine sac; (v) vulva.

Table 3.
Morphometrics of female Filenchus sheri (Khan and Khan) Siddiqi [21,22] examined in this study and from the original and subsequently published descriptions. All measurements are in µm and in the form: mean ± standard deviation and/or range.
Table 3.
Morphometrics of female Filenchus sheri (Khan and Khan) Siddiqi [21,22] examined in this study and from the original and subsequently published descriptions. All measurements are in µm and in the form: mean ± standard deviation and/or range.
| Characteristics | This Study | Khan and Khan [21] | Karegar and Geraert [45] |
|---|---|---|---|
| Locality | Alberta, Canada | Afghanistan | Iran |
| n | 17 | 5 | 4 |
| Body length | 618.6 ± 28.0 (567.0–679.0) | 510 (460–550) | 515 (490–535) |
| a | 35.2 ± 3.0 (31.5–42.3) | 33 (31–38) | 35.3 (31.1–39.9) |
| b | 6.8 ± 0.3 (6.1–7.3) | 502 (5–6) | 5.6 (5.3–6.1) |
| c | 5.5 ± 0.3 (5.0–6.2) | 4.5 (4–5) | 3.8 (3.6–3.9) |
| c′ | 11.5 ± 1.1 (9.8–14.0) | – | 14.8 (13.6–15.8) |
| MB | 41.2 ± 3.1 (34.5–47.0) | 40–45 | 40.4 (38.9–43.2) |
| V | 69.0 ± 1.3 (66.0–71.0) | 60.2 (61–63) | 56.1 (56.1–60.7) |
| Lip height | 2.5 ± 0.2 (2.0–3.0) | – | – |
| Lip width | 5.8 ± 0.4 (5.0–6.5) | – | – |
| Stylet length | 7.7 ± 0.4 (7.0–8.5) | 7.6 (7–8) | – |
| Anterior end to excretory pore | 80.6 ± 1.4 (78.0–83.0) | – | 72.5 (66–77) |
| Pharynx length | 91.6 ± 3.4 (85.0–98.0) | – | – |
| Maximum body width | 17.7 ± 1.2 (15.0–19.0) | – | – |
| Vulva body width | 15.3 ± 0.7 (14.0–17.0 | – | – |
| Post-uterine sac (PUS) length | 9.5 ± 1.5 (7.5–13.0) | – | – |
| Distance from vulva to anus | 78.4 ± 6.4 (71.0–90.0) | – | – |
| Distance from vulva to tail terminus | 191.8 ± 9.0 (173.0–206.0) | – | – |
| Anal body width | 9.9 ± 0.5 (9.0–10.5) | – | – |
| Tail length | 113.4 ± 7.9 (98.0–126.0) | – | 136 (127–142) |
Abbreviations: n, number of specimens on which the measurements are based; a, body length/greatest body diameter; b, body length/distance from anterior end to pharyngo-intestinal junction; c, body length/tail length; c’, tail length/tail diameter at anus; MB, distance between the anterior end of the body and center of the median pharyngeal bulb expressed as a percentage (%) of the pharynx length; V, distance from the body anterior end to the vulva expressed as a percentage (%) of the body length.
Remarks: This species was described by Khan and Khan [21] in the rhizosphere of orange plants in Afghanistan. After the formal description, F. sheri was recorded from Iran [45] in the rhizosphere of the horsetail plant. In the present study, F. sheri was recovered from common mallow growing on the headland of a cultivated potato field in southern Alberta, Canada (Table 3). We observed that the morphological and morphometrical characteristics of the Canadian population of F. sheri are consistent with the original and the subsequent descriptions except for the presence of males. Males were reported in the original description; however, no males were detected in the present study. This is the first integrative identification of this species, and consequently, this population of F. sheri is proposed here as a standard and reference population for this species until topotype material becomes available and molecularly characterized.
3.4. Description of Filenchus thornei
Female: Body cylindrical, straight, when heat relaxed. Cuticle finely annulated with 4 lateral lines. Lip region narrow, conical, anteriorly truncated, 5.5–7.0 µm wide and 3.0–4.0 µm high, continuous with body contour. Stylet straight, with rounded knobs. Median bulb elongated–oval with refractive valve plates, situated at ca. 39–49% of the pharyngeal length. Isthmus slender, encircled with nerve ring gradually expanding into a small pyriform basal pharyngeal bulb. Excretory pore at the anterior end of the basal pharyngeal bulb. Reproductive system mono-prodelphic, composed of an outstretched ovary with oocytes mostly in a single row; vulva smooth, vagina straight; spermatheca axial with an offset sac, filled with sperm; post-vulval uterine sac (PUS) shorter than the vulval body diameter. Anus a minute pore. Tail elongated, filiform, ending in a fine terminus (Figure 4, Table 4).
Male: Not found.
Juveniles: Present but not studied.
Remarks: This species was described by Andrássy [1] from Hungary. The same author described F. thornei from Bulgaria [46]; then, the species was recorded from the USA [6], the Netherlands [47], and Spain [48]; however, the host association was not indicated [8]. In the present study, F. thornei was recovered from a post-harvest bean field in southern Alberta, Canada. The presence of males was detected in the Spanish population; however, no males were found in the Canadian population. Morphological and morphometrical characteristics of the Canadian population of F. thornei are consistent with the original and subsequent descriptions. Small differences in the measurements may be attributed to intraspecific geographical variability (Table 4). A 28S sequence of F. thornei has been reported from Wyoming, USA [49], without morphological details. The Canadian population of F. thornei is the first integrative identification of this species. Since molecular differences among both the Canadian and Wyoming, USA populations were detected, additional studies on the Wyoming population are needed to clarify whether F. thornei is composed of a species complex or cryptic species that could be confirmed when topotype material becomes available and molecularly characterized.
3.5. Molecular Characterization and Phylogenetic Relationships of Detected Filenchus Species with Related Filenchus Species
Using the partial 18S, D2–D3 of the 28S, and ITS rRNA sequences, we molecularly characterized the four Filenchus species recovered in this study. The newly obtained sequences were edited and submitted to NCBI under the following accession numbers: partial 18S ([OM230085] for F. cylindricus, [OM230086] for F. hazenensis, [OM230087–OM230088] for F. sheri, and [OM230089–OM230090] for F. thornei); D2–D3 of 28S ([OM230091–OM230092] for F. cylindricus, [OM230093–OM230094] for F. hazenensis, [OM230095–OM230097] for F. sheri, and [OM230098–OM230100] for F. thornei); and ITS ([OM230105–OM230106] for F. hazenensis and [OM230107] for F. thornei). Filenchus is a large genus, but not all the species were characterized with DNA sequence-based information. Therefore, the 18S, D2–D3 of 28S, and ITS trees were constructed with the available Filenchus species and the related Tylenchidae species sequences obtained through a BLASTN search.
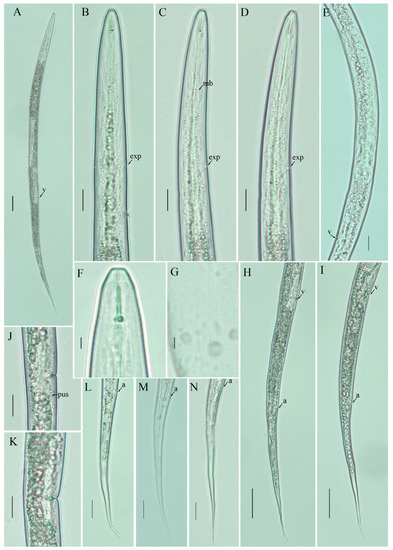
Figure 4.
Photomicrographs of female Filenchus thornei (Andrássy) Andrássy [1,23]. (A) Entire body; (B–D) pharyngeal regions; (E) gonad; (F) lip region; (G) lateral lines; (H,I) posterior body to tail terminus; (J,K) vulval regions; (L–N) tail region. Scale bars: (A) 50 µm; (B–E,H–N) 20 µm; (F,G) 5 µm. Arrowheads: (a) anus; (exp) excretory pore; (mb) median bulb; (PUS) post-uterine sac; (v) vulva.

Table 4.
Morphometrics of female Filenchus thornei (Andrássy) Andrássy [1,23] examined in this study and from the original and subsequently published descriptions. All measurements are in µm and in the form: mean ± standard deviation and/or range.
Table 4.
Morphometrics of female Filenchus thornei (Andrássy) Andrássy [1,23] examined in this study and from the original and subsequently published descriptions. All measurements are in µm and in the form: mean ± standard deviation and/or range.
| Characteristics | This Study | Andrássy [1] | Raski and Geraert [6] | Castillo et al. [48] |
|---|---|---|---|---|
| Locality | Alberta, Canada | Bulgaria | Colorado, USA | Spain |
| n | 16 | 1 | 15 | 24 |
| Body length | 633.2 ± 37.1 (582.0–701.0) | 739 | 780 (710–870) | 734 (616–840) |
| a | 30.4 ± 2.6 (26.0–35.5) | 36.7 | 40 (37–46) | 37.5 (32.2–42.9) |
| b | 6.1 ± 0.3 (5.6–6.7) | 7.8 | 6.8 (6.4–8.2) | 6.5 (5.4–7.4) |
| c | 4.5 ± 0.4 (3.9–5.4) | 3.9 | 3.6 (3.4–4.1) | 4.0 (3.5–4.7) |
| c′ | 11.4 ± 1.4 (8.7–13.9) | – | – | 14.6 (12.1–17.5) |
| MB | 42.7 ± 3.3 (38.8–49.1) | 41 | 40 (38–42) | 40 (37–46) |
| V | 59.0 ± 2.5 (53.0–63.4) | 58.1 | 56 (53–60) | 59 (57–63) |
| Lip height | 3.2 ± 0.2 (3.0–4.0) | – | – | – |
| Lip width | 6.0 ± 0.3 (5.5–7.0) | – | – | – |
| Stylet length | 10.3 ± 0.8 (9.0–11.5) | 10.5 | 9.6 (9–11) | 11 (10–12) |
| Anterior end to excretory pore | 84.1 ± 3.5 (78.0–90.0) | – | 89 (81–97) | 86 (79–109) |
| Pharynx length | 103.0 ± 3.8 (96.0–112.0) | 95 | 115 (101–122) | 113 (99–127) |
| Maximum body width | 21.1 ± 2.1 (18.5–27.0) | – | – | – |
| Vulva body width | 18.9 ± 2.0 (15.5–25.0) | – | – | – |
| Post-uterine sac (PUS) length | 12.7 ± 1.5 (10.5–15.0) | – | – | – |
| Distance from vulva to anus | 120.6 ± 19.9 (102.0–178.0) | 123 | 132 (193–233) | 119 (93–146) |
| Distance from vulva to tail terminus | 262.9 ± 24.0 (238.0–317.0) | – | – | – |
| Anal body width | 12.5 ± 1.2 (10.0–14.0) | – | – | – |
| Tail length | 142.3 ± 11.9 (123.0–160.0) | 187 | 218 (193–233) | 182 (171–209) |
Abbreviations: n, number of specimens on which the measurements are based; a, body length/greatest body diameter; b, body length/distance from anterior end to pharyngo-intestinal junction; c, body length/tail length; c’, tail length/tail diameter at anus; MB, distance between the anterior end of the body and center of the median pharyngeal bulb expressed as a percentage (%) of the pharynx length; V, distance from the body anterior end to the vulva expressed as a percentage (%) of the body length.
3.5.1. 18S Phylogeny
Figure 5 presents an 18S Bayesian phylogenetic tree constructed with sequences of the Canadian populations of F. cylindricus, F. hazenensis, F. sheri, F. thornei, and other species. In this tree, the aforementioned species are grouped with related Filenchus species and distributed throughout the tree.
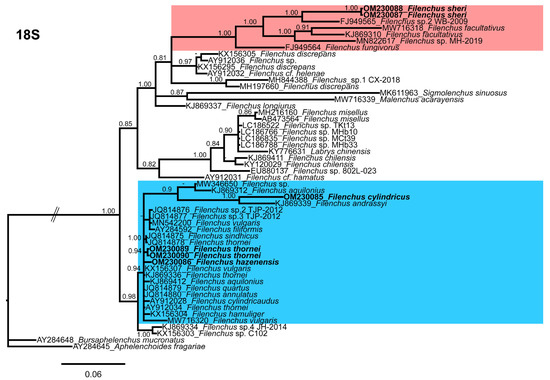
Figure 5.
Phylogenetic relationships of the Canadian population of Filenchus species with related Tylenchidae species. Bayesian 50% majority rule consensus tree as inferred from 18S rRNA sequence alignment under the general time-reversible model with invariable sites and a gamma-shaped distribution (GTR + G + I). Posterior probabilities of more than 0.70 are given for appropriate clades. The sequences produced in this study are shown in bold, and the colored boxes indicate the clade association of recovered Filenchus species.
Filenchus cylindricus clusters with F. andrassyi (Szczygiel) Andrássy [50,51](KJ869339) and shares a branch with F. aquilonius (Wu) Lownsberry and Lownsberry [16,52] (KJ869312) and an unidentified Filenchus sp. (MW346650) from Iran in the middle position of the tree. The sequence identity of F. cylindricus with the clustered species is 93–94% with 40–42 nucleotide differences and 1–2% indels. Filenchus hazenensis groups independently within Filenchus species and does not share a branch with other species. Filenchus sheri groups with an unidentified Filenchus sp. (FJ949565) from Belgium and shares a branch with two populations of F. facultativus (Szczygiel), Raski and Geraert [6,50] (MW716318, KJ869310), F. fungivorus Bert, Okada, Tavernier, Borgonie, and Houthoofd [10] (FJ949564), and an unidentified Filenchus sp. (MN822617). The sequence identity of F. sheri with the clustered species is 93–94% with 42–50 nucleotide differences and 0–1% indels. The Canadian population of F. thornei groups independently within Filenchus species and does not share a branch with other sequences of F. thornei. The other three populations of F. thornei were reported from Iran (JQ814878), the Netherlands (KJ869336), and the USA (AY912034) without morphological and morphometrical information available. The sequence identity of the Canadian population of F. thornei with the other populations of F. thornei is 98–99% with 2–7 nucleotide differences and 0% indels. It is evident that the sequence identities of other populations of F. thornei are close to the Canadian population; thus, their position is unlikely. This may be due to geographical distribution, misidentification, or cryptic species with similar morphology and morphometry. The identification of Filenchus species is challenging, and the misidentification and the presence of cryptic species within the widely published sequences cannot be ruled out. Consequently, we refer to the Canadian population of F. thornei as the reference population for future studies, until topotypes of this species can be sequenced.
3.5.2. 28S Phylogeny
Figure 6 presents a 28S Bayesian phylogenetic tree constructed with sequences of the Canadian populations of F. cylindricus, F. hazenensis, F. sheri, F. thornei, and related species. In this tree, F. cylindricus clusters with, but is well separated from, F. hazenensis; the sequence identity between both species is 77% with 139 nucleotide differences and 8% indels.
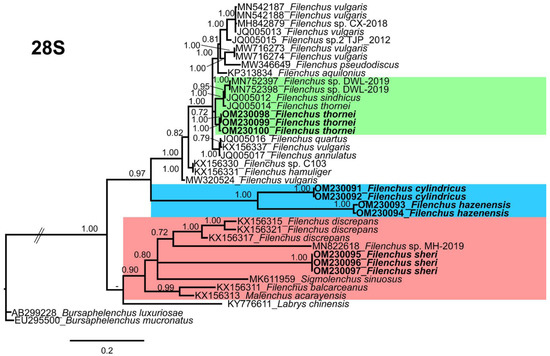
Figure 6.
Phylogenetic relationships of the Canadian population of Filenchus species with related Tylenchidae species. Bayesian 50% majority rule consensus tree as inferred from D2–D3 expansion domains of the 28S rRNA sequence alignment under the Tamura and Nei model with invariable sites and a gamma-shaped distribution (TrN + I + G). Posterior probabilities of greater than 0.70 are given for appropriate clades. The sequences produced in this study are shown in bold, and the colored boxes indicate the clade association of the recovered Filenchus species.
Filenchus sheri groups independently in a clade with F. balcarceanus Torres and Geraert [53] (KX156311), three populations of F. discrepans (Andrássy) Andrássy [1,54] (KX156315, KX156317, KX156321), an unidentified Filenchus sp. (MN822618) from Iran, Malenchus acarayensis Andrássy [46] (KX156313), Sigmolenchus sinuosus Gharakhani, Pourjam, Abolafia, Castillo and Pedram [55] (MK611959), and Labrys chinensis Qing and Bert [34] (KY776611). Though F. sheri is distinct from the clustered species, the sequence identity of F. sheri with the clustered species is 78–95% with 8–60 nucleotide differences and 0–3% indels. The Canadian population of F. thornei groups with F. sindhicus Shahina and Maqbool [56] (JQ005012), the F. thornei (JQ005014) population from Iran, and two unidentified Filenchus sp. (MN752397, MN752398) from Korea. The sequence identity of the Canadian population of F. thornei with the clustered species is 95–96% with 28–36 nucleotide differences and 0% indels. The Iranian population of F. thornei was reported without morphological and morphometrical information; therefore, we suggest a re-evaluation on the identity of this population, as it may be a cryptic species or misidentified.
3.5.3. ITS Phylogeny
There is a limited number of phylogeny studies based on ITS gene sequences of Filenchus species; only F. vulgaris (Brzeski) Lownsberry and Lownsberry [52,57] (MZ959287, MZ959288, MH842880) sequences are available for comparative studies. Therefore, we obtained the closest Tylenchidae species sequences through a BLASTN search for constructing an ITS tree (Figure 7). In this tree, the Canadian population of F. hazenensis and F. thornei group with F. vulgaris, and this Filenchus clade further shares a branch with a Coslenchus Siddiqi [58] species clade.
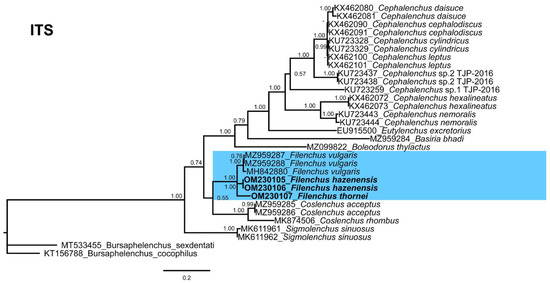
Figure 7.
Phylogenetic relationships of the Canadian population of Filenchus species with related Tylenchidae species. Bayesian 50% majority rule consensus tree as inferred from ITS sequence alignment under the general time-reversible model with a gamma-shaped distribution (GTR + G). Posterior probabilities of greater than 0.70 are given for appropriate clades. The sequences produced in this study are shown in bold, and the colored box indicates the clade association of the recovered Filenchus species.
Due to the lack of ITS sequences, the prediction of phylogenetic relationships is uncertain. We anticipate that the inclusion of new sequences in the future will likely change the position of Filenchus species; therefore, we omitted the calculation of sequence identity for the ITS gene sequences. Unfortunately, ITS sequences for F. cylindricus and F. sheri from Canada cannot be obtained.
4. Discussion
The genus Filenchus contains over 90 species. Among these, some are of economic significance (being fungal feeders) whilst others do not affect plants [8,12,13,14]. However, we anticipate that the presence of the latter species in the rhizosphere of cultivated plants can impact soil ecosystems. A comprehensive taxonomical compilation of 95 Filenchus species was provided by Geraert [8], after which only two more species (F. fungivorus and F. pseudodiscus Mortazavi, Heydari, Abolafia, Castillo, and Pedram [32]) were added to the genus, indicating the difficulties of studying this group of nematodes, which may be due, at least in part, to the absence of sufficient representatives. Alternatively, the paucity of descriptions in the literature may stem from research objectives [35]. Several studies have become increasingly focused on examining potential nematode species rather than mild parasitic species, such as Filenchus or other Tylenchidae genera [15,35,59,60,61].
Regarding habitat, the nematodes we describe here were collected from postharvest wheat and bean fields or from headland vegetation; therefore, we cannot associate these nematodes to one host. These nematodes may have fed on the roots of the previous crop or on the fungal propagules present in the soil. In addition, we did not observe any significant mycelial growth on the crop residues or on the rhizosphere samples at the time of soil collection. Consequently, the feeding preferences of these nematodes may have been plants or soil microbes.
The phylogenetic analyses conducted in this study raised questions regarding the existence of cryptic species or incorrect species identification. For instance, in the 18S tree, we noted the same populations of F. aquilonius, F. facultativus, and F. discrepans grouped distantly from each other. Similarly, several populations of F. vulgaris in the 28S tree were distributed throughout the first major clade. In addition, in the 18S and 28S trees, F. thornei populations from different countries showed aberrant positions; for example, the population from Iran was consistently grouped with F. sindhicus, whereas the Netherlands and USA populations held a basal position in the F. thornei clade. Such doubtful positions indicate that cryptic species may be present or a substantial part of the existing sequence data appears to be incorrect. In this regard, there is a need for the expansion and curation of rRNA sequences that are obtained through an integrative taxonomical approach. The Filenchus species characterized in this study appeared distinct in phylogenetic analyses and grouped with other members of the genus. However, it is evident that most Filenchus species have yet to be sequenced. We anticipate that, with the availability of new sequences, the phylogenetic positioning of Filenchus species will likely change.
The sustainable management of agricultural areas is becoming more challenging due to economic pressures and changes in the regulations of pesticides. Among fungal, bacterial, and insect pests, nematodes are probably the least understood and most often overlooked parasites. Due to a lack of understanding of the nematodes associated with cropping systems and their potential impact on cultivated plants, it is very difficult to accurately diagnose a nematode problem. Therefore, in the present work, we focused on the diagnostics of Filenchus spp. to enhance the visibility of this group of soil nematodes. The discovery of four Filenchus species from cultivated areas suggests that these species are common in agricultural soils, but may have been overlooked in prior nematode inventory surveys. Our work enhances the existing index to accommodate commonly occurring, mild parasitic nematodes to adequately uncover the existing nematode diversity. Moreover, the addition of molecular characterization of these species from Canada, in comparison to a reference dataset (NCBI) of Tylenchidae nematodes, provides insight into the biogeography of nematodes.
5. Conclusions
A rigorous understanding of the existing nematode biodiversity is of significant concern because nematodes divert nutrients from plants and use them for their own development and reproduction. Once a nematode problem is identified, it is difficult to overcome; the continuous presence and multiple generations of phytoparasitic nematodes can have a significant effect on plant vigor and growth, ultimately impacting the crops in the affected area. The presence and diagnostics of Filenchus species occurring in southern Alberta have not been addressed in previous studies, so, here, we conducted a comprehensive characterization of adult females of four Filenchus species from southern Alberta, three of which are new records in Canada. These results suggest that the known diversity of Canadian nemato-fauna has increased. However, more research is needed to further identify other genera and species of phytoparasitic nematodes that might occur in grasses, weeds, and wild plants present in cultivated areas. Due to our limited knowledge of the nematodes present in our cultivated areas, it is very difficult to accurately diagnose and assess the impact of nematode infestations problems. We anticipate that the results obtained in this study may help to determine if reduced crop yield may be the result of cumulative nematode infestation, fungal disease, or environmental factors.
Author Contributions
Conceptualization, M.M. and D.P.Y.; methodology, M.M., P.C. and D.P.Y.; software, M.M. and P.C.; validation, M.M., P.C. and D.P.Y.; formal analysis M.M., P.C. and D.P.Y.; investigation, M.M. and D.P.Y.; resources, D.P.Y.; data curation, M.M.; writing—original draft preparation, M.M., P.C. and D.P.Y.; writing—review and editing, M.M., P.C. and D.P.Y.; visualization, M.M., P.C. and D.P.Y.; supervision, D.P.Y.; project administration, D.P.Y.; funding acquisition, D.P.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Potato Early Dying Complex project funded by the University of Lethbridge Research Operating Fund, and the Canadian Potato Early Dying Network project funded by the Canadian Agri-Science Cluster for Horticulture 3 grant to D.P.Y., in collaboration with the Potato Growers of Alberta (Taber, AB, Canada), McCain Foods Canada Ltd. (Chin, AB, Canada), Cavendish Farms Corp. (Lethbridge, AB, Canada), and Lamb Weston Inc. (Purple Springs, AB, Canada).
Data Availability Statement
Not applicable.
Acknowledgments
We thank potato growers in Alberta, Canada, for providing access to their fields and Atta Ur Rahman (University of Lethbridge, AB, Canada) for the collection of soil samples. We also thank Carolina Cantalapiedra-Navarrete (Institute for Sustainable Agriculture (IAS), CSIC, Córdoba, Spain) for excellent technical assistance in molecular analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Andrássy, I. Revision der Gattung Tylenchus Bastian, 1865 (Tylenchidae, Nematoda). Acta Zool. Hung 1954, 1, 5–42. [Google Scholar]
- Husain, S.I.; Khan, A. A new subfamily, a new subgenus and eight new species of nematodes from India belonging to superfamily Tylenchoidea. Proc. Helminthol. Soc. Wash. 1967, 34, 175–186. [Google Scholar]
- Wu, L.Y. Dactylotylenchus crassacuticulus, a new genus and new species (Tylenchinae: Nematoda). Can. J. Zool. 1968, 46, 831–834. [Google Scholar] [CrossRef]
- Brzeski, M. Lambertia uliginosa g. n. sp. n. (Nematoda, Nothotylenchidae). Nematol. Mediterr. 1977, 5, 51–55. [Google Scholar]
- Siddiqi, M.R. Seven new species in a new nematode subfamily Duosulciinae (Tylenchidae), with proposals for Duosulcius gen. n., Zanenchus gen. n. and Neomalenchus gen. n. Nematologica 1979, 25, 215–236. [Google Scholar] [CrossRef]
- Geraert, E.; Raski, D. Review of the genus Filenchus Andrassy, 1954 and descriptions of dix new species (Nemata: Tylenchidae). Nematologica 1986, 32, 265–311. [Google Scholar] [CrossRef] [Green Version]
- Siddiqi, M. Tylenchida: Parasites of Plants and Insects; CABI: Wallingford, UK, 2000; Volume 2, p. 800. [Google Scholar]
- Geraert, E. The Tylenchidae of the World: Identification of the Family Tylenchidae (Nematoda); Academia Press: Gent, Belgium, 2008; pp. 246–356. [Google Scholar]
- Yeates, G.W.; Bongers, T.; De Goede, R.G.; Freckman, D.W.; Georgieva, S.S. Feeding habits in soil nematode families and genera—An outline for soil ecologists. J. Nematol. 1993, 25, 315–331. [Google Scholar] [PubMed]
- Bert, W.; Okada, H.; Tavernier, I.; Borgonie, G.; Houthoofd, W. Morphological, morphometrical and molecular characterisation of Filenchus fungivorus n. sp., a fungivorous nematode from Japan in a most likely polyphyletic genus (Nematoda: Tylenchina). Nematology 2010, 12, 235–246. [Google Scholar] [CrossRef]
- Sawahata, T.; Nakamura, H.; Okada, H.; Sasaki, A.; Kanematsu, S. Nonlethal ectoparasitism of the mycophagous nematode Filenchus discrepans (Nematoda: Tylenchidae). Nematology 2012, 14, 159–164. [Google Scholar] [CrossRef]
- Okada, H.; Harada, H.; Kadota, I. Fungal-feeding habits of six nematode isolates in the genus Filenchus. Soil Biol. Biochem. 2005, 37, 1113–1120. [Google Scholar] [CrossRef]
- Okada, H.; Kadota, I. Host status of 10 fungal isolates for two nematode species, Filenchus misellus and Aphelenchus avenae. Soil Biol. Biochem. 2003, 35, 1601–1607. [Google Scholar] [CrossRef]
- Okada, H.; Tsukiboshi, T.; Kadota, I. Mycetophagy in Filenchus misellus (Andrássy, 1958) Lownsbery & Lownsbery, 1985 (Nematoda: Tylenchidae), with notes on its morphology. Nematology 2002, 4, 795–801. [Google Scholar]
- Munawar, M.; Yevtushenko, D.P.; Palomares-Rius, J.E.; Castillo, P. Species diversity of pin nematodes (Paratylenchus spp.) from potato growing regions of southern Alberta, Canada. Plants 2021, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.Y. Five new species of Tylenchus Bastian, 1865 (Nematoda: Tylenchidae) from the Canadian high Arctic. Can. J. Zool. 1969, 47, 1005–1010. [Google Scholar] [CrossRef]
- Brzeski, M.W. Nematodes of Tylenchina in Poland and Temperate Europe; Muzeum i Instytut Zoologii Polska Adademia Nauk: Warszawa, Poland, 1998. [Google Scholar]
- Niblack, T.L.; Bernard, E.C. Plant-parasitic nematode communities in dogwood, maple, and peach nurseries in Tennessee. J. Nematol. 1985, 17, 132–139. [Google Scholar] [PubMed]
- Thorne, G.; Malek, R.B. Nematodes of the northern Great Plains. Part I. Tylenchida (Nemata: Secernentea). Tech. Bull. S. Dak. Agric. Exp. Stn. 1968, 1, 72–76. [Google Scholar]
- Andrássy, I. Evolution as a Basis for the Systematization of Nematodes; Pitman Publishing: Budapest, Hungary, 1976; pp. 1–288. [Google Scholar]
- Khan, M.; Khan, S. Two new and a known species of Tylenchus Bastian (Nematoda: Tylenchinae) from Afghanistan. Nematol. Mediterr. 1978, 6, 213–221. [Google Scholar]
- Siddiqi, M.; Siddiqi, M.R. Tylenchida Parasites of Plants and Insects; CABI: Slough, UK, 1986; p. 646. [Google Scholar]
- Andrássy, I. Freilebende nematoden aus Angola I. Einige moosbewohnende Nematoden. Publicaçōes Cult. Da cia. de Diam. de Angola 1963, 66, 55–80. [Google Scholar]
- Jenkins, W. A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Dis. Report. 1964, 48, 692. [Google Scholar]
- Seinhorst, J. On the Killing, fixation and transferring to glycerin of nematodes. Nematologica 1962, 8, 29–32. [Google Scholar] [CrossRef] [Green Version]
- De Grisse, A.T. Redescription ou modification de quelques techniques utilisees dans l’etude des nematodes phytoparasitaires. Meded. Rijksfakulteit Landbowwetenschappen Gent. 1969, 34, 351–369. [Google Scholar]
- Maria, M.; Powers, T.; Tian, Z.; Zheng, J. Distribution and description of criconematids from Hangzhou, Zhejiang Province. China. J. Nematol. 2018, 50, 183–206. [Google Scholar]
- Holterman, M.; Van der Wurff, A.; van den Elsen, S.; Van Megen, H.; Bongers, T.; Holovachov, O.; Bakker, J.; Helder, J. Phylum-Wide analysis of SSU rDNA reveals deep phylogenetic relationships among nematodes and accelerated evolution toward crown clades. Mol. Biol. Evol. 2006, 23, 1792–1800. [Google Scholar] [CrossRef]
- De Ley, P.; Felix, M.-A.; Frisse, L.; Nadler, S.; Sternberg, P.; Thomas, W.K. Molecular and morphological characterisation of two reproductively isolated species with mirror-image anatomy (Nematoda: Cephalobidae). Nematology 1999, 1, 591–612. [Google Scholar] [CrossRef]
- Ferris, V.; Ferris, J.; Faghihi, J. Variation in spacer ribosomal DNA in some cyst-forming species of plant parasitic nematodes. Fundam. Appl. Nematol. 1993, 16, 177–184. [Google Scholar]
- Curran, J.; Driver, F.; Ballard, J.; Milner, R. Phylogeny of Metarhizium: Analysis of ribosomal DNA sequence data. Mycol. Res. 1994, 98, 547–552. [Google Scholar] [CrossRef]
- Mortazavi, P.; Heydari, F.; Abolafia, J.; Castillo, P.; Pedram, M. Morphological and molecular characterization of Filenchus pseudodiscus n. sp. from east Golestan province, north Iran; with an updated phylogeny of Malenchus Andrássy, 1968 (Tylenchomorpha: Tylenchidae). J. Nematol. 2021, 53, e2021-069. [Google Scholar] [CrossRef] [PubMed]
- Munawar, M.; Yevtushenko, D.P.; Castillo, P. First report of three Tylenchidae taxa from southern Alberta, Canada. Horticulturae 2021, 7, 449. [Google Scholar] [CrossRef]
- Qing, X.; Bert, W. 3D printing in zoological systematics: Integrative taxonomy of Labrys chinensis gen. nov., sp. nov. (Nematoda: Tylenchomorpha). J. Zool. Syst. Evol. Res. 2017, 56, 35–47. [Google Scholar] [CrossRef]
- Qing, X.; Bert, W. Family Tylenchidae (Nematoda): An overview and perspectives. Org. Divers. Evol. 2019, 19, 391–408. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2017, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, T. In BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Castresana, J. Selection of conserved clocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rambaut, A. FigTree v1. 4.2, A Graphical Viewer of Phylogenetic Trees. Available online: http://tree.bio.ed.ac.uk/software/figtree/rightanglebracket (accessed on 30 December 2021).
- Elmiligy, I.A. Two New Species of Tylenchidae, Basiroides nortoni n. sp. and Tylenchus hageneri n. sp. (Nematoda: Tylenchida). J. Nematol. 1971, 3, 108–112. [Google Scholar] [PubMed]
- Geraert, E.; Zeidan, A. The genera Filenchus Andrassy, 1954, Sakia Khan, 1964, Boleodorus Thorne, 1941 and Basiria Siddiqi, 1959 (Nemata: Tylenchida) from Sudan. Nematologica 1991, 37, 185–212. [Google Scholar] [CrossRef]
- Dobrin, I.; Geraert, E. Some tylenchida from Romania. Biol. Jaarb. 1994, 62, 121–133. [Google Scholar]
- Brzeski, M. Redescription of some species of the genus Filenchus Andrássy, 1954 (Nematoda, Tylenchidae). Miscel. Zoològica 1997, 20, 45–64. [Google Scholar]
- Karegar, A.; Geraert, E. The genus Filenchus Andrássy, 1954 (Nemata: Tylenchidae) from Iran. Belg. J. Zool. 1995, 125, 363–382. [Google Scholar]
- Andrássy, I. Fauna Paraguayensis. 2. Nematoden aus den Galeriewäldern des Acaray-Flusses. Opusc. Zool. Bp. 1968, 8, 167–312. [Google Scholar]
- Bongers, T. De Nematoden Van Nederland: Een Identificatietabel Voor de in Nederland Aangetroffen Zoetwater-En Bodembewonende Nematoden; Stichting Uitgeverij Koninklijke Nederlandse Natuurhistorische Vereniging: Zeist, The Netherlands, 1988. [Google Scholar]
- Castillo, P.; Gomez-Barcina, A.; Gonzalez, M.A. Nematodes of the family Tylenchidae Orley, 1880 (Nematoda: Tylenchida), in Cazorla Mountains, Spain. Zool. Baetica. 1991, 2, 137–161. [Google Scholar]
- Atighi, M.R.; Pourjam, E.; Pereira, T.J.; Okhovvat, S.M.; Alizada, B.A.; Mundo-Ocampo, M.; Baldwin, J.G. Redescription of Filenchus annulatus (Siddiqui & Khan, 1983) Siddiqi, 1986 based on specimens from Iran with contributions to the molecular phylogeny of the Tylenchidae. Nematology 2013, 15, 129–141. [Google Scholar]
- Szczygiel, A. A new genus and four new species of the subfamily Tylenchinae de Man, 1876 (Nematoda: Tylenchidae) from Poland. Opusc. Zool. 1969, 9, 159–170. [Google Scholar]
- Andrássy, I. The genera and species of the family Tylenchidae Örley, 1880 (Nematoda). The genus Tylenchus Bastian, 1865. Acta Zool. Hung. 1979, 25, 1–33. [Google Scholar]
- Lownsbery, J.W.; Lownsbery, B.F. Plant-parasitic nematodes associated with forest trees in California. Hilgardia 1985, 53, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Torres, M.; Geraert, E. Tylenchidae from Buenos Aires, Argentina. Nematologica 1996, 42, 42–61. [Google Scholar] [CrossRef]
- Andrássy, I. A Magyarországról eddig kimutatott szabadon élő fonálférgek (Nematoda) jegyzéke. Állattani Közlemények 1972, 59, 161–171. [Google Scholar]
- Gharahkhani, A.; Pourjam, E.; Abolafia, J.; Castillo, P.; Pedram, M. Sigmolenchus sinuosus n. gen., n. sp. (Tylenchidae: Ecphyadophorinae), a new member of the family. Nematology 2020, 22, 985–997. [Google Scholar] [CrossRef]
- Maqbool, M.; Shahina, F. Description of Filenchus sindhicus sp.n. and observations on three known species (Nematoda: Tylenchidae) on banana in Pakistan. Nematologica 1994, 40, 69–77. [Google Scholar] [CrossRef]
- Brzeski, M. On the taxonomic status of Tylenchus filiformis Butschli, 1873, and description of T. vulgaris sp. n. (Nematoda, Tylenchidae). Bulletin de 1′Academic Polonaise des Sciences. Cl. U. Ser. Des Sci. Biol. 1963, 11, 531–535. [Google Scholar]
- Siddiqi, M.R. The unusual position of the phasmids in Coslenchus costatus (De Man, 1921) gen. n., comb. n. and other Tylenchidae (Nematoda: Tylenchida). Nematologica 1978, 24, 449–455. [Google Scholar] [CrossRef]
- Forge, T.A.; Larney, F.J.; Kawchuk, L.M.; Pearson, D.C.; Koch, C.; Blackshaw, R.E. Crop rotation effects on Pratylenchus neglectus populations in the root zone of irrigated potatoes in southern Alberta. Can. J. Plant Pathol. 2015, 37, 363–368. [Google Scholar] [CrossRef]
- Mahran, A.; Tenuta, M.; Shinners-Carenelly, T.; Mundo-Ocampo, M.; Daayf, F. Prevalence and species identification of Pratylenchus spp. in Manitoba potato fields and host suitability of ‘Russet Burbank’. Can. J. Plant Pathol. 2010, 32, 272–282. [Google Scholar] [CrossRef]
- Munawar, M.; Yevtushenko, D.P.; Castillo, P. Integrative taxonomy, distribution, and host associations of Geocenamus brevidens and Quinisulcius capitatus from southern Alberta, Canada. J. Nematol. 2021, 53, 1–13. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).