Abstract
Festuca rubra is one of the dominant species in mountain high natural values grasslands. Most herbaceous plants are involved in a symbiotic partnership with arbuscular mycorrhizas for maintaining their abundance and cover. This research was conducted to explore the changes in mycorrhizal colonization patterns and structure development due to the long-term application of treatments. A large database of microscopic images was deeply analyzed with the MycoPatt tool, for the extraction of clear mycorrhizal maps that present particular colonization strategies. The overall colonization frequency and intensity varied largely between treatments, with a maximum in organic conditions. The presence of both arbuscules and vesicles in the same root area indicate a continuous alternance of fungal strategy, from storage to enhanced transfer of nutrients. A low-mineral organic treatment permits a clear separation of colonization strategy in different parts of roots. The nutrient availability due to mineral treatment induced a restriction in mycorrhizal development, which was maintained colonization by a resistance conditions strategy. The use of mycorrhizal maps permits a deep scanning of colonized roots, identifying the real positioning of fungal structures, along with their developmental potential and the assessment of the colonization strategy.
1. Introduction
Grassland ecosystems represent the largest biomass, covering over 45% of the Earth’s dry surface [1]. These ecosystems are dominated by species of the Poaceae family [2], most of which are perennial. This has led to the formation of nutrient-poor soil over time due to the high quantities of nutrient removed, a phenomenon called oligotrophy. This phenomenon is an organism or habitat feature that develops and even thrives on low nutrient levels [3]. The primary consequence of this phenomenon is that the biota hierarchy, both above-ground and below-ground, has adapted to a slower lifestyle and with limited sources of nutrients. Plants found in mountain grassland are thus forced to adopt different survival strategies. The most convenient process is to accept a microbial partner that, in exchange for a proportion of the photosynthetic assimilates, provides a higher amount of nutrients and water for the plant. During their life cycle, plants interact with various organisms, but one of the most important positive interactions is with arbuscular mycorrhizal fungi [4]. Abiotic stresses, which include heavy metals, drought, salinity, cold, and heat stress, pose a severe threat to plant ecosystems, which greatly reduce crop productivity and also damage the environment [5]. It is absolutely necessary to understand the magnitude of this process because mycorrhizal fungi, in addition to their higher intake of nutrients, a role explained in most articles, provide protection to the host from pathogens [6,7] and drought [8,9,10], limit the absorption of heavy metals [11,12], or intervene in the stability of floristic composition in these ecosystems [13].
Mycorrhizal fungi represent key mutualists in two-thirds of all terrestrial plants, including most plants of major interest [14]. These fungi are an essential part of the rhizosphere biota, and form a group that plays a crucial role in maintaining the productivity and stability of grasslands [15,16]. Because the biodiversity of these ecosystems is still high, and mycorrhizal fungi do not have a high selectivity for host choice, a fungal species can colonize more roots of plants that occupy the same niche. Mycorrhizal fungi regulate species’ distribution through uneven nutrient distribution. Thus, the fungal community in the grassland ecosystems directs the cycling of nutrients, governing the absorption of nutrients for plants, as well as their loss, through leaching. Bender et al. [17] demonstrated in a study that the fungal community reduced phosphorus leaching by 31%, improved the content of the element in plants by 15%, and increased its soil-to-soil mobilization by 18%. Regarding nitrogen, the same authors claim that the main factor is the soil type; the leaching of this element was reduced by 24% and the concentration of nitrogen in the practical species increased by 13%. In addition to the present oligotrophy, we also mention the global changes that led to the reduction of biodiversity and change in the presence proportion of certain species [18]. The biodiversity present in grassland ecosystems, both above ground, represented by plants, and that given by the composition of the microbial community is closely related [19]. Any changes made to the two communities will affect the proper functioning of the ecosystem as a whole. At the plant community level, it is also clear that changes in plant diversity and community structure can have an action–reaction effect on soil microorganisms and their functioning, again by changing the amount and quality of resources entering from the soil [20].
The process of plant symbiotic partnership with mycorrhizas is highlighted by the presence of specific structures developed by the fungal component: hyphae, appressorium, vesicles, arbuscules, or spores [21]. Mycorrhiza begins the first stage of symbiosis, that of pre-symbiosis [22], in which it encounters inoculum source (fungal hyphae, spores, vesicles, or infected roots), which performs an exchange of signals with the plant roots [23]. When the signals between the two partners have been established, the symbiosis proceeds. Depending on the permissiveness of the host species, the fungal component forms different structures after rooting [24,25]. Intraradicular hyphae play the role of colonizing the cells of the root cortex, and they come from extraradicular hyphae that can be run or absorbed [26]. If the host is permissive enough, it allows mycorrhizal fungi to form arbuscules and vesicles. Arbuscules are the structures formed inside the root cells, where nutrient transfer takes place [27], in intimate contact between the two partners. They are short-lived structures [28] and can be digested after the end of their cycle. The vesicles are thick-walled structures of different shapes, and as a constituent they are lipid in nature [29]. They act as storage and infectious propagules [30]. Spores are the most important structures from a genetic point of view, based on their characteristics assessed between mycorrhizal species, and they have a significant role in fungal resistance and dispersion [31].
The symbiotic relationship between plants and mycorrhizal fungi is governed by the production of phosphorus, an essential element for the good development of plant species [32], but the main problem is its form, with it not being accessible to plants in its raw form due to the autotrophic component. A concern that has arisen in recent years is the limitation of this element in the chemical industry as well. Plant adaptations need to be stepped up to maximize the use of soil-rich phosphorus [33]. Phosphate deposits are limited, and high-quality reserves for fertilizer production could be depleted before the end of this century. Fertilizers are still quite inexpensive, leading to high or even excessive use. High phosphorus from increased fertilizer intake can suppress mycorrhizal colonization and subsequent plant–mycorrhiza function [34]. To increase/maintain this symbiotic association, it is recommended to keep the accessible phosphorus level in soil solution low, as higher concentrations may block the development of mycorrhizae [35].
The purpose of this article was to assess the characteristics and stability of the mycorrhizal mechanism in Festuca rubra as a response to the long-term application of differentiated treatments. In order to fulfill the proposed goal, the following concepts and hypotheses were established: (i) defining the mycorrhizal strategy according to the structures developed in roots; (ii) analyze the correlation of mycorrhizal expansion with the applied treatments; (iii) assessment of the relative colonization strategy and their extension on mycorrhizal maps; (iv) identification of distinct mycorrhizal patterns in control vs. treatment.
2. Materials and Methods
2.1. Experimental Design
The experimental area is part of a large experimental field established in 2001, in a high natural value grassland form the Apuseni Mountains, with the coordinates 46.49064 N–22.81418 E (DD) [36]. The location of the field is in Ghețari Village, 1130 m above sea level, and the soil type is a preluvosol (terra rossa). Soil reaction is acidic (5.1) and the texture is loamy–clayey, with low bulk density and a medium porosity. The humus content is low (2.8); the content in macro-elements is medium for N (0.195%) and extremely low for P (2.1 ppm) and K (23 ppm) [37].
Root samples were extracted from 5 experimental variants. The treatments were applied each year and have the following structure: V1 (control variant)—untreated; V2—organic (manure) treatment—10 t ha−1 manure; V3—low-mineral organic treatment—10 t ha−1 manure + 50 N 25 P2O5 25 K2O; V4—mineral treatment—100 N 50 P2O5 50 K2O; V5—high-mineral organic treatment—10 t ha−1 manure + 100 N 50 P2O5 50 K2O. Prior to root extraction, Festuca rubra plants were identified by two grassland specialists. A large pool of roots was sampled in 2019, at the flowering stage of plants (15 July). Soil cores containing F. rubra plants were extracted from each of the 4 replications of experimental field. At the site, entire plants were gently extracted from each soil core and washed to eliminate large soil aggregates. Clean roots were detached from plants and frozen at −20 °C until processing in the laboratory.
Festuca rubra is a species of grass commonly known as red fescue. The systematic classification of the species is in the family Poaceae (Graminee). F. rubra is a perennial herbaceous plant; it has a stem of 30–90 cm high, and is an essential indicator species for the mountain grasslands from Romania. It generally indicates grasslands with an average–high ecological value. This species has a good performance under oligotrophic conditions [38], and has a native ability to establish symbiotic partnerships with mycorrhizal fungi [39].
2.2. Laboratory Analysis
At harvest, each root sample from each replication was collected in a separate bag. The plant has a shallow root system, with short rhizomes. For mycorrhizal analysis, all roots were cleared and stained before microscopy. The staining method was performed at room temperature, in the absence of any heating source, and followed the steps described by Stoian and Florian [40], with four stages: clearing (48 h) in a 10% solution of NaOH; tap water washing to remove the clearing solution; staining with 5% of Pelikan 4001 ink [41] +5% vinegar solution for another 48 h. This procedure permits good visualization of intra-radical mycorrhizal structures, with slow and delicate entry of solutions in tissues, in the absence of heating. Another benefit of this method is that root samples can be analyzed multiple times during the clearing and staining procedure and the moment of optimum staining can be identified separately for each root batch. This permits one to obtain the same staining quality independently of root dimensions or thickness, and multiple batches can be processed in the same time interval.
Microscopic quantification of mycorrhizas and the image extraction was performed with an Optika microscope, at a magnification of 40×. Colonization parameters and the analysis of raw data were extracted based on the method proposed by Stoian et al., with the MycoPatt tool (Figure S1), [42]. This tool is an Excel based one, with 3 different active sheets: the first one represents the raw data, where each of the mycorrhizal structures are coded with values from 1–6, according to their position in the previous extracted microscopic images; the second sheet convert the codes into 6 colors, and creates the mycorrhizal map with the real positioning of each structure in analyzed roots; the third sheet converts the codes into colonization parameters, both horizontally and vertically, and then calculates transversally to obtain a real average for each image/segment analyzed. The six codes used in the MycoPatt tool represent: 1—hyphae, 2—arbuscules, 3—vesicles, 4—spores, 5—auxiliary cells, 6—entry points. For each 1 cm of root segment, 15 microscopic fields were analyzed and assembled to provide the mycorrhizal colonization expression of an entire segment. A total number of 15 segments were analyzed for each replication. Overall, 900 observations were made for each experimental variant, and for each segment a mycorrhizal map was extracted. For the entire experiment a large database was set up and contained a total number of 4500 observations for each of the following parameters: colonization frequency (%); colonization intensity (%); arbuscules and vesicles abundance (%); the overall colonization degree (%); the share of non-mycorrhizal areas (%) in roots; the report between mycorrhized and non-mycorrhized areas. To these parameters, a new one was proposed to show the report between arbuscules and vesicles in each root segment. Each mycorrhizal map is constructed from a grid of 15 blocks of 10 × 10 squares, which results in the real positioning of each fungal structure in the colonized roots.
2.3. Data Analysis
The entire database was analyzed with RStudio software version, 1.4.1106 [43], on R platform [44]. For each of the analyzed experimental variants, a set of histograms (package “graphics” [44]) was extracted for the analysis of variations trends. Basic statistics were done with the package “psych” [45], from which means and their standard errors and median values were used. All differences between the means of each parameter, grouped by treatments, were analyzed with ANOVA and LSD tests, available in the package “agricolae” [46]. The database containing all mycorrhizal parameters from each variant was analyzed with the Principal Component Analysis (PCA), package “vegan” [47], and vectors of each parameter were projected based on the “envfit” function from the same package. A series of scatterplots were constructed for the assessment of relationships between two parameters, which were further explored through regressions with “stats” package from R platform [44,48] and verified with “MASS” package [49,50]. For the description of fungal strategy, the median frequency and intensity of colonization from each set of data was used as the “general median model” and compared to the same value of 2 other models which were extracted: the low colonization model, which represents half (½) of the median value, and the high colonization model which represents the double (2×) value of the median. Additional to these three models, the maps that show the maximum of arbuscules and vesicles abundance were extracted separately. This data analysis process was used to explore the colonization pattern and strategies in different root samples, in order to define their direction in colonization and to compare the structure formation.
3. Results
3.1. Global Dynamics of Mycorrhizal Parameters as Affected by Applied Treatments
A histogram analysis of each parameter, in relation to applied treatments, was chosen as a pairwise comparison between data distribution and their potential to indicate the colonization strategy. Colonization frequency (Figure 1a–e) varies largely in the interval 0–100%, with sequential similar distributions. The control variant presented 30% of the observations in the interval 0–20%, compared to the highest values of treatments (V4 and V5), where this parameter has a similar distribution and a supplemental 100 observations in the same interval. For these three variants, the remaining two-thirds of observations are split within the rest of frequency classes, with at least 100 observations for each class. Contrasting, organic and low-mineral organic variants (V2 and V3) show more equilibrated colonization frequencies. There is a visible polarity of colonization frequency, with equal observations in the interval 0–20% and 80–100%. The entire colonization presence is visible for these two variants.
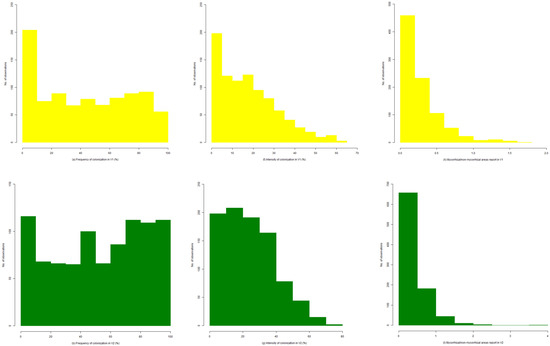
Figure 1.
Histograms of colonization parameters F. rubra roots for each experimental variant: (a–e) colonization frequency (%); (f–j) colonization intensity (%); (k–o) mycorrhizal/non-mycorrhizal report. Treatments V1–V5: V1 (control variant)—untreated; V2—organic (manure) treatment—10 t ha−1 manure; V3—low-mineral organic treatment—10 t ha−1 manure + 50 N 25 P2O5 25 K2O; V4—mineral treatment—100 N 50 P2O5 50 K2O; V5—high-mineral organic treatment—10 t ha−1 manure + 100 N 50 P2O5 50 K2O.
Intensity of colonization brings an even more different image of colonization development in roots due to the applied treatments (Figure 1i,j). More than half of the observations from control and high-treated variants have intensities lower than 20%, with abrupt decreases in higher intensity classes (Figure S1). In contrast, the use of low quantity treatments (V2 and V3) (Figure S1), shows a smooth decrease of intensities and a maximum of observations in the interval 10–20%, which is associated with the point-local development of hyphae in most of the areas where they are present in colonized roots.
Control and high-mineral organic treated variant (V5) show punctual colonization intensities that reach 60%, and the mineral treated variant even reached 90% (Figure 1i,j). The maximum values observed in organic (V2) (Figure S1) and low-mineral organic (V3) (Figure S1) variants do not exceed 80%, but this value is reached in more cases than the rest of variants.
For both arbuscules (Figure S2a–e) and vesicles (Figure S2i,j), almost all observations show a reduced development of these structures, regardless of the applied treatments. The differences between variants are the maximum percentage of arbuscules and vesicles developed. For control and mineral fertilized variants (V4), the maximum of arbuscules is 40% of the mycorrhized area, while for the rest of variants the maximum limit is set to 50–60%. Vesicles show even a deeper difference between variants. The high-treated ones (V4 and V5) have around 15% vesicles, while the control and V3 reached 25%, and for the organic treated variant (V2), 40%.
The volume of roots explored by mycorrhizas, presented by colonization degree, shows a combined pattern of data distribution between intensity and frequency (Figure S2k–o). The differences between variants are of 100 observations in the interval 0–5%. There were approximately 300 observations for organic variant (V2), 400 for control and low-mineral organic variant (V3), 100 for high-mineral organic variant (V5), and a maximum of 600 for mineral variant (V4).
The analysis of non-mycorrhizal areas revealed a higher blocking potential of colonization in roots of control and highly treated variants than in lower treated ones (Figure S2p–t). All variants presented uncolonized areas which indicates the continuous evolution of the symbiotic partnership.
The most sensitive synthetic colonization indicator, the report between mycorrhized and non-mycorrhized areas, shows high differences between treatments (Figure 1k–o). Both control and high-mineral organic treated variants show values for this report under 0.5 for the large majority of the observations. The rest of the three variants have approximately 800 observations, with a report lower than 1.0, but with different maximums reached. The variant with mineral treatment reached 4.0–5.0 values of this report, compared to the organic treatment one, where this report is in the interval 3.0–4.0, and decreases by 0.5 in the case of low-mineral organic variant.
3.2. Specific Influence of Long-Term Application of Treatments on Mycorrhizal Parameters
Data analysis of differences between variants highlighted the influence of treatments regarding the average values of colonization parameters (Table 1). Colonization frequency varied greatly with 20% from the high value in organic treatment to 35.6% in high-mineral organic variant (V5). The intensity of colonization followed the same trend as frequency, with control variant being in the middle of the variation interval and with a significant 2% gradual decreases between variants. Both arbuscules and vesicles have a low presence in roots of F. rubra. In the case of arbuscules, the same hierarchy of variants, as in frequency and intensity, was observed. Vesicles present clear significant differences between organic and control variants, with values starting from 1.8% when compared to the rest of treatments, where the observed decrease is 0.5% as the amounts of fertilizers increase. The general colonization degree reaches a maximum of 17.7% in the case of organic fertilized variant, and significantly decreases below 10% in high-mineral organic fertilized variant. The native potential is set to an intermediate value of 12.3%, which can be improved by organic treatments. Non-mycorrhized areas varies in a 10% interval, with a maximum of 85.14% in V5 and a decrease of approximately 2% from one variant to another, up to the minimum of 75.87% in organic treated variant (V2). There are three significantly different groups of variants based on the differences in mycorrhizal/non-mycorrhizal areas report. The group of low-input variants (V2 and V3), with values of this report above 0.35, a group formed by control and mineral treated variant, with very similar values, and the variant high-mineral organic treated variant (V5) with a value of only 0.21.

Table 1.
Differences in mycorrhizal parameters in roots of F. rubra induced by long-term treatments.
Pearson correlation between mycorrhizal parameters shows different relations due to applied treatments Table S1. Due to the large database analyzed, the large majority of correlations are significant at p < 0.05. Frequency and intensity of colonization shows values of 0.91 and 0.93 for control and high-treated variants (V4 and V5), respectively. In the case of low-treated variants (V2 and V3), this relation decreases bellow 0.90. Frequency is more correlated with arbuscules presence (0.24–0.35) than with vesicles (0.16–0.24). Intensity shows very different values of correlation with arbuscules and vesicles. This relation is in the favor of arbuscules, and when compared to the intensity–vesicles correlation the highest difference is observed in high-mineral organic treatment (V5). For the correlation between arbuscules and vesicles, all the recorded values are negative for high-treated variants (V4 and V5), with very low values (−0.03) and the only correlation with lack of significance.
3.3. Spatial Ordination of Mycorrhizal Colonization Due to Applied Treatments
By plotting each data set in a particular PCA ordination, this makes visible the differences between colonization potential and registered parameters (Figure 2a–e). The total variance explained by each PCA varies between 95.43 (V2) and 98.11% (V4), which represent, respectively, the two opposite treatments: organic vs. mineral. The PCA analysis applied separately shows a different vector orientation, specific to each treatment. Each PCA has an intensity–nonmycorrhizal areas combination of vectors (colonization intensity and non-mycorrhizal areas) as a gradient of parameters, which are always in an 180° opposition. While for the control variant (V1), the gradient of intensity–nonmycorrhizal areas is oriented form +− to −+ quadrants, the application of organic (V2) or mineral treatment (V4) change the gradient direction to an opposite one (Figure 2a,b,d). The combination of organic + mineral treatments (V3 and V5) changes the direction of intensity–nonmycorrhizal areas gradient to the quadrants −− to ++, in the case of low-mineral organic variant, and from ++ to −− in the case of high-mineral organic variant (Figure 2c,e).
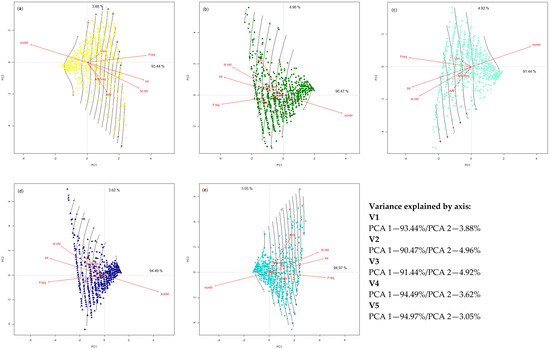
Figure 2.
PCA ordinations of mycorrhizal patterns in roots of F. rubra as projected on colonization degree based on the influence of applied treatments. (a) V1 (control variant)—untreated; (b) V2—organic (manure) treatment—10 t ha−1 manure; (c) V3—low-mineral organic treatment—10 t ha−1 manure + 50 N 25 P2O5 25 K2O; (d) V4—mineral treatment—100 N 50 P2O5 50 K2O; (e) V5—high-mineral organic treatment—10 t ha−1 manure + 100 N 50 P2O5 50 K2O. Legend: Freq—colonization frequency; Int—colonization intensity; Arb—arbuscules abundance; Ves—vesicles abundance; Arb.Ves—arbuscules/vesicles report; nonM—non-mycorrhizal areas; M.nM—mycorrhizal/non-mycorrhizal areas report.
The control variant (V1) shows a positive correlation between colonization frequency and the presence of vesicles (Figure 2a). Arbuscule development is conditioned by the higher values of intensity and mycorrhizal–nonmycorrhizal areas report. The vesicles and arbuscules/vesicles report have both equal, short vectors in the ordination space, at the half of arbuscules vector length. The data associated with the quadrant of vesicles vector are more agglomerate when compared to the arbuscule vector quadrant, where the data are more dispersed. The PCA ordination of organic treatment (V2) shows a very dispersed image of data associated with the arbuscules vector (Figure 2b). For this treatment, the vector of arbuscules/vesicles report is shorter than those of both vesicles and arbuscules, in a different angle from each one. The arbuscules and vesicles vector show a 90° angle, with a positioning of the vesicles vector near to the center of PCA and in the middle of the quadrant for arbuscules. For this treatment, arbuscules show a long vector associated with a highly dispersed set of data. When compared to this, the vesicles vector has almost half of length but is associated with a homogeneous set of data. This variant shows the most correlated vectors of synthetic reports: arbuscules/vesicles and mycorrhizal/nonmycorrhizal areas. The variation between arbuscule or vesicle development is determined by the fungal capacity to colonize larger areas of roots.
The differences between low (V3) and high (V5) mineral doses applied with organic treatments are highly visible in the position of PCA vectors and the dispersion of data (Figure 2c,e). The isolines of colonization degree are larger in the case of low-mineral treatment (V3), with a short vector of arbuscules, which restrict the formation of these structures. As an opposite, a lower colonization degree and tight isolines are associated with high-mineral treatment (V5). For this treatment, the arbuscules vector is long, but it is associated with a highly dispersed set of data.
Half of the dataset form the mineral treatment (V4) are grouped in the middle of the PCA ordination (Figure 2d). For this treatment, the vectors of arbuscules have the same direction and arbuscules/vesicles report are fully overlaid. This phenomenon is associated with the nutrient transfer to host roots, without their storage in vesicles. All the data associated with arbuscules development present a high dispersion, which sustain the idea of an assailant process in their development.
3.4. Colonization Strategies and Fungal Patterns in F. rubra Roots
Colonization process and the development of fungal structures in F. rubra roots shows high differences due to the long-term applied treatments. The level of hyphal extension in roots and their potential transformation into arbuscules or vesicles indicates an adapttive mycorrhizal strategy. Based on a transversal increase in frequency and intensity of colonization [51], mycorrhizal associations vary from lax (casual) to strong (obligatory). Based on the values of colonization parameters and their combination with arbuscules and/or vesicles, four types of colonization strategies can be proposed (Table 2).

Table 2.
Mycorrhizal colonization strategies and the guide to fungal patterns in roots of F. rubra.
- The propagative strategy (PS) shows a proliferation of mycorrhizas in roots, with the development of extensive hyphal networks; hyphae may develop continuously or around an entry point with discontinuities between colonized areas; arbuscules and vesicles are rarely present or absent in this case.
- The transfer strategy (TS) provides an image of a strong hyphal penetration in root cells and the formation of arbuscules; roots can present areas with both hyphae and arbuscules and alternate areas with hyphae and arbuscules or hyphae; vesicles are absent.
- The Storage strategy (SS) shows high densities of vesicles in roots; vesicles are uniform, being distributed along roots, or alternate areas of hyphae and vesicles with hyphae only.
- The plant resistance conditions strategy (RS) implies a reduced development of hyphae within the root cortex and large areas without fungal structures are present; several scenarios of development can be observed in roots—high punctual hyphal extension surrounded by non-mycorrhizal areas, numerous entry points with a reduced hyphal extension, and numerous discontinuities.
Observed values of each colonization parameter can be completed with values obtained from two reports: mycorrhizal/non-mycorrhizal areas and arbuscules/vesicles. The first one, mycorrhizal/non-mycorrhizal areas report, is integrated in MycoPatt tool and its variations can be used as an indicator of the stability in colonization process. It shows both variations in actual mycorrhizal development and the future potential development. This indicator can be used to explain the variation from extensive to intense (proliferative or parasitic) colonization. The second report is proposed for the analysis of arbuscules’ and vesicles’ shares in the colonization process. Even at low presence of these structures, their report can provide a forecast of future development of one in the detriment of the other. The arbuscules/vesicles report provides an image of three possible scenarios. When this report is in the interval 0.0 up to 0.90, the colonization process is oriented toward the development of vesicles to the detriment of arbuscules, and the fungal strategy is oriented toward a storage strategy. A value of report near 1.00 (0.90–1.10), indicates that mycorrhizas have a balanced development, with a similar allocation of resources for the transfer of nutrients toward the host and the storage of excess nutrients. In the case when the report is higher than 1.10, mycorrhizas are oriented toward a faster transfer of nutrients to host, which is associated with intense growth processes.
Both arbuscules and vesicles are dependent on the intensity of colonization (Figure 3a,b). The regression equation for the arbuscules show a normal presence of these structure of up to 10% from the colonized area of the root (Figure 3a). Based on the regression, the minimum intensity required to have this process initiated is almost 5%. By scanning the entire root with MycoPatt tool, this process can be deeper analyzed and used to identify the areas where these structures are highly present. Overall, half of the data present higher arbuscules’ abundance, non-related to the associated intensity of colonization. This phenomenon is due to root cells’ punctual permissiveness, which stimulates arbuscule formation.
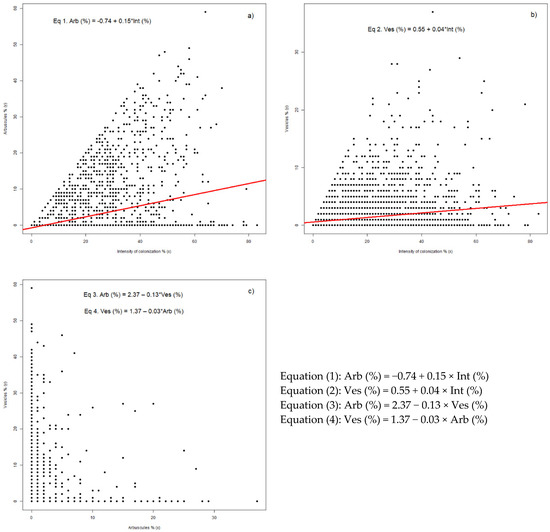
Figure 3.
Interdependency between colonization parameters in F. rubra involved in the direction of colonization strategy: (a) Arbuscules vs. Intensity of colonization; (b) Vesicles vs. Intensity of colonization; (c) Arbuscules vs. Vesicles. Legend: Int—colonization intensity; Arb—arbuscules abundance; Ves—vesicles abundance; * means multiply.
The vesicles development case is associated with the native potential of colonization (Figure 3b). The regression curve establishes the general value for this structure abundance up to 5%, with half of data associated. Based on the regression equation, there is a possibility to have 0.59% vesicles for each 1.00% of intensity. This value suggests that the colonization strategy is more oriented to storage than to transfer. An interesting observation is the increase of vesicles when compared to intensity of colonization: there is a parity between the two parameters of up to 10% for both, and the maintaining of this value for vesicles is independent to the increase in intensity. After this value, vesicles are sporadic, and not related to a specific intensity.
The arbuscules vs. vesicles development shows a continuous alteration of fungal strategies (Figure 3c). With values of up to 10% for arbuscules and only 5% for vesicles, both structures can occur in the same area. Over these values, the development of one structure is to the detriment of the other. Up to a 30% abundance, arbuscules can be associated with 1% vesicles, over this value vesicles are present only sporadically. As an opposite note, both structures are complementary up to 10% vesicle abundance and 1% arbuscules. A change in the fungal strategy was visible only in few isolate cases, where high values of both structures are present in the same area. By using vesicles as a descriptor for arbuscule presence, the general regression equation set the arbuscules presence at 2.37%, a value which is lowered by 0.13% for each 1.00% of vesicles. In this case, after 18.23% vesicles, the arbuscules could disappear from the colonized roots. The case of using arbuscules as a descriptor for vesicles set the base value at 1.37% vesicle presence. This value decreases by 0.03% for each 1.00% of arbuscules, which set the value of arbuscules abundance, concomitant with the absence of vesicles, at 45.67%. Up to this value, the storage strategy is still viable in the colonized areas of roots.
3.5. Mapping the Mycorrhizal Strategy in Long-Term Fertilized F. rubra
The large number of segments and the possibility to analyze them in-depth through MycoPatt allows the extraction maps with different colonization strategies (Figure 4a–e). Each of the treatments provide punctual changes in root colonization, with a segment direction toward a strategy.
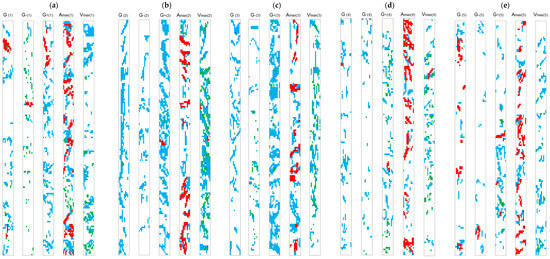
Figure 4.
Mycorrhizal patterns and fungal strategy in roots of F. rubra as shaped by long-term applied treatments. (a) V1 (control variant)—untreated; (b) V2—organic (manure) treatment—10 t ha−1 manure; (c) V3—low-mineral organic treatment—10 t ha−1 manure + 50 N 25 P2O5 25 K2O; (d) V4—mineral treatment—100 N 50 P2O5 50 K2O; (e) V5—high-mineral organic treatment—10 t ha−1 manure + 100 N 50 P2O5 50 K2O. Legend to color codes and colonization strategies: blue—hyphae; red—arbuscules; green—vesicles (as detailed in Table 2). Legend to map codding: (1–5)—treatments V1 to V5; G[1–5]—general median model; G−[1–5]−½ of general model; G+[1–5]—2× general median model; Amax[1–5]—maximum arbuscules model; Vmax[1–5]—maximum vesicles model.
The control variant shows five different colonization strategies (Figure 4a—G[1]). The median model of colonization presents an alternation of large areas colonized by mycorrhizas and areas where fungal structures are absent. Arbuscules are present together with vesicles, which sustain the plant’s need for a dual colonization strategy—a combined storage and transfer. However, there are areas where only vesicles are present, a phenomenon related to the perennial character of F. rubra, which implies the need for a reserve of nutrients. This model is generally oriented toward a propagative strategy, with the development of both transfer and storage structures. A decrease at the half frequency and intensity median (Figure 4a—G−[1]) shows a storage colonization strategy, which is assessed to occur in the least colonized fragments of the roots. This colonization map shows a homogeneous distribution of vesicles toward the roots, with a reduced punctual development and smaller distance between colonized areas. One interesting observation is related to arbuscule presence. These structures are located in only one area and present a punctual proliferation of a separate group of hyphae. An increase in frequency and intensity median up to a double value (Figure 4a—G+[1]) shows an opposite change in colonization strategy. In this context, a combined proliferative–transfer strategy was observed, which is possible in conditions of high values of both intensity and frequency. The colonization map shows an unbalanced development of hyphae and hyphae + arbuscules over the entire root, with a pole of arbuscules and the rest of the segment with both linear–ramified and punctual–irregular hyphal networks. Arbuscules are present in a very small proportion, outside the arbuscular area. The extraction of colonization maps based on maximum recorded arbuscularity permitted the extensive analysis of transfer strategy (Figure 4a—Amax[1]). The overall transfer strategy is based on irregular–linear development of both hyphae and arbuscules, with very small discontinuity areas. The visual arbuscules/hyphae presence report suggests an equilibrium of hyphae formation continued by the penetration of root cells to form arbuscules. One important observation is related to the presence of vesicles, with most of these structures separated by the areas where arbuscules are well developed. The use of maximum recorded vesicles development (Figure 4a—Vmax[1]) for the extraction of the transfer strategy map, shows a generally irregular hyphal development in colonized roots, followed by an irregular development of vesicles. This model present large discontinuities, with areas colonized only by hyphae, which suggests the formation of vesicles as a secondary structure in the entire colonization process and only after the colonization of a larger area.
The median colonization map as a response to organic treatments shows a proliferative colonization strategy (Figure 4b—G[2]), this model being completed by a small amount of storage structures. The entire hyphal network has a longitudinal development, toward the length of the root. There are present only few discontinuity areas and arbuscules are absent. By decreasing the colonization model to half of the frequency and intensity median, the entire colonization map shows a thumbnail strategy performed by the symbiotic fungus (Figure 4b—G−[2]). There is a mix between linear and irregular–radial development of hyphae in roots, with large discontinuities between mycorrhized areas. This small-scale strategy is oriented toward proliferation, but without the formation of both arbuscules and vesicles. The use of organic treatment stimulates a high-scale proliferative colonization strategy of mycorrhiza (Figure 4b—G+[2]). The high median values of frequency and intensity show large areas colonized by hyphae, with an irregular development around multiple points and small discontinuity areas between colonized ones. Arbuscules are present, in a very small proportion and localized in only one area. Vesicles are present in a double proportion and are scattered in multiple points inside the root. The maximum value of arbuscules shows a different colonization map for this treatment (Figure 4b—Amax[2]). These structures are developed around root areas with an irregular development of hyphae. An alternation of colonized and uncolonized areas, with the presence of areas where only hyphae are developed, was observed. In the absence of arbuscules, hyphal development is linear along root length.
The highest level of storage strategy is associated with the maximum of recorded vesicles (Figure 4b—Vmax[2]). Linear hyphal development is completed by lateral branching with the development of vesicles. In this colonization strategy, vesicles appear in groups and more rarely solitary. Arbuscules are present in a very small proportion, but their development is outside the areas where vesicles are present. Discontinuity areas have a reduced dimension, hyphae being connected along almost entire root.
Due to the combination of organic with low-mineral fertilizers, a change in the median colonization patterns was observed (Figure 4c—G[3]). Hyphal development is both transversal to the root as a linear intersection with lateral branched hyphae. Vesicles are present in a small proportion and high dimension non-colonized areas alternate with colonized ones. The entire model shows a potential confluence of adjacent colonized areas. In the context of reduced frequency and intensity, by half of their median (Figure 4c—G−[3]), an increase in the presence of vesicles was observed. For this case, the strategy of development is promoted by resistance conditions, which is related to first stages of colonization. The colonization model described by the increased values of frequency and intensity shows a good image of a storage colonization strategy (Figure 4c—G+[3]). There are still visible the general development patterns of hyphae, with an increased number of vesicles spread over all colonized areas. The connections between different colonized areas are visible and for the isolated ones, an irregular round development was observed. The transfer strategy associated with this treatment shows both the presence of arbuscules on linear developed hyphae and on irregularly developed ones (Figure 4c—Amax[3]). Arbuscules tend to appear in large groups, rarely solitary. This phenomenon suggests that solitary arbuscules represent an area of root cells with a high availability for hyphal penetration and arbuscules will be further developed. The maximum value of vesicles, in areas of root where storage strategy is prevalent, shows a more linear development of hyphae and the vesicles intercalated between these lines (Figure 4c—Vmax[3]). The entire model of storage strategy shows a homogeneous distribution of vesicles, the interconnections between colonized areas, and the presence in a small proportion of fully non-colonized areas. The high availability of nutrients from the mineral treatment induce a general median model of a strategy developed in resistance conditions (Figure 4d—G[4]). Both arbuscules and vesicles are present, but in a very small proportion. Large, non-colonized areas create high discontinuities between the colonized ones. Both the linear and round irregular colonization patterns are present.
In the case of frequency and intensity reductions, a more intense resistance for colonization is visible (Figure 4d—G−[4]). In this case, the fungal partner is stimulated to produce vesicles, as storage structures. The non-colonized areas occupy a large share of root. Even at high values of frequency and intensity, the resistance of root to the development of mycorrhizas is highly visible (Figure 4d—G+[4]). The hyphal development model is mostly irregular, with a reduction of the dimension of non-colonized areas. Both vesicles and arbuscules are present, with the highest share of vesicles. Arbuscules are located in one place when compared to the homogeneous distribution of vesicles. The mechanism of arbuscules’ development indicates a powerful transfer strategy (Figure 4d—Amax[4]). The appearance of these structures in the colonization map is heterogeneous, all being developed around hyphal clusters. The dimension of non-colonized areas is reduced, indicating an extension potential of mycorrhizas in the entire root segment. The mineral treatment restricted the storage strategy of mycorrhizas to a small-scale development (Figure 4d—Vmax[4]). Large discontinuities are present between colonized areas and an irregular pattern of punctual development is visible. Hyphal networks have a limited extension and arbuscules are present, even if in a small share.
Application of high-mineral organic treatment reduced drastically the median extension of fungal partners in colonized roots (Figure 4e—G[5]). The overall colonization strategy for this case is oriented toward an increased transfer, with half of the visible structures being arbuscules. Most hyphae are developed in clusters, with an irregular extension and a heterogeneous distribution. The development of arbuscules starts very quickly after the appearance of hyphae. Non-colonized areas have a large proportion in these roots and act as a segmentation between colonized ones. The transfer strategy is still visible at low values of frequency and intensity, but is restricted by the highly resistant strategy (Figure 4e—G−[5]). Hyphae are present only in small groups, separated by large non-colonized areas. At high values of frequency and intensity, both arbuscules and vesicles are present (Figure 4e—G+[5]). A triple strategy was observed: small areas characterized by a transfer strategy and development of arbuscule groups; vesicles appear in numerous groups, associated with hyphae; hyphae are grouped and irregularly developed—non-colonized areas surround small hyphal clusters. The transfer strategy is clearly visible at the maximum observed arbuscules (Figure 4e—Amax[5]). Three types of areas could be identified in these root segments: areas where all or almost all hyphae develop further into arbuscules; areas with a balanced association between hyphae and arbuscules; areas where only hyphal groups were observed. The entire roots segment is characterized by a lateral–irregular development of both structures. The maximum number vesicles are present near to the limit of resistance condition strategy (Figure 4e—Vmax[5]). Vesicles appeared to be associated with irregular developed hyphae, in small groups, heterogeneously distributed across the root segment. Non-colonized areas have a similar pattern and split the colonized areas across the entire root.
4. Discussion
4.1. Mycorrhizal Structure Development in Roots of F. rubra Shaped by Long-Term Treatments
Long-term application of each treatment induces a different distribution pattern of colonization parameter values. An interesting phenomenon was observed for the control variant, as well as the mineral and high-mineral organic ones. A group of one-third of their analyzed root samples presented an incipient colonization, which is related to two different root mechanisms. The one from control variant is based on root growth for nutrient acquisition, which occurs at a higher speed than the potential growth of fungal symbionts. The mechanism of high treated variants (V4 and V5) shows a reduction of root permissiveness for mycorrhizas due to the higher amount of available nutrients. Low input-treated variants (V2 and V3) show a more balanced colonization frequency, which implies a reduction of root growth completed by an increase in permissiveness for fungal colonization. Arbuscular mycorrhizas are widespread in the grassland ecosystem and are particularly relevant as they can act as natural fertilizers and increase plant yield. Low organically fertilized soils have a high potential to maintain active mycorrhizal services, toward keeping the soil fertile and productive [52]. Also, these fungi can function in ecosystems as a sustainable biocontrol agent against pathogens, a bioprotection against toxicity, and as an anti-erosional solution [53]. Long-term fertilization, especially with high levels of manure and N, lead to a decline in species richness in the fungal community, along with spore density and colonization potential [54]. The low input conditions restrict the number of arbuscular mycorrhizal species to a small group that show a large range of hosts, with an increase in mutualistic potential and a more important share in the stability and productivity of hosts [55,56,57].
The divergence between treatments, based on differences observed in colonization frequency, were amplified when the intensity of colonization was analyzed. Low-treated variants present a higher root permissiveness for hyphal development than the ones observed in control and high-treated ones. This indicates that mycorrhizal spots in the roots of first ones develops continuously, with lower fluctuations along root length. Organic amendments increase the production of spores, provide extra radical proliferation of hyphae, and improve root colonization [58]. Both arbuscules and vesicles are structures related to available nutrients and root permissiveness. Arbuscules indicate a deep, intimate contact between plant and fungal symbionts, and an increased and efficient exchange of substances. The maximum development potential of these structures, observed in our experiment indicates at least a 10% reduction between variants. Control variant have a reduced permissiveness due to the nutrient acquisition strategy and the necessity for growth in oligotrophic conditions. The similar value observed in the mineral-treated variant indicates the reduced permissiveness due to the root access to soluble nutrients. Both situations act as a shock event for the success of colonization. All the variants that have organic treatments show an increased potential for arbuscule development, due to the balanced rate of nutrient release, which sustains a smoother development of fungal structures in roots. Vesicles, which are associated with nutrient deposits, indicate the reduction of plant necessities for fungal storage. Plants grown in variants with high mineral treatments have a reduced necessity for nutrient storage. For these structures, both the control and low-mineral organic (V3)-treated variants have a similar strategy. The control required higher deposit rates due to the oligotrophic conditions, which act as a nutritional imbalance, while in the case of V3, vesicle formation was stimulated by the slower release of nutrients from organic source. This mechanism is amplified in the roots of plants unilaterally fertilized with manure. Our results are in line with the results of Porras-Alfaro et al. [59], where long-term nitrogen fertilization has led to decreased root colonization in a dominant grass species as well as a lack of fungal-specific structures. Mineral fertilization in grasslands induces a negative effect on root colonization in dominant species [60]. Their results show that the percentage of vesicles is negatively affected by the amount of nitrogen [61]. Changes in vesicle abundance may be caused by changes in the fungal community, as Scutellospora and Gigaspora species do not form vesicles, while Glomus and Acaulospora do [62]. The tillage system can improve the productivity of organic farming through the pronounced colonization of arbuscular mycorrhizal roots [63]. Amaya-Carpio et al. [64] concluded in an experiment with organic fertilization that the fungal community provided plants with a higher amount of nutrients by facilitating phosphatase activity, which led to a higher yield of photosynthesis and thus improving the growth of plants. Long-term fertilization with NPK has negative effects on the fungal community, which can be attributed to nutrients that are directly accessible to plants. But at high concentrations of these elements, there may be differences in pH that give rise to other blocking elements, such as Al, so there may be positive fluctuations for the acceptance of the symbiont, which is also supported by Cassman et al. [65].
Non-mycorrhizal areas show the two opposite conditions that can lead to an altered colonization process: the lack of nutrients, which implies root development as a continuous search for more efficient partners and the maintenance of non-colonized areas for the new symbionts; or the higher availability of nutrients, in which case the plant will block the proliferation of fungal partners. In the case of slowly released nutrients, the colonization is more balanced and non-mycorrhizal areas show a smoother pattern of distribution with our observations. The overall phenomenon is related to the capacity of mycorrhizas to improve nutrient acquisition and sustainability of host growth, reducing the need of the host for high quantities of external nutrients, and maintaining a balanced and constant flux of nutrients [66,67]. The report of mycorrhizal/non-mycorrhizal areas is the most suggestive indicator of colonization. Based on observed values and environmental traits, it can be related to root permissiveness, discontinuities in colonization process, the identification of areas of high mycorrhizal proliferation potential, the response to external pressure, or even the change in symbiotic status toward a parasitic form. Experimental observations revealed that 80–90% of the roots have a maximum of 35% the area allocated for colonization. A value of 0.5 of this report is associated with a 33–34% in colonization intensity, which can grow up to a value of 1.0 when half of root is colonized. Up to this value, there are large discontinuities in colonization, or the hyphal network is weakly branched, or unbranched, and extended parallel with root length. Values of this report between 1.0 and 2.5 correspond to an intensity of colonization between 51 and 71%, which indicates an amplified effect on root economy with each 1% increase in intensity. After this level of intensity, increases in report are lower, with 0.5 (rep = 2.5–3.0) allocated for a 5% increase in intensity, and an equal increase in intensity for 1.0 of report (between 3.0 and 4.0) between 4.0 and 5.0. Values of this report above 5.0 are correlated with highly proliferative areas, a phenomenon that can be observed in early stages of root growth, a change from symbiont to parasite in fungal component, a decrease in plant health, or a root system developed by an invasive species which connects to existent hyphal network from soil. Mineral fertilization reduced the community of mycorrhizal fungi, while organic fertilization led to an increase in the fungal component [68]. At the level of structural development, increases in fertilizer stimulate a higher allocation of resources to a mycorrhizal storage mechanisms [69]. An increase in nutrient availability stimulates the development of roots, but concomitantly acts toward a reduction of colonization potential [70,71].
4.2. Heterogeneity of Mycorrhizal Development due to Long-Term Applied Treatments in F. rubra
The addition of low dose of mineral fertilizers to organic treatment does not alter greatly the colonization frequency when compared to the unilateral application of the organic one. This supplement is important for the increase in biomass production, and it will act as a low-input solution that does not affect the overall frequency of colonization. The native colonization frequency is set to 44.5% of the total roots; the application of low-input treatments (V2 and V3) will increase this parameter by almost 10%. Both the mineral treatment (V4) and high-mineral organic one (V5) act as intensive inputs and decrease gradually, with 5% the colonization frequency. As colonization frequency decrease along with intensification in treatments, the potential extension of colonizing hyphae is reduced. This phenomenon results in a higher number of areas of roots where the colonization is blocked or slowed down. The future extension of hyphae for the development of arbuscules is also restricted, with less than 4% on average of intercellular hyphae able to penetrate root cells. Vesicles show an interesting mechanism of colonization, with high values in the case of organic fertilized variant (V2), where the nutrients are slowly released, and they stimulate their formation. The next position is occupied by the control variant, where the reduced availability of nutrients and the dominant character of the analyzed species sustain the storage of nutrients. Along with the intensification of treatments, this structure is formed in less than 1% of the colonized areas. The average volume of the roots that can be natively colonized by mycorrhizas is only 12.3%. As the colonization degree presents the product between frequency and intensity, it set the maximum available volume to be colonized. Also, it presents the prolificacy of fungal symbionts to extend in roots and the depth of the colonization process. Overall, roots have an average of 15–25% of colonization acceptance, with increases in non-colonized areas in higher treated variants. The mycorrhizal/non-mycorrhizal areas report shows a colonization restricted to less than 30% of the entire root, with higher values in the case of low-input treatments. The native permissiveness of roots for symbionts is 20–21% and decreases drastically with 5% when intensification occurs. This report is more sensitive to changes produced by fertilizer gradients, and it also explains the magnitude of even 1% modification in intensity in the entire economy of colonization. For the interval of 0–50% colonization intensity, this report shows small increases, which are associated with a generally balanced colonization process sustained by both partners. After 50% of colonization intensity, the values of this report increase with unequal steps, which is associated with a proliferative stage of mycorrhizas or to a plant anomaly produced by a decrease in health or a critical necessity for nutrients. The application of organic manure was the most important factor that influenced the composition of the fungal community between the samples with and without organic manure, followed by fertilizer N and fertilizer inputs P [72]. In this sense, the literature indicates that in sites with a phosphorus and nitrogen deficiency, plant species are highly mycorrhizal, and mycorrhizae act as a biological solution to satisfy nutrients [73]. The lack of time-series data bases, focused on specific AM intraradical structures and representative for a specific climate, plant, and soil, makes it very difficult to interpret the significance of hyphae, arbuscules, and vesicles proportions in roots as reported in various studies [74].
The correlation value between frequency and colonization degree indicates a constant colonizing potential rather than the development of already colonized areas and the extension of existing hyphae. This is also consistent with the number of mycorrhizal spots resulting from multiple penetration points or the growth of roots, which results in fragmentation of hyphal networks. The higher correlation between colonization intensity and arbuscules when compared to vesicles indicates a 2–3 times higher investment of fungal symbiont in transfer structures than in storage ones. This phenomenon is related to the oligotrophic conditions in studied area, where plant is forced to accept the formation of arbuscules for a higher transfer rate of nutrients, which is necessary for species’ dominance in grassland. Vesicle production may be related to the species with a lower abundance and slower metabolism, which will result in a necessity for stored nutrients. The most important correlation analyzed is between arbuscules and vesicles, the negative sign of this relation indicating a geographical separation between the two structures. Even though both are developed on the same hyphal network, their presence in different areas can be traced and used as a historical analysis of root traits and development. Based on arbuscules and vesicles position, the entire root development can be traced back, with indications of period with higher transfer rates or the storage ones and that can be further used to create the life cycle of colonization from emergence to a specific point.
4.3. Long-Term Application of Treatments in F. rubra Induce Multiple Punctual Colonization Strategies
Native mycorrhizal colonization of perennial plants implies a more sustained vesicle development, simultaneous with the frequency of colonization. For this case, the development of arbuscules is conditioned by higher values of colonization intensity. Based on PCA analysis, the arbuscules/vesicles report begin to have a sense only when arbuscules have higher values when compared to vesicles. The development of vesicles is a more homogeneous process related to the natural character of colonization, compared to arbuscule development, which is a punctual, heterogeneous process. For the root parts with a colonization degree between 0 and 10%, the development of fungal structures is oriented for both arbuscules and vesicles. After this value, a more specialized structure development is visible with a lower correlation between one structure and colonization degree. The same value of maximum colonization degree (55%) is achieved in control and high-mineral organic treatment. The application of organic and low-mineral organic treatments establishes the maximum value of this parameter at 70%, and +10% more for the mineral treatment. Any treatment applied stimulates a deviation from the native mycorrhizal colonization process. The data associated with higher vesicles abundance, which is characteristic to the native colonization, have a regular display on PCA. The higher arbuscule abundance stimulates a higher dispersion of data, which is associated with a higher fungal activity and acceptance of symbionts by plants. The conversion of results in a biological mechanism shows that the organic treatment implies a decomposition process prior to the release of nutrients. This treatment provides a good biological basis for the proliferative strategy of mycorrhizal development and colonization, and shows a good development of fungal component along the entire root. The slow decomposition rate of manure, which implies a slow release of nutrients in soil, decreases the nutritional flux in soil and restricts the formation of arbuscules. It is a process that oscillates in time, which favors the development of vesicles as a storage option for the periods with higher amounts of nutrients than the plants can accept [75]. The mixture between high-mineral doses and organic treatments acts as a restrictor for colonization degree but stimulates the punctual development of arbuscules.
Arbuscules and vesicles appear only at high frequency and intensity values, which sustain their secondary structure characteristics. Based on observed values, in the organic treatment context, arbuscules and vesicles can be developed only after a powerful proliferation of fungus in the root. This phenomenon implies the development of a stable and large hyphal system, before the development of storage or transfer strategies. The general native trend in the development of both arbuscules and vesicles is characterized by a 2:1 report in roots [76]. The model of hyphal development is linear along root length. The formation of arbuscules implies a change in this development toward an irregular one, which sustains the colonization strategy of arbuscule formation on branched hyphae. Fungal symbiotic species are important drivers of arbuscule development related to colonization rate, varying largely in reports from 1:3 up to 1:7, in the case of F. rubra [77]. The development of abundant vesicles implies a change in colonization strategy of fungal partners, from a proliferative to a storage-based one. Habitat also acts as a restrictor in the formation of one or both of these structures, up to the total elimination of one of them from roots [78]. The resistance conditions strategy stimulates the appearance of vesicles, a colonization pattern related to the need of fungal partner to deposit the excess of nutrients up to the development of the fungal network. The absence of vesicles indicates a clear strategy selection by fungal partners, which is oriented either to arbuscules’ production—transfer strategy—or to vesicles’ production—storage strategy. Plant root acts differently according to its length, with areas where cells permit the penetration and arbuscules formation and areas where the penetration is blocked, and vesicles are developed intercellularly. This phenomenon shows the importance of non-colonized areas as a buffer between different strategies, which permit the appearance of an opposite colonization strategy. Previous research has described the colonization strategy from the perspective of hyphae (length, diameter, etc.) or infectious propagules (hyphae, spores, and vesicles) [79,80], which suggests the continuous changes in arbuscules/vesicles report and presence in roots.
The mineral fertilizer favors resistance to colonization. In this case, the fungal partner produces limited amounts of hyphae and vesicles. These storage structures will act as a support for the development of future hyphal networks, after the effect of resistance to colonization is mitigated. For this treatment, in the root segments where transfer strategy occurs, there is a dominance of the fungal partner over the entire area. As an opposite note, the vesicle development potential is restricted and only at a low level can a storage strategy be identified, with numerous discontinuities between colonized areas. In root areas where transfer strategy is dominant, the storage strategy is completely restricted. The opposite case is not valid, the storage strategy only restricting the presence of the transfer strategy. At high arbuscule levels, the transfer strategy debuts immediately after the proliferative stage. In these root segments, a succession of fungal strategies can be observed: proliferative—proliferative + transfer—transfer strategy. For the maximum vesicle development, a short succession from resistance condition to small-scale storage strategy can be observed. The potential expansion of hyphae and vesicles is reduced, which suggests the character of storage for these areas. A clear separation is visible between root segments, where a high amount of arbuscules are formed and the segments where vesicles are present.
5. Conclusions
The use of root mycorrhizal pattern method permits a deep and realistic analysis of fungal colonization in F. rubra, with large database construction and the possibility to assess colonization strategies. Organic and low-mineral organic treatments increase the native mycorrhizal potential by 10% when compared to native status, in contrast to mineral and high-mineral treatments, which acts with a 5 and 10% reduction, respectively. Arbuscules and vesicles have the highest presence in roots treated with organic inputs, sustained by a 0.39 mycorrhizal/non-mycorrhizal areas report. The use of the intensity–nonmycorrhizal areas cumulative vector, along with colonization degree isolines, enabled a good analysis of PCA rotation and the dispersion of data due to treatment specificity. Colonization strategy is a highly visible phenomenon on mycorrhizal maps and allows the full exploration of fungal structural development. The native colonization strategy varies from proliferative to both transfer and storage ones. The application of organic treatment stimulates the expansion of arbuscules in root segment oriented to a transfer strategy, but in the presence of vesicles, this strategy is overcome by a storage one. Mineral fertilizers induce a colonization strategy in resistance conditions, with large uncolonized areas and a reduced potential of vesicles development. As the analysis of colonization goes deeper, there are visible clear and distinct fungal colonization strategies, which turn the entire root in a heterogeneous assemblage of segments with different functionalities and symbiotic permissiveness.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy12030650/s1, Figure S1: Details of mycorrhizal colonization and structures in F. rubra roots, for each experimental variant: (a) V1 (control variant)—untreated; (b) V2—organic (manure) treatment—10 t ha−1 manure; (c) V3—low-mineral organic treatment—10 t ha−1 manure + 50 N 25 P2O5 25 K2O; (d) V4—mineral treatment—100 N 50 P2O5 50 K2O; (e) V5—high-mineral organic treatment—10 t ha−1 manure + 100 N 50 P2O5 50 K2O; Figure S2: Histograms of colonization parameters in roots of F. rubra for each experimental variant: (a–e) arbuscules abundance (%); (f–j) vesicles abundance (%); (k–o) colonization degree; (p–t) non-mycorrhizal areas (%). Treatments V1–V5: V1 (control variant)—untreated; V2—organic (manure) treatment—10 t ha−1 manure; V3—low-mineral organic treatment—10 t ha−1 manure + 50 N 25 P2O5 25 K2O; V4—mineral treatment—100 N 50 P2O5 50 K2O; V5—high-mineral organic treatment—10 t ha−1 manure + 100 N 50 P2O5 50 K2O; Table S1: Pearson correlation between mycorrhizal parameters as influenced by long-term treatments.
Author Contributions
Conceptualization, L.C., V.S. and R.V.; methodology, L.C., F.P., I.V., A.P. and C.M.; software, C.M. and V.S.; validation, F.P., I.V. and A.P.; formal analysis, L.C., V.S. and R.V.; investigation, L.C., C.M., V.S. and R.V.; data curation, L.C., F.P., I.V. and A.P.; writing—original draft preparation, L.C., F.P., I.V., A.P., C.M., V.S. and R.V.; writing—review and editing, L.C., F.P., I.V., A.P., C.M., V.S. and R.V.; visualization, L.C., F.P., I.V. and A.P.; supervision, V.S. and R.V.; L.C., F.P., C.M., V.S. and R.V. contributed equally to this paper, all being considered first authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Acknowledgments
This paper is part of a PhD study in the thematic area of Mycorrhizal Status and Development of Colonization in Mountain Grassland Dominant Species, conducted by the first author L.C., under the coordination of Roxana Vidican (R.V.).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gibson, D.J. Grasses and Grassland Ecology; OUP: Oxford, UK, 2008; ISBN 978-0-19-154609-9. [Google Scholar]
- Dornak, L.L.; Barve, N.; Peterson, A.T. Spatial Scaling of Prevalence and Population Variation in Three Grassland Sparrows. Condor 2013, 115, 186–197. [Google Scholar] [CrossRef]
- Sângeorzan, D.; Rotar, I.; Pacurar, F.; Ioana, V.; Alina, S.; Valeria, D. The definition of oligotrophic grasslands. Rom. J. Grassl. Forage Crops 2018, 2018, 17. [Google Scholar]
- Veresoglou, S.D.; Rillig, M.C.; Johnson, D. Responsiveness of Plants to Mycorrhiza Regulates Coexistence. J. Ecol. 2018, 106, 1864–1875. [Google Scholar] [CrossRef]
- Sushma; Verma, R.K.; Thakur, S.; Singh, H.; Kapur, D. Chapter 6—The Role of Fungi in Abiotic Stress Tolerance of Plants. In Fungi Bio-Prospects in Sustainable Agriculture, Environment and Nano-Technology; Sharma, V.K., Shah, M.P., Parmar, S., Kumar, A., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 117–154. ISBN 978-0-12-821394-0. [Google Scholar]
- Pozo, M.J.; Azcón-Aguilar, C. Unraveling Mycorrhiza-Induced Resistance. Curr. Opin. Plant Biol. 2007, 10, 393–398. [Google Scholar] [CrossRef]
- Tian, L.; Zou, Y.-N.; Wu, Q.-S.; Kuča, K. Mycorrhiza-Induced Plant Defence Responses in Trifoliate Orange Infected by Phytophthora Parasitica. Acta Physiol. Plant 2021, 43, 45. [Google Scholar] [CrossRef]
- Jongen, M.; Albadran, B.; Beyschlag, W.; Unger, S. Can Arbuscular Mycorrhizal Fungi Mitigate Drought Stress in Annual Pasture Legumes? Plant Soil 2022. [Google Scholar] [CrossRef]
- Fu, W.; Chen, B.; Rillig, M.C.; Jansa, J.; Ma, W.; Xu, C.; Luo, W.; Wu, H.; Hao, Z.; Wu, H.; et al. Community response of arbuscular mycorrhizal fungi to extreme drought in a cold-temperate grassland. New Phytol. 2021. [Google Scholar] [CrossRef]
- Ilyas, M.; Nisar, M.; Khan, N.; Hazrat, A.; Khan, A.H.; Hayat, K.; Fahad, S.; Khan, A.; Ullah, A. Drought Tolerance Strategies in Plants: A Mechanistic Approach. J. Plant Growth Regul. 2021, 40, 926–944. [Google Scholar] [CrossRef]
- Riaz, M.; Kamran, M.; Fang, Y.; Wang, Q.; Cao, H.; Yang, G.; Deng, L.; Wang, Y.; Zhou, Y.; Anastopoulos, I.; et al. Arbuscular Mycorrhizal Fungi-Induced Mitigation of Heavy Metal Phytotoxicity in Metal Contaminated Soils: A Critical Review. J. Hazard. Mater. 2021, 402, 123919. [Google Scholar] [CrossRef]
- Rasheed, A.; Hassan, M.U.; Fahad, S.; Aamer, M.; Batool, M.; Ilyas, M.; Shang, F.; Wu, Z.; Li, H. Heavy Metals Stress and Plants Defense Responses. In Sustainable Soil and Land Management and Climate Change; CRC Press: Boca Raton, FL, USA, 2021; ISBN 978-1-00-310889-4. [Google Scholar]
- Zhou, J.; Wilson, G.W.T.; Cobb, A.B.; Zhang, Y.; Liu, L.; Zhang, X.; Sun, F. Mycorrhizal and Rhizobial Interactions Influence Model Grassland Plant Community Structure and Productivity. Mycorrhiza 2022, 32, 15–32. [Google Scholar] [CrossRef]
- Cardoso, I.M.; Kuyper, T.W. Mycorrhizas and Tropical Soil Fertility. Agric. Ecosyst. Environ. 2006, 116, 72–84. [Google Scholar] [CrossRef]
- Moora, M.; Zobel, M. Arbuscular Mycorrhizae and Plant–Plant Interactions Impact of Invisible World on Visible Patterns. In Positive Plant Interactions and Community Dynamics; Taylor & Francis Group: Boca Raton, FL, USA, 2010; pp. 79–98. ISBN 978-1-4398-2495-5. [Google Scholar]
- Asmelash, F.; Bekele, T.; Birhane, E. The Potential Role of Arbuscular Mycorrhizal Fungi in the Restoration of Degraded Lands. Front. Microbiol. 2016, 7, 1095. [Google Scholar] [CrossRef] [PubMed]
- Bender, S.F.; van der Heijden, M.G.A. Soil Biota Enhance Agricultural Sustainability by Improving Crop Yield, Nutrient Uptake and Reducing Nitrogen Leaching Losses. J. Appl. Ecol. 2015, 52, 228–239. [Google Scholar] [CrossRef]
- Wilby, A.; Orwin, K.H. Herbivore Species Richness, Composition and Community Structure Mediate Predator Richness Effects and Top-down Control of Herbivore Biomass. Oecologia 2013, 172, 1167–1177. [Google Scholar] [CrossRef]
- Li, H.; Xu, Z.; Yan, Q.; Yang, S.; Van Nostrand, J.D.; Wang, Z.; He, Z.; Zhou, J.; Jiang, Y.; Deng, Y. Soil Microbial Beta-Diversity Is Linked with Compositional Variation in Aboveground Plant Biomass in a Semi-Arid Grassland. Plant Soil 2018, 423, 465–480. [Google Scholar] [CrossRef]
- Grigulis, K.; Lavorel, S.; Krainer, U.; Legay, N.; Baxendale, C.; Dumont, M.; Kastl, E.; Arnoldi, C.; Bardgett, R.D.; Poly, F.; et al. Relative Contributions of Plant Traits and Soil Microbial Properties to Mountain Grassland Ecosystem Services. J. Ecol. 2013, 101, 47–57. [Google Scholar] [CrossRef]
- Gois, L.D.S.; Mendonça, J.D.J.; Teixeira, J.L.; Prado, C.M.D.O.; Holanda, F.S.R.; Marino, R.H. Exotic arbuscular mycorrhizal fungi and native dark septate endophytes on the initial growth of Paspalum Millegrana GRASS. Rev. Caatinga 2019, 32, 607–615. [Google Scholar] [CrossRef]
- Wilkes, T.I. Arbuscular Mycorrhizal Fungi in Agriculture. Encyclopedia 2021, 1, 1132–1154. [Google Scholar] [CrossRef]
- Willis, A.; Rodrigues, B.F.; Harris, P.J.C. The Ecology of Arbuscular Mycorrhizal Fungi. Crit. Rev. Plant Sci. 2013, 32, 1–20. [Google Scholar] [CrossRef]
- Jane Barker, S.; Tagu, D.; Delp, G. Regulation of Root and Fungal Morphogenesis in Mycorrhizal Symbioses1. Plant Physiol. 1998, 116, 1201–1207. [Google Scholar] [CrossRef]
- Genre, A.; Bonfante, P. The Making of Symbiotic Cells in Arbuscular Mycorrhizal Roots. In Arbuscular Mycorrhizas: Physiology and Function; Koltai, H., Kapulnik, Y., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 57–71. ISBN 978-90-481-9489-6. [Google Scholar]
- Gollotte, A.; van Tuinen, D.; Atkinson, D. Diversity of Arbuscular Mycorrhizal Fungi Colonising Roots of the Grass Species Agrostis Capillaris and Lolium Perenne in a Field Experiment. Mycorrhiza 2004, 14, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Parniske, M. Mycorrhiza: The Mother of Plant Root Endosymbioses|Nature Reviews Microbiology. Available online: https://www.nature.com/articles/nrmicro1987 (accessed on 28 February 2022).
- Roth, R.; Chiapello, M.; Montero, H.; Gehrig, P.; Grossmann, J.; O’Holleran, K.; Hartken, D.; Walters, F.; Yang, S.-Y.; Hillmer, S.; et al. A Rice Serine/Threonine Receptor-like Kinase Regulates Arbuscular Mycorrhizal Symbiosis at the Peri-Arbuscular Membrane. Nat. Commun. 2018, 9, 4677. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: Cambridge, MA, USA, 2008; ISBN 978-0-08-055934-6. [Google Scholar]
- Ohtomo, R.; Kobae, Y.; Morimoto, S.; Oka, N. Infection Unit Density as an Index of Infection Potential of Arbuscular Mycorrhizal Fungi. Microbes Environ. 2018, 33, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Soka, G.E.; Ritchie, M.E. Arbuscular Mycorrhizal Spore Composition and Diversity Associated with Different Land Uses in a Tropical Savanna Landscape, Tanzania. Appl. Soil Ecol. 2018, 125, 222–232. [Google Scholar] [CrossRef]
- Vance, C.P. Symbiotic Nitrogen Fixation and Phosphorus Acquisition. Plant Nutrition in a World of Declining Renewable Resources. Plant Physiol. 2001, 127, 390–397. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Introduction. In Mycorrhizal Symbiosis, 2nd ed.; Academic Press: Cambridge, MA, USA, 1997; pp. 1–8. [Google Scholar] [CrossRef]
- Qu, L.; Wang, M.; Biere, A. Interactive Effects of Mycorrhizae, Soil Phosphorus, and Light on Growth and Induction and Priming of Defense in Plantago Lanceolata. Front. Plant Sci. 2021, 12, 647372. [Google Scholar] [CrossRef]
- Grant, C.; Bittman, S.; Montreal, M.; Plenchette, C.; Morel, C.; Bittman, C.; Montreal, S. Soil and Fertilizer Phosphorus: Effects on Plant P Supply and Mycorrhizal Development. Can. J. Plant Sci. 2005, 85, 3–14. [Google Scholar] [CrossRef]
- Vaida, I.; Păcurar, F.; Rotar, I.; Tomoș, L.; Stoian, V. Changes in Diversity Due to Long-Term Management in a High Natural Value Grassland. Plants 2021, 10, 739. [Google Scholar] [CrossRef]
- Vaida, I. The Influence of Management on the Agronomic and Ecological Value of Mountain Grasslands. Ph.D. Thesis, University of Agricultural Sciences and Veterinary Medicine, Cluj-Napoca, Romania, 2018. Available online: https://rei.gov.ro/ (accessed on 10 November 2021).
- Stoian, V.; Vidican, R.; Rotar, I.; Păcurar, F. Mycorrhizal Induced Domination of Festuca Rubra in Grasslands. Rom. J. Grassl. Forage Crop. 2016, 13, 77–84. [Google Scholar]
- Corcoz, L.; Păcurar, F.; Pop-Moldovan, V.; Vaida, I.; Stoian, V.; Vidican, R. Mycorrhizal Patterns in the Roots of Dominant Festuca Rubra in a High-Natural-Value Grassland. Plants 2021, 11, 112. [Google Scholar] [CrossRef]
- Stoian, V.H.; Florian, V. Mycorrhiza–Benefits, Influence, Diagnostic Method. Bull. UASMV Agric. 2009, 66, 2009. [Google Scholar] [CrossRef]
- Available online: https://www.pelikan.com/int/products/writing/supplies-accesories/156-inks/125-pelikan-4001-ink-glass-jar.html (accessed on 4 February 2022).
- Stoian, V.; Vidican, R.; Crişan, I.; Puia, C.; Şandor, M.; Stoian, V.A.; Păcurar, F.; Vaida, I. Sensitive approach and future perspectives in microscopic patterns of mycorrhizal roots. Sci. Rep. 2019, 9, 10233. [Google Scholar] [CrossRef] [PubMed]
- RStudio Team. RStudio: Integrated Development Environment for R. RStudio Inc.; RStudio Team: Boston, MA, USA, 2019. [Google Scholar]
- RCore Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Revelle, W. Psych: Procedures for Psychological, Psychometric, and Personality Research, R Package Version 1.9.12; Northwestern University: Evanston, IL, USA, 2019. [Google Scholar]
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. 2019. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 10 November 2021).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legrende, P.; McGlinn, D.; Wagner, H. 633 Vegan: Community Ecology Package, R Package Version 2.5-2. 2019. Available online: https://CRAN.R-project.org/package=vegan (accessed on 10 November 2021).
- Vidican, R.; Păcurar, F.; Vâtcă, S.D.; Pleșa, A.; Stoian, V. Arbuscular mycorrhizas traits and yield of winter wheat profiled by mineral fertilization. Agronomy 2020, 10, 846. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Vâtcă, S.; Vidican, R.; Gâdea, Ș.; Horvat, M.; Vâtcă, A.; Stoian, V.A.; Stoian, V. Blackcurrant Variety Specific Growth and Yield Formation as a Response to Foliar Fertilizers. Agronomy 2020, 10, 2014. [Google Scholar] [CrossRef]
- Stoian, V.; Vidican, R.; Rotar, I.; Păcurar, F.; Morea, A. Mycorrhizas in Trifolium Repens—A Short Term High Experiment Approach. Agric. Agric. Sci. Procedia 2016, 10, 39–46. [Google Scholar] [CrossRef]
- Mahmood, I.; Rizvi, R. Mycorrhiza and Organic Farming. Asian J. Plant Sci. 2010, 9, 241–248. [Google Scholar] [CrossRef][Green Version]
- Vosátka, M.; Albrechtová, J.; Patten, R. The International Market Development for Mycorrhizal Technology. In Mycorrhiza: State of the Art, Genetics and Molecular Biology, Eco-Function, Biotechnology, Eco-Physiology, Structure and Systematics; Varma, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 419–438. ISBN 978-3-540-78826-3. [Google Scholar]
- Wang, M.-Y.; Hu, L.-B.; Wang, W.-H.; Liu, S.-T.; Li, M.; Liu, R.-J. Influence of Long-Term Fixed Fertilization on Diversity of Arbuscular Mycorrhizal Fungi. Pedosphere 2009, 19, 663–672. [Google Scholar] [CrossRef]
- Vályi, K.; Rillig, M.C.; Hempel, S. Land-use intensity and host plant identity interactively shape communities of arbuscular mycorrhizal fungi in roots of grassland plants. New Phytol. 2015, 205, 1577–1586. [Google Scholar] [CrossRef]
- Johnson, N.C.; Wilson, G.W.; Bowker, M.A.; Wilson, J.A.; Miller, R.M. Resource limitation is a driver of local adaptation in mycorrhizal symbioses. Proc. Natl. Acad. Sci. USA 2010, 107, 2093–2098. [Google Scholar] [CrossRef]
- Ceulemans, T.; Merckx, R.; Hens, M.; Honnay, O. A trait-based analysis of the role of phosphorus vs. nitrogen enrichment in plant species loss across North-west European grasslands. J. Appl. Ecol. 2011, 48, 1155–1163. [Google Scholar] [CrossRef]
- Muthukumar, T.; Udaiyan, K. Influence of organic manures on arbuscular mycorrhizal fungi associated with Vigna unguiculata (L.) Walp. in relation to tissue nutrients and soluble carbohydrate in roots under field conditions. Biol. Fertil. Soils 2000, 31, 114–120. [Google Scholar] [CrossRef]
- Porras-Alfaro, A.; Herrera, J.; Natvig, D.O.; Sinsabaugh, R.L. Effect of Long-Term Nitrogen Fertilization on Mycorrhizal Fungi Associated with a Dominant Grass in a Semiarid Grassland. Plant Soil 2007, 296, 65–75. [Google Scholar] [CrossRef]
- Bradley, K.; Drijber, R.A.; Knops, J. Increased N Availability in Grassland Soils Modifies Their Microbial Communities and Decreases the Abundance of Arbuscular Mycorrhizal Fungi. Soil Biol. Biochem. 2006, 38, 1583–1595. [Google Scholar] [CrossRef]
- Treseder, K.K.; Allen, M.F. Direct Nitrogen and Phosphorus Limitation of Arbuscular Mycorrhizal Fungi: A Model and Field Test. New Phytol. 2002, 155, 507–515. [Google Scholar] [CrossRef]
- Brundrett, M.; Bougher, N.; Dell, B.; Grove, T.; Malajczuk, N. Working with Mycorrhizas in Forestry and Agriculture; ACIAR monograph 32; Australian Centre for International Agricultural Research: Canberra, Australia, 1996.
- Bilalis, D.J.; Karamanos, A.J. Organic Maize Growth and Mycorrhizal Root Colonization Response to Tillage and Organic Fertilization. J. Sustain. Agric. 2010, 34, 836–849. [Google Scholar] [CrossRef]
- Amaya-Carpio, L.; Davies, F.T., Jr.; Fox, T.; He, C. Arbuscular Mycorrhizal Fungi and Organic Fertilizer Influence Photosynthesis, Root Phosphatase Activity, Nutrition, and Growth of Ipomoea Carnea ssp. fistulosa; Springer: Berlin/Heidelberg, Germany, 2009; Available online: https://link.springer.com/article/10.1007/s11099-009-0003-x (accessed on 5 February 2022).
- Cassman, N.A.; Leite, M.F.A.; Pan, Y.; de Hollander, M.; van Veen, J.A.; Kuramae, E.E. Plant and Soil Fungal but Not Soil Bacterial Communities Are Linked in Long-Term Fertilized Grassland. Sci. Rep. 2016, 6, 23680. [Google Scholar] [CrossRef]
- Fornara, D.A.; Flynn, D.; Caruso, T. Improving phosphorus sustainability in intensively managed grasslands: The potential role of arbuscular mycorrhizal fungi. Sci. Total Environ. 2020, 706, 135744. [Google Scholar] [CrossRef]
- Sielaff, A.C.; Polley, H.W.; Fuentes-Ramirez, A.; Hofmockel, K.; Wilsey, B.J. Mycorrhizal colonization and its relationship with plant performance differs between exotic and native grassland plant species. Biol. Invasions 2019, 21, 1981–1991. [Google Scholar] [CrossRef]
- Gryndler, M.; Larsen, J.; Hršelová, H.; Řezáčová, V.; Gryndlerová, H.; Kubát, J. Organic and Mineral Fertilization, Respectively, Increase and Decrease the Development of External Mycelium of Arbuscular Mycorrhizal Fungi in a Long-Term Field Experiment. Mycorrhiza 2006, 16, 159–166. [Google Scholar] [CrossRef]
- Nijjer, S.; Rogers, W.E.; Siemann, E. The impacts of fertilization on mycorrhizal production and investment in Western Gulf Coast Grasslands. Am. Midl. Nat. 2010, 163, 124–133. [Google Scholar] [CrossRef]
- Liu, B.; Li, H.; Zhu, B.; Koide, R.T.; Eissenstat, D.M.; Guo, D. Complementarity in nutrient foraging strategies of absorptive fine roots and arbuscular mycorrhizal fungi across 14 coexisting subtropical tree species. New Phytol. 2015, 208, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Van Der Heijden, M.G. Mycorrhizal fungi reduce nutrient loss from model grassland ecosystems. Ecology 2010, 91, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Ling, N.; Guo, J.; Wang, M.; Guo, S.; Shen, Q. Impacts of Fertilization Regimes on Arbuscular Mycorrhizal Fungal (AMF) Community Composition Were Correlated with Organic Matter Composition in Maize Rhizosphere Soil. Front. Microbiol. 2016, 7, 1840. [Google Scholar] [CrossRef] [PubMed]
- Sellal, Z.; Ouazzani Touhami, A.; Chliyeh, M.; Dahmani, J.; Benkirane, R.; Douira, A. Arbuscular Mycorrhizal Fungi Species Associated with Rhizosphere of Argania spinosa (L.) Skeels in Morocco. Int. J. Pure Appl. Biosci. 2014, 4, 82–99. [Google Scholar] [CrossRef]
- Juge, C.; Cossette, N.; Jeanne, T.; Hogue, R. Long-Term Revegetation on Iron Mine Tailings in Northern Québec and Labrador and Its Effect on Arbuscular Mycorrhizal Fungi. Appl. Soil Ecol. 2021, 168, 104145. [Google Scholar] [CrossRef]
- Bücking, H.; Liepold, E.; Ambilwade, P. The Role of the Mycorrhizal Symbiosis in Nutrient Uptake of Plants and the Regulatory Mechanisms Underlying These Transport Processes; IntechOpen: London, UK, 2012; ISBN 978-953-51-0905-1. [Google Scholar]
- Sonnemann, I.; Baumhaker, H.; Wurst, S. Species Specific Responses of Common Grassland Plants to a Generalist Root Herbivore (Agriotes Spp. Larvae). Basic Appl. Ecol. 2012, 13, 579–586. [Google Scholar] [CrossRef]
- Stein, C.; Rißmann, C.; Hempel, S.; Renker, C.; Buscot, F.; Prati, D.; Auge, H. Interactive effects of mycorrhizae and a root hemiparasite on plant community productivity and diversity. Oecologia 2009, 159, 191–205. [Google Scholar] [CrossRef]
- Hildebrandt, U.; Janetta, K.; Ouziad, F.; Renne, B.; Nawrath, K.; Bothe, H. Arbuscular mycorrhizal colonization of halophytes in Central European salt marshes. Mycorrhiza 2001, 10, 175–183. [Google Scholar] [CrossRef]
- Hart, M.M.; Reader, R.J. Taxonomic basis for variation in the colonization strategy of arbuscular mycorrhizal fungi. New Phytol. 2002, 153, 335–344. [Google Scholar] [CrossRef]
- Klironomos, J.N.; Hart, M.M. Colonization of roots by arbuscular mycorrhizal fungi using different sources of inoculum. Mycorrhiza 2002, 12, 181–184. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).