Effects of Nutrient Levels and Rice Cultivation on Taxonomic and Functional Diversity of Bacterial Communities in Flooded Soils of the Hanon Maar Crater, Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Soil Sampling and Analysis of Chemical Properties
2.3. DNA Extraction, PCR Amplification, and Sequencing
2.4. Bioinfomatics Analysis
2.5. Statistical Analysis
3. Results
3.1. Soil Chemical Properties
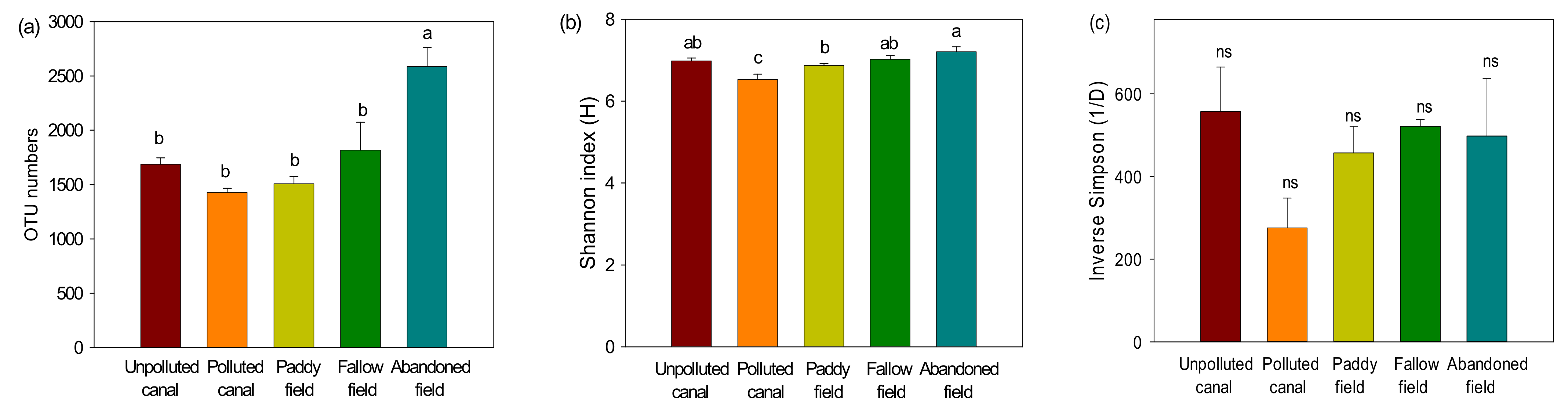
3.2. Pyrosequencing Analysis of Bacterial Community
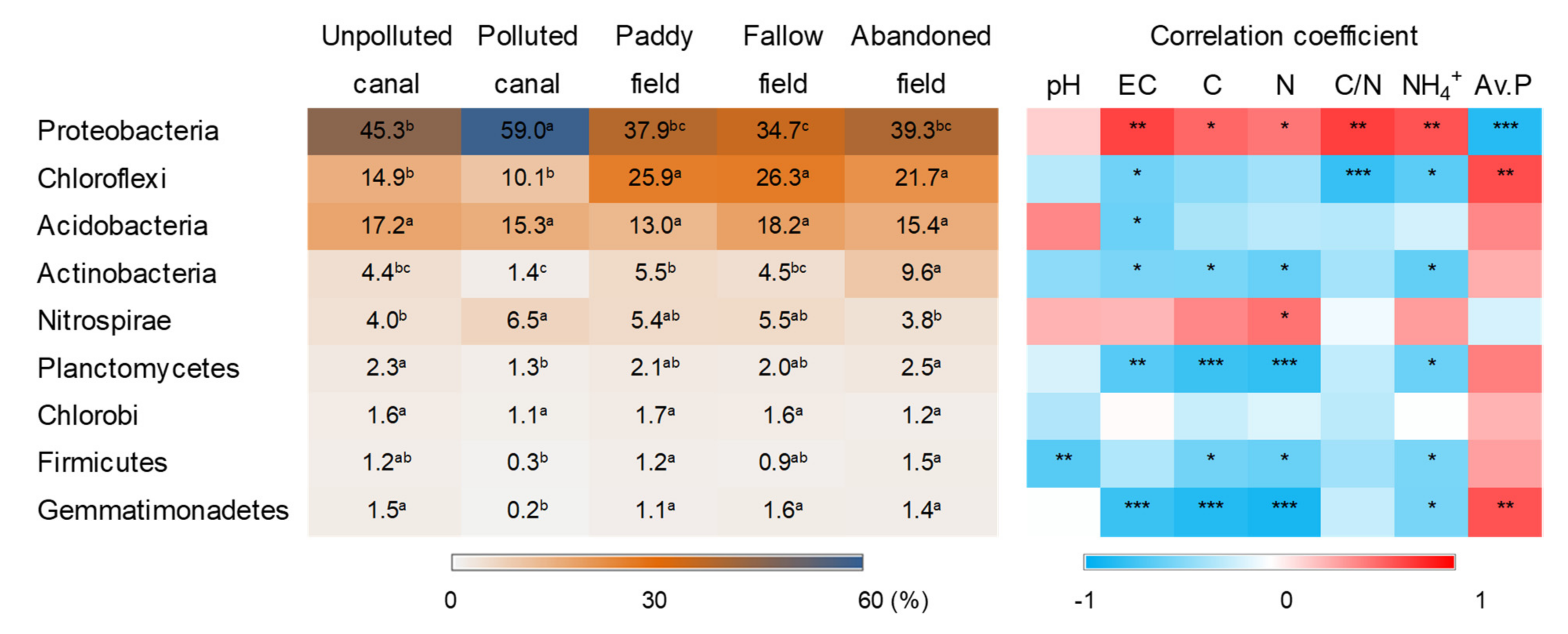
3.3. Taxonomic Diversity
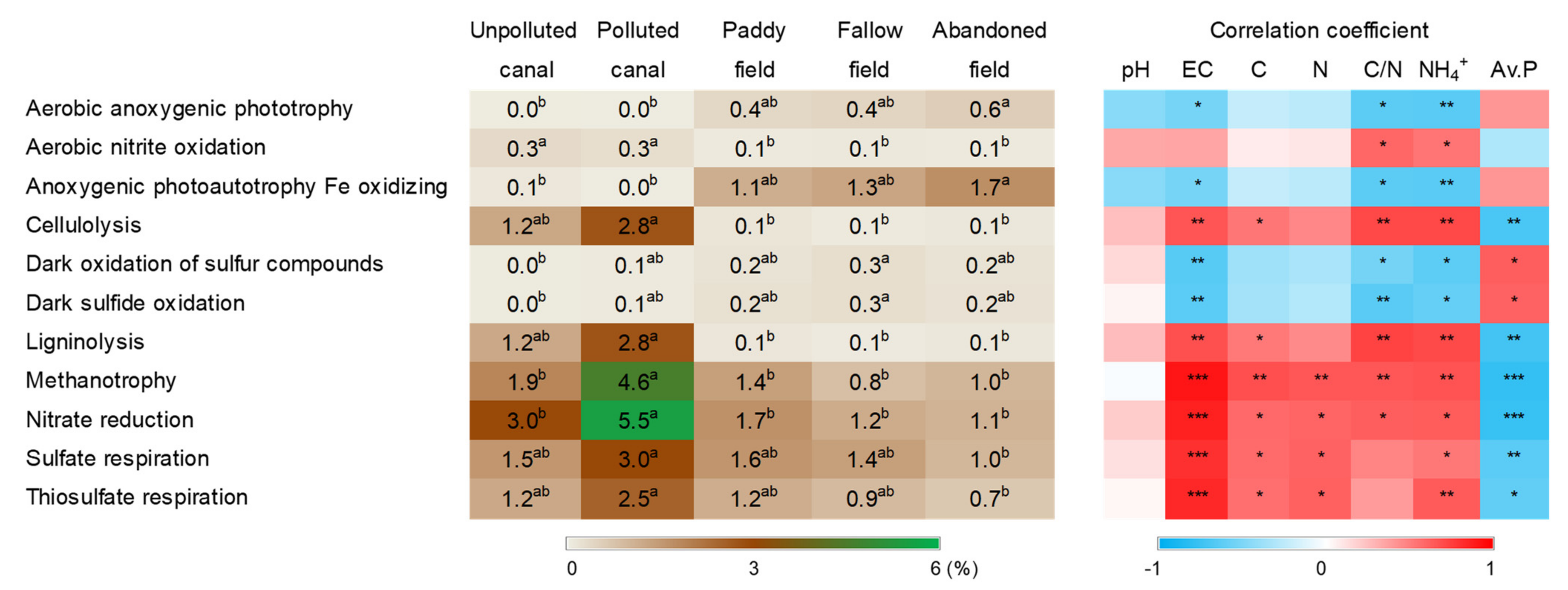
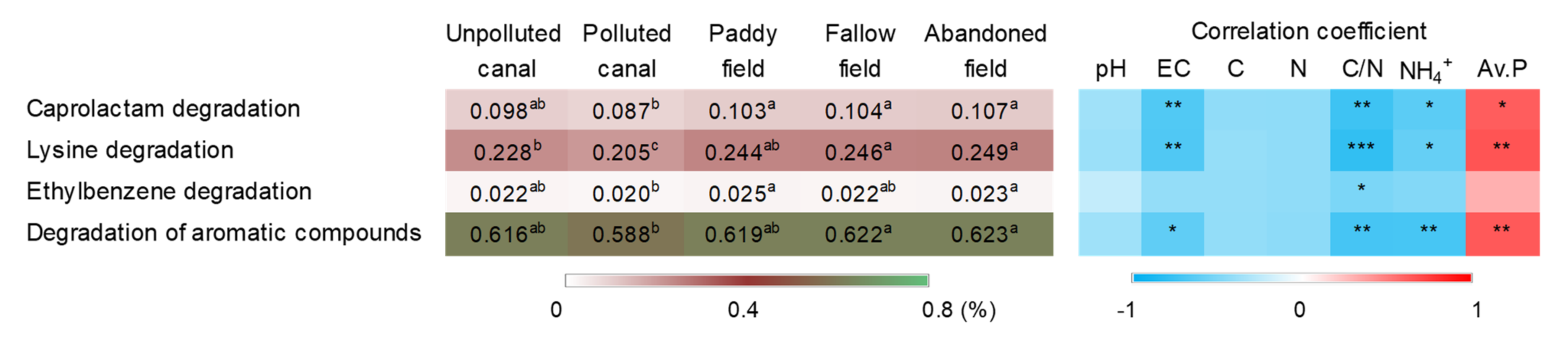
3.4. Putative Functions of Soil Bacteria
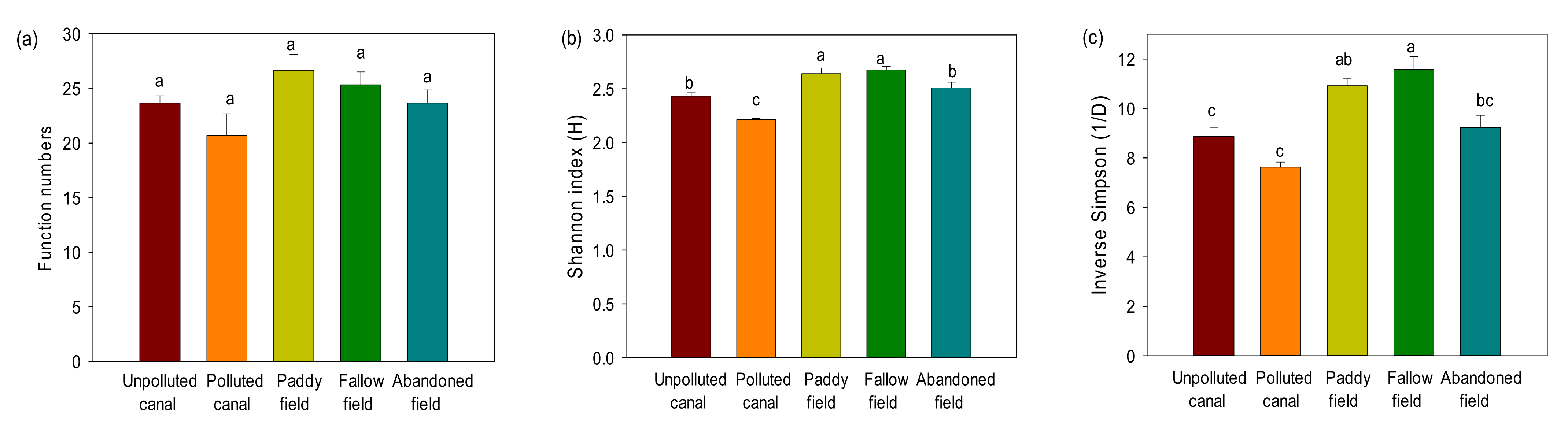
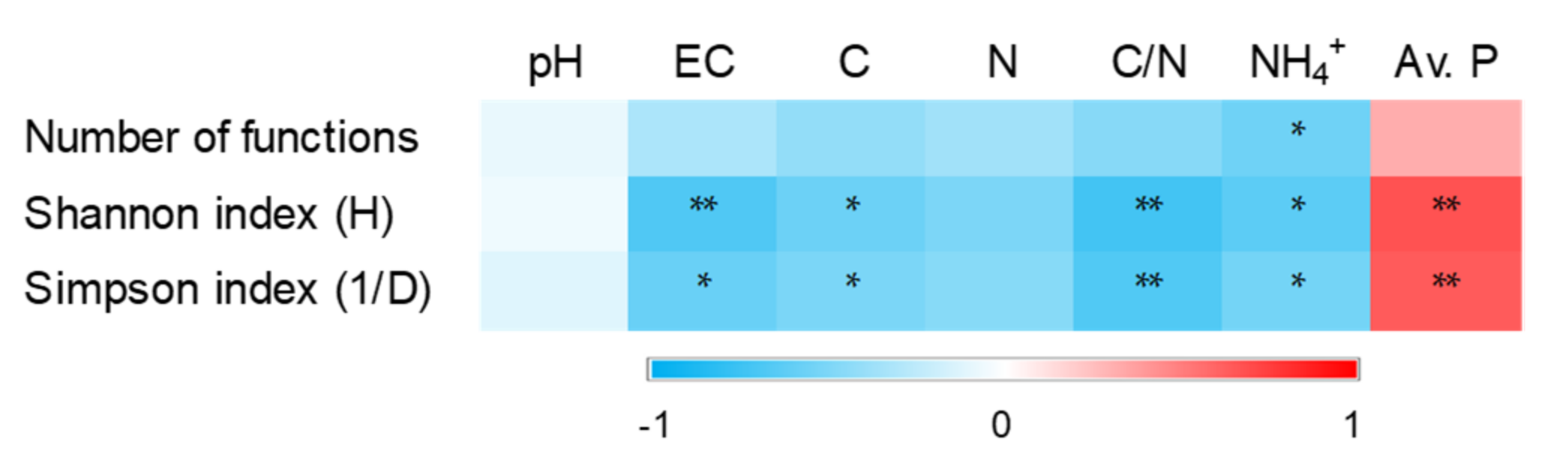
3.5. Diversity of Putative Function
4. Discussion
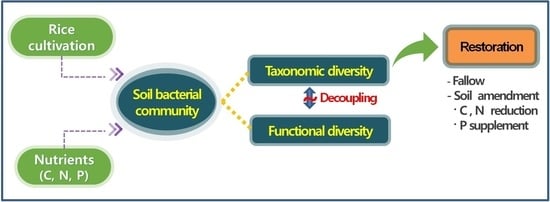
5. Conclusions
- o Keeping land fallow for more than 5 years.
- o Reducing the input of C and N from fertilizers.
- o Supplementing P in P-deficient soil.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beget, J.E.; Hopkins, D.M.; Charron, S.D. The Largest Known Maars on Earth, Seward Peninsula, Northwest Alaska. Arctic 1996, 49, 62–69. [Google Scholar] [CrossRef]
- Yoon, S.H.; Lee, B.G.; Sohn, Y.K. Geomorphic and geological characteristics and eruption process of the Hanon volcano, Jeju Island. J. Geol. Soc. Korea 2006, 42, 19–30. [Google Scholar]
- Bowers, K.; Kim, E.S.; Yang, Y.C.; Lee, S.C. A study on restoration plans of Jeju Hanon maar crater. World Environ. Isl. Stud. 2014, 4, 45–82. [Google Scholar]
- Kim, M.; Nam, H.; Eo, J.; Kwon, S.; Song, Y. Flora and restoration plan of Hanon paddy fields made in maar crater, Jeju island, South Korea. Korea J. Environ. Biol. 2018, 36, 439–455. [Google Scholar] [CrossRef]
- Barluenga, M.; Stölting, K.N.; Salzburger, W.; Muschick, M.; Meyer, A. Sympatric speciation in Nicaraguan crater lake cichlid fish. Nature 2006, 439, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Lanzén, A.; Simachew, A.; Gessesse, A.; Chmolowska, D.; Jonassen, I.; Øvreås, L. Surprising Prokaryotic and Eukaryotic Diversity, Community Structure and Biogeography of Ethiopian Soda Lakes. PLoS ONE 2013, 8, e72577. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.-H. Vegetation response to climate change on Jeju Island, South Korea, during the last deglaciation based on pollen record. Geosci. J. 2007, 11, 147–155. [Google Scholar] [CrossRef]
- Trivedi, P.; Delgado-Baquerizo, M.; Jeffries, T.C.; Trivedi, C.; Anderson, I.C.; Lai, K.; McNee, M.; Flower, K.; Singh, B.P.; Minkey, D.; et al. Soil aggregation and associated microbial communities modify the impact of agricultural management on carbon content. Environ. Microbiol. 2017, 19, 3070–3086. [Google Scholar] [CrossRef] [PubMed]
- Chiba, A.; Uchida, Y.; Kublik, S.; Vestergaard, G.; Buegger, F.; Schloter, M.; Schulz, S. Soil bacterial diversity is positively correlated with decomposition rates during early phases of maize litter decomposition. Microorganisms 2021, 9, 357. [Google Scholar] [CrossRef]
- Eo, J.; Park, K.-C. Long-term effects of imbalanced fertilization on the composition and diversity of soil bacterial community. Agric. Ecosyst. Environ. 2016, 231, 176–182. [Google Scholar] [CrossRef]
- Singh, B. Are nitrogen fertilizers deleterious to soil health? Agronomy 2018, 8, 48. [Google Scholar] [CrossRef]
- Adekiya, A.O.; Ejue, W.S.; Olayanju, A.; Dunsin, O.; Aboyeji, C.M.; Aremu, C.; Adegbite, K.; Akinpelu, O. Different organic manure sources and NPK fertilizer on soil chemical properties, growth, yield and quality of okra. Sci. Rep. 2020, 10, 16083. [Google Scholar] [CrossRef]
- Zhu, Z.L.; Chen, D.L. Nitrogen fertilizer use in China: Contributions to food production impacts on the environment strategies. Nutr. Cycl. Agroecosystems 2002, 63, 117–127. [Google Scholar] [CrossRef]
- Dybowski, D.; Dzierzbicka-Glowacka, L.A.; Pietrzak, S.; Juszkowska, D.; Puszkarczuk, T. Estimation of nitrogen leaching load from agricultural fields in the Puck Commune with an interactive calculator. PeerJ 2020, 8, e8899. [Google Scholar] [CrossRef] [PubMed]
- Wade, M.R.; Gurr, G.M.; Wratten, S.D. Ecological restoration of farmland: Progress and Prospects. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 831–847. [Google Scholar] [CrossRef]
- Pander, J.; Geist, J. Ecological indicators for stream restoration success. Ecol. Indic. 2013, 30, 106–118. [Google Scholar] [CrossRef]
- Benayas, J.M.R.; Newton, A.C.; Diaz, A.; Bullock, J.M. Enhancement of biodiversity and ecosystem services by ecological res-toration: A Meta-Analysis. Science 2009, 325, 1121–1124. [Google Scholar] [CrossRef]
- Laurila-Panta, M.; Lehikoinen, A.; Uusitalo, L.; Venesjarvi, R. How to value biodiversity in environmental management? Ecol. Indic. 2015, 55, 1–11. [Google Scholar] [CrossRef]
- Kennedy, A.C.; Smith, K.L. Soil microbial diversity and the sustainability of agricultural soils. Plant Soil 1995, 170, 75–86. [Google Scholar] [CrossRef]
- Griffiths, B.S.; Philippot, L. Insights into the resistance and resilience of the soil microbial community. FEMS Microbiol. Rev. 2013, 37, 112–129. [Google Scholar] [CrossRef]
- Tautges, N.E.; Sullivan, T.S.; Reardon, C.L.; Burke, I.C. Soil microbial diversity and activity linked to crop yield and quality in a dryland organic wheat production system. Appl. Soil Ecol. 2016, 108, 258–268. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Tsai, C.-F.; Rekha, P.D.; Ghate, S.D.; Huang, H.-Y.; Hsu, Y.-H.; Liaw, L.-L.; Young, C.-C. Agricultural management practices influence the soil enzyme activity and bacterial community structure in tea plantations. Bot. Stud. 2021, 62, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, D. Nitrogen and phosphorus losses by surface runoff and soil microbial communities in a paddy field with different irrigation and fertilization managements. PLoS ONE 2021, 16, e0254227. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; He, P.; Ai, C.; Zhao, S.; Ding, W.; Song, D.; Zhang, J.; Huang, S.; Abbas, T.; Zhou, W. How do soil bacterial diversity and community composition respond under recommended and conventional nitrogen fertilization regimes? Microorganisms 2020, 8, 1193. [Google Scholar] [CrossRef]
- Pulleman, M.; Creamer, R.; Hamer, U.; Helder, J.; Pelosi, C.; Peres, G.; Rutgers, M. Soil biodiversity, biological indicators and soil ecosystem services—An overview of European approaches. Curr. Opin. Environ. Sustain. 2012, 4, 529–538. [Google Scholar] [CrossRef]
- Behnke, G.D.; Kim, N.; Zabaloy, M.; Riggins, C.W.; Rodriguez-Zas, S.; Villamil, M.B. Soil Microbial Indicators within Rotations and Tillage Systems. Microorganisms 2021, 9, 1244. [Google Scholar] [CrossRef]
- Checcucci, A.; Luise, D.; Modesto, M.; Correa, F.; Bosi, P.; Mattarelli, P.; Trevisi, P. Assessment of Biolog EcoplateTM method for functional metabolic diversity of aerotolerant pig fecal microbiota. Appl. Microbiol. Biotechnol. 2021, 105, 6033–6045. [Google Scholar] [CrossRef]
- Kang, D.; Jacquiod, S.; Herschend, J.; Wei, S.; Nesme, J.; Sørensen, S.J. Construction of Simplified Microbial Consortia to Degrade Recalcitrant Materials Based on Enrichment and Dilution-to-Extinction Cultures. Front. Microbiol. 2020, 10, 3010. [Google Scholar] [CrossRef]
- Fadrosh, D.W.; Ma, B.; Gajer, P.; Sengamalay, N.; Ott, S.; Brotman, R.M.; Ravel, J. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2014, 2, 6. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Yoon, S.-H.; Ha, S.-M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A Taxonomically United Database of 16S rRNA Gene Sequences and Whole-Genome Assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef]
- Louca, S.; Parfrey, L.W.; Doebeli, M. Decoupling function and taxonomy in the global ocean microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, G.; Friendly, M.; Kindt, R.; Lagendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.; Solymos, P. Vegan: Community Ecology, R Package. Available online: https://craan.r-project.org/web/packages/vegta/vegan (accessed on 8 December 2021).
- Ho, A.; Di Lonardo, D.P.; Bodelier, P.L.E. Revisiting life strategy concepts in environmental microbial ecology. FEMS Microbiol. Ecol. 2017, 93. [Google Scholar] [CrossRef]
- Xun, W.; Zhao, J.; Xue, C.; Zhang, G.; Ran, W.; Wang, B.; Shen, Q.; Zhang, R. Significant alteration of soil bacterial communities and organic carbon decomposition by different long-term fertilization management conditions of extremely low-productivity arable soil in South China. Environ. Microbiol. 2016, 18, 1907–1917. [Google Scholar] [CrossRef]
- Li, F.; Chen, L.; Zhang, J.; Yin, J.; Huang, S. Bacterial Community Structure after Long-term Organic and Inorganic Fertilization Reveals Important Associations between Soil Nutrients and Specific Taxa Involved in Nutrient Transformations. Front. Microbiol. 2017, 8, 187. [Google Scholar] [CrossRef]
- Yao, F.; Yang, S.; Wang, Z.; Wang, X.; Ye, J.; Wang, X.; DeBruyn, J.M.; Feng, X.; Jiang, Y.; Li, H. Microbial Taxa Distribution Is Associated with Ecological Trophic Cascades along an Elevation Gradient. Front. Microbiol. 2017, 8, 2071. [Google Scholar] [CrossRef]
- Will, C.; Thurmer, A.; Wollherr, A.; Nacke, H.; Herold, N.; Schrumpf, M.; Gutknecht, J.; Wubet, T.; Buscot, F.; Daniel, R. Horizon-specific community composition of German grassland soils, as revealed by pyrosequencing-based analysis of 16S rDNA genes. Appl. Environ. Microb. 2010, 76, 6751–6759. [Google Scholar] [CrossRef]
- Ren, N.; Wang, Y.; Ye, Y.; Zhao, Y.; Huang, Y.; Fu, W.; Chu, X. Effects of continuous nitrogen fertilizer application on the di-versity and composition of rhizosphere soil bacteria. Front. Microbiol. 2020, 11, 1948. [Google Scholar] [CrossRef]
- Song, J.; Min, L.; Wu, J.; He, Q.; Chen, F.; Wang, Y. Response of the microbial community to phosphate-solubilizing bacterial inoculants on Ulmus chenmoui Cheng in Eastern China. PLoS ONE 2021, 16, e0247309. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, C.; Yu, W.; Turak, A.; Chen, D.; Huang, Y.; Ao, J.; Jiang, Y.; Huang, Z. Effects of Nitrogen and Phosphorus Inputs on Soil Bacterial Abundance, Diversity, and Community Composition in Chinese Fir Plantations. Front. Microbiol. 2018, 9, 1543. [Google Scholar] [CrossRef] [PubMed]
- Leff, J.W.; Jones, S.E.; Prober, S.M.; Barberán, A.; Borer, E.T.; Firn, J.; Harpole, W.S.; Hobbie, S.; Hofmockel, K.; Knops, J.M.H.; et al. Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. Proc. Natl. Acad. Sci. USA 2015, 112, 10967–10972. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tremblay, J.; Bainard, L.D.; Cade-Menun, B.; Hamel, C. Long-term effects of nitrogen and phosphorus fertilization on soil microbial community structure and function under continuous wheat production. Environ. Microbiol. 2020, 22, 1066–1088. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Q.; Han, X. Soil Bacterial Communities Respond to Mowing and Nutrient Addition in a Steppe Ecosystem. PLoS ONE 2013, 8, e84210. [Google Scholar] [CrossRef]
- Thirukkumaran, C.M.; Parkinson, D. Microbial respiration, biomass, metabolic quotient and litter decomposition in a lodgepole pine forest floor amended with nitrogen and phosphorous fertilizers. Soil Biol. Biochem. 2000, 32, 59–66. [Google Scholar] [CrossRef]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Trivedi, P.; Osanai, Y.; Liu, Y.; Hamonts, K.; Jeffries, T.C.; Singh, B.K. Carbon content and climate variability drive global soil bacterial diversity patterns. Ecol. Monogr. 2016, 86, 373–390. [Google Scholar] [CrossRef]
- Fierer, N.; Ladau, J.; Clemente, J.C.; Leff, J.W.; Owens, S.M.; Pollard, K.S.; Knight, R.; Gilbert, J.A.; McCulley, R.L. Reconstructing the microbial diversity and function of pre-agricultural tallgrass prairie soils in the United States. Science 2013, 342, 621–624. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, X.; Song, L.; Lin, X.; Zhang, H.; Shen, C.; Chu, H. Nitrogen fertilization directly affects soil bacterial diversity and indirectly affects bacterial community composition. Soil Biol. Biochem. 2015, 92, 41–49. [Google Scholar] [CrossRef]
- Liang, R.; Hou, R.; Li, J.; Lyu, Y.; Hang, S.; Gong, H.; Ouyang, Z. Effects of different fertilizers on rhizosphere bacterial communities of winter wheat in the North China plain. Agronomy 2020, 10, 93. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Guo, Z.; Jia, Z.; Wang, S.; Ding, K. Response of soil microbes to a reduction in phosphorus fertilizer in rice-wheat rotation paddy soils with varying soil P levels. Soil Tillage Res. 2018, 181, 127–135. [Google Scholar] [CrossRef]
- Pathan, S.I.; Scibetta, S.; Grassi, C.; Pietramellara, G.; Orlandini, S.; Ceccherini, M.T.; Napoli, M. Response of soil bacterial community to application to organic and inorganic phosphate based fertilizers under Vicia faba L. cultivation at two different phenological stages. Sustainability 2020, 12, 9706. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, X.; Lin, Q.; Li, G. Decrease in diversity and shift in composition of the soil bacterial community were closely related to high available phosphorus in agricultural Fluvisols of North China. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2019, 69, 618–630. [Google Scholar] [CrossRef]
- Xia, Z.; Yang, J.; Sang, C.; Wang, X.; Sun, L.; Jiang, P.; Wang, C.; Bai, E. Phosphorus Reduces Negative Effects of Nitrogen Addition on Soil Microbial Communities and Functions. Microorganisms 2020, 8, 1828. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Xing, F.; Zhao, H.; Gao, Y.; Bai, Z.; Dong, Y. Response of bacterial community to simulated nitrogen deposition in soils and a unique relationship between plant species and soil bacteria in the Songnen grassland in Northeastern China. J. Soil Sci. Plant Nutr. 2014, 14, 565–580. [Google Scholar] [CrossRef][Green Version]
- García-Orenes, F.; Morugán-Coronado, A.; Zornoza, R.; Scow, K. Changes in Soil Microbial Community Structure Influenced by Agricultural Management Practices in a Mediterranean Agro-Ecosystem. PLoS ONE 2013, 8, e80522. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, M.; Wu, C. Short-term fallow practices drive soil bacterial community changes: A case study from China. Appl. Soil Ecol. 2021, 165, 103988. [Google Scholar] [CrossRef]
- Zhang, K.; Cheng, X.; Shu, X.; Liu, Y.; Zhang, Q. Linking soil bacterial and fungal communities to vegetation succession following agricultural abandonment. Plant Soil 2018, 431, 19–36. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, K.; Wurzburger, N.; Zhang, J. Relationships between plant diversity and soil microbial diversity vary across taxonomic groups and spatial scales. Ecosphere 2020, 11, e02999. [Google Scholar] [CrossRef]
- Curd, E.E.; Martiny, J.B.H.; Li, H.; Smith, T.B. Bacterial diversity is positively correlated with soil heterogeneity. Ecosphere 2018, 9, e02079. [Google Scholar] [CrossRef]
- Eo, J.; Kim, M.H.; Kim, M.K.; Choi, S.K. Shift of dominant species in plant community and soil chemical properties shape soil bacterial community characteristics and putative functions: A Case Study on Topographic Variation in a Mountain Pasture. Microorganisms 2021, 9, 961. [Google Scholar] [CrossRef] [PubMed]
- Sansupa, C.; Wahdan, S.F.M.; Hossen, S.; Disayathanoowat, T.; Wubet, T.; Purahong, W. Can we use functional annotation of prokaryotic taxa (FAPROTAX) to assign the ecological functions of soil bacteria? Appl. Sci. 2021, 11, 688. [Google Scholar] [CrossRef]
- Li, Y.; Wu, J.; Shen, J.; Liu, S.; Wang, C.; Chen, D.; Huang, T.; Zhang, J. Soil microbial C:N ratio is a robust indicator of soil productivity for paddy fields. Sci. Rep. 2016, 6, 35266. [Google Scholar] [CrossRef] [PubMed]
- Bengtsso, G.; Bengtson, P.; Mansson, K.F. Gross nitrogen mineralization, immobilization, and nitrification rates as a functional of soil C/N ratio and microbial activity. Soil Biol. Biochem. 2003, 35, 143–154. [Google Scholar] [CrossRef]
- Ananderud, Z.; Saurey, S.; Ball, B.A.; Wall, D.H.; Barrett, J.E.; Muscarella, M.E.; Griffin, N.A.; Virainia, R.A.; Barberan, A.; Adams, B.J. Stoichiometric shifts in soil C: N: P promote bacterial taxa dominance, maintain biodiversity, and deconstruct community assemblages. Front Microbiol. 2018, 9, 1401. [Google Scholar]
- Entry, J.A. Influence of nitrogen on cellulose and lignin mineralization in blackwater and redwater forested wetland soils. Biol. Fertil. Soils 2000, 31, 436–440. [Google Scholar] [CrossRef]
- Güsewell, S.; Gessner, M.O. N:P ratios influence litter decomposition and colonization by fungi and bacteria in microcosms. Funct. Ecol. 2009, 23, 211–219. [Google Scholar] [CrossRef]
- Sun, S.; Jones, R.B.; Fodor, A.A. Inference-based accuracy of metagenome prediction tools varies across sample types and functional categories. Microbiome 2020, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Damon, C.; Lehembre, F.; Oger-Desfeux, C.; Luis, P.; Ranger, J.; Fraissinet-Tachet, L.; Marmeisse, R. Metatranscriptomics Reveals the Diversity of Genes Expressed by Eukaryotes in Forest Soils. PLoS ONE 2012, 7, e28967. [Google Scholar] [CrossRef] [PubMed]
- Hickman, Z.A.; Reid, B.J. Increased microbial catabolic activity in diesel contaminated soil following addition of earthworms (Dendrobaena veneta) and compost. Soil Biol. Biochem. 2008, 40, 2970–2976. [Google Scholar] [CrossRef]
- Schleuter, D.; Daufresne, M.; Massol, F.; Argillier, C. A user’s guide to functional diversity indices. Ecol. Monogr. 2010, 80, 469–484. [Google Scholar] [CrossRef]
- Fierer, N.; Leff, J.W.; Adams, B.J.; Nielsen, U.N.; Bates, S.T.; Lauber, C.L.; Owens, S.; Gilbert, J.A.; Wall, D.H.; Caporaso, J.G. Cross-biome metagenomic analyses of soil microbial communities and their functional attributes. Proc. Natl. Acad. Sci. USA 2012, 109, 21390–21395. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; Field, D.; Swift, P.; Thomas, S.; Cummings, D.; Temperton, B.; Weynberg, K.; Huse, S.; Hughes, M.; Joint, I.; et al. The Taxonomic and Functional Diversity of Microbes at a Temperate Coastal Site: A ‘Multi-Omic’ Study of Seasonal and Diel Temporal Variation. PLoS ONE 2010, 5, e15545. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Delgado-Baquerizo, M.; Anderson, I.C.; Singh, B.K. Response of soil properties and microbial communities to ag-riculture: Implications for Primary Productivity and Soil Health Indicators. Front. Plant Sci. 2016, 7, 990. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.C.; Mendes, C.; Reis-Junior, F.B.; Carvalho, F.M.; Nogueira, M.A.; Vasconcelos, A.T.R.; Vicente, V.A.; Hungria, M. Shifts in taxonomic and functional microbial diversity with agriculture: How fragile is the Brazilian Cerrado? BMC Microbiol. 2016, 16, 42. [Google Scholar] [CrossRef] [PubMed]
- He, X.-Y.; Wang, K.-L.; Zhang, W.; Chen, Z.-H.; Zhu, Y.-G.; Chen, H.-S. Positive correlation between soil bacterial metabolic and plant species diversity and bacterial and fungal diversity in a vegetation succession on Karst. Plant Soil 2008, 307, 123–134. [Google Scholar] [CrossRef]
- Zhang, X.; Johnston, E.R.; Barberán, A.; Ren, Y.; Wang, Z.; Han, X. Effect of intermediate disturbance on soil microbial functional diversity depends on the amount of effective resources. Environ. Microbiol. 2018, 20, 3862–3875. [Google Scholar] [CrossRef] [PubMed]
- Roth-Schulze, A.J.; Zozaya-Valdes, E.; Steinberg, P.D.; Thomas, T. Partitionaing of functional and taxonomic diversity in surface-associated microbial communities. Environ. Microbiol. 2016, 18, 4391–4402. [Google Scholar] [CrossRef] [PubMed]
- Cheaib, B.; Le Boulch, M.; Mercier, P.; Derome, N. Taxon-function decoupling as an adaptive signature of lake microbial metacommunites under a chronic polymetallic pollution gradient. Front. Microbiol. 2018, 9, 869. [Google Scholar] [CrossRef]
- Kiersztyn, B.; Chróst, R.; Kaliński, T.; Siuda, W.; Bukowska, A.; Kowalczyk, G.; Grabowska, K. Structural and functional microbial diversity along a eutrophication gradient of interconnected lakes undergoing anthropopressure. Sci. Rep. 2019, 9, 11144. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, C.; Delgado-Baquerizo, M.; Hamonts, K.; Lai, K.; Reich, P.B.; Singh, B.K. Losses in microbial functional diversity reduce the rate of key soil processes. Soil Biol. Biochem. 2019, 135, 267–274. [Google Scholar] [CrossRef]
- Philippot, L.; Spor, A.; Hénault, C.; Bru, D.; Bizouard, F.; Jones, C.M.; Sarr, A.; Maron, P.-A. Loss in microbial diversity affects nitrogen cycling in soil. ISME J. 2013, 7, 1609–1619. [Google Scholar] [CrossRef] [PubMed]
- Hartman, W.H.; Richardson, C.J.; Vilgalys, R.; Bruland, G.L. Environmental and anthropogenic controls over bacterial communities in wetland soils. Proc. Natl. Acad. Sci. USA 2008, 105, 17842–17847. [Google Scholar] [CrossRef]
- Jangid, K.; Williams, M.A.; Franzluebbers, A.J.; Blair, J.M.; Coleman, D.C.; Whitman, W.B. Development of soil microbial communities during tallgrass prairie restoration. Soil Biol. Biochem. 2010, 42, 302–312. [Google Scholar] [CrossRef]
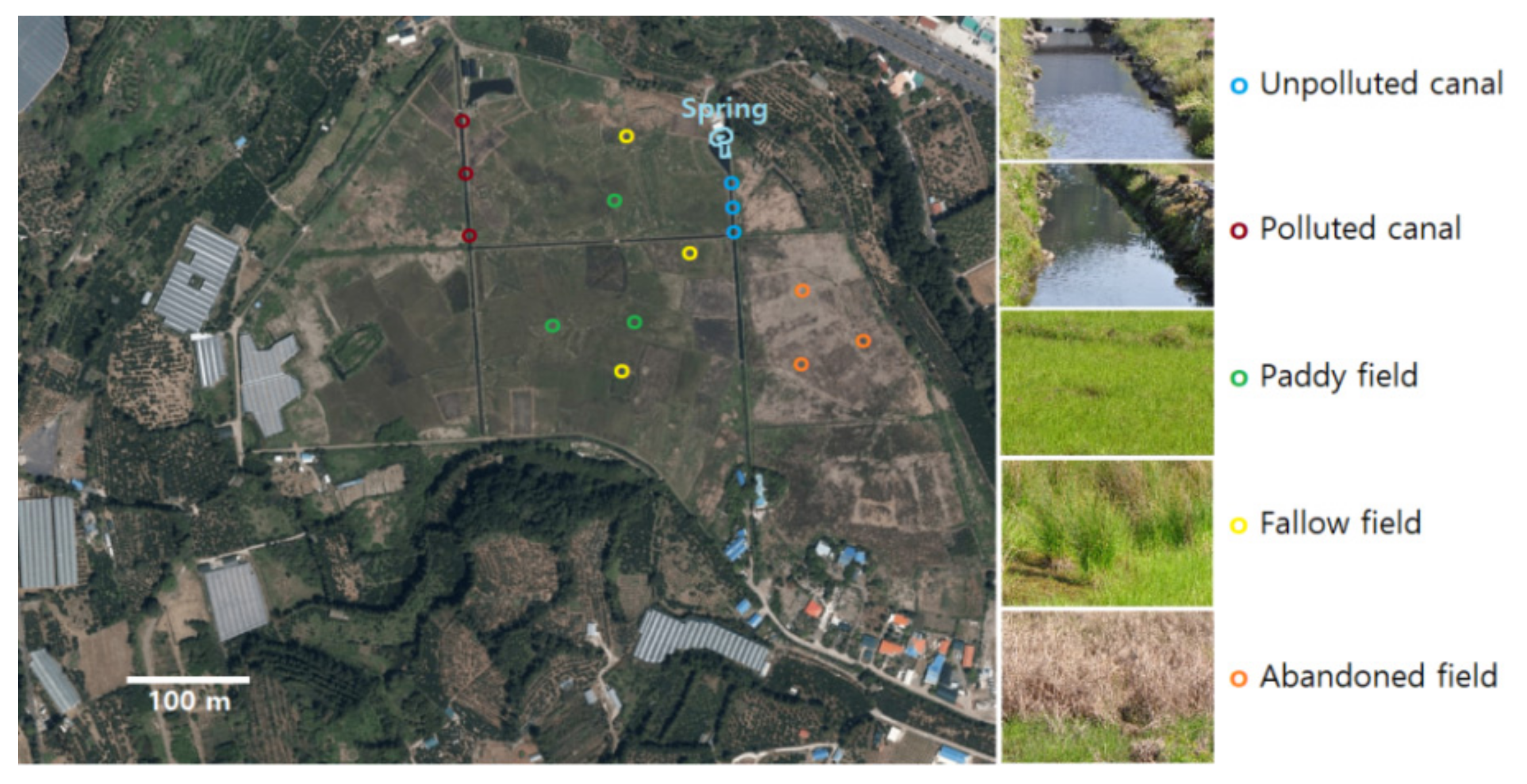


| Dominant Species | |
|---|---|
| Unpolluted canal | Zizania latifolia, Hydrilla verticillata, Potamogeton cristatus |
| Polluted canal | Hydrilla verticillata, Leersia japonica, Nasturtium officinale |
| Paddy field | Alopecurus aequalis, Aneilema keisak, Eclipta prostrata |
| Fallow field | Juncus effuses, Hydrocotyle sibthorpioides, Stellaria alsine, Aneilema keisak |
| Abandoned field | Echinochloa crusgalli, Aneilema keisak, Lemna perpusilla, Eleocharis acicularis |
| pH | EC (ds m−1) | N (%) | C (%) | NH4+ (mg kg−1) | Av.P (mg kg−1) | |
|---|---|---|---|---|---|---|
| Unpolluted canal | 5.4 ± 0.2 a | 0.6 ± 0.1 b | 0.3 ± 0.2 b | 6.0 ± 2.0 b | 55.5 ± 5.5 b | 18.2 ± 3.4 ab |
| Polluted canal | 5.4 ± 0.1 a | 1.5 ± 0.1 a | 1.0 ± 0.1 a | 20.1 ± 2.5 a | 98.3 ± 11.9 a | 2.8 ± 0.6 b |
| Paddy field | 5.2 ± 0.1 a | 0.6 ± 0.0 b | 0.6 ± 0.1 ab | 8.4 ± 0.4 b | 49.2 ± 7.1 b | 17.6 ± 5.7 ab |
| Fallow field | 5.3 ± 0.1 a | 0.4 ± 0.0 b | 0.5 ± 0.1 ab | 7.6 ± 0.5 b | 41.9 ± 9.7 b | 30.6 ± 7.6 a |
| Abandoned field | 5.1 ± 0.1 a | 0.5 ± 0.0 b | 0.5 ± 0.1 ab | 7.3 ± 1.0 b | 44.0 ± 3.8 b | 18.5 ± 3.3 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eo, J.; Kim, M.-H. Effects of Nutrient Levels and Rice Cultivation on Taxonomic and Functional Diversity of Bacterial Communities in Flooded Soils of the Hanon Maar Crater, Korea. Agronomy 2022, 12, 651. https://doi.org/10.3390/agronomy12030651
Eo J, Kim M-H. Effects of Nutrient Levels and Rice Cultivation on Taxonomic and Functional Diversity of Bacterial Communities in Flooded Soils of the Hanon Maar Crater, Korea. Agronomy. 2022; 12(3):651. https://doi.org/10.3390/agronomy12030651
Chicago/Turabian StyleEo, Jinu, and Myung-Hyun Kim. 2022. "Effects of Nutrient Levels and Rice Cultivation on Taxonomic and Functional Diversity of Bacterial Communities in Flooded Soils of the Hanon Maar Crater, Korea" Agronomy 12, no. 3: 651. https://doi.org/10.3390/agronomy12030651
APA StyleEo, J., & Kim, M.-H. (2022). Effects of Nutrient Levels and Rice Cultivation on Taxonomic and Functional Diversity of Bacterial Communities in Flooded Soils of the Hanon Maar Crater, Korea. Agronomy, 12(3), 651. https://doi.org/10.3390/agronomy12030651