Mitigation of Calcium-Related Disorders in Soilless Production Systems
Abstract
1. Climate Change and Agriculture
2. Soilless Production Systems: Challenges and Solutions
2.1. Soilless Cropping Systems
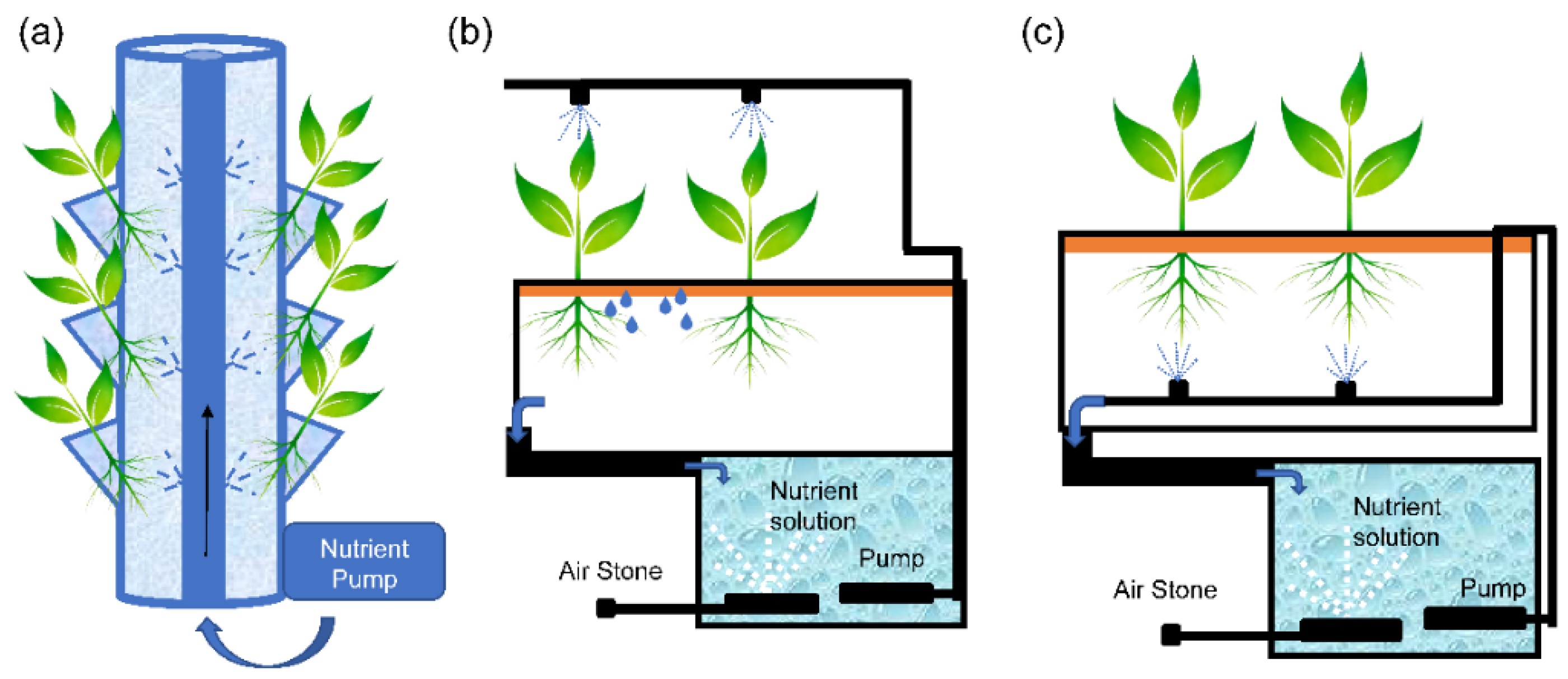
2.1.1. Aeroponic Cultivation
- Spray column system: This consists of a cylindrical platform made of opaque polyvinyl chloride, with lateral perforations through which the plants are introduced. The nutrient solution is sprayed over the upper part of the roots to ensure a permanent contact with the nutrient solution while the lower part of the root is well aerated (Figure 2a).
- Schwalbach system: This consists of a growth chamber in which the roots grow in the air and are kept in complete darkness. The nutrient solution is sprayed at different distribution points located near the leaves to ensure optimal foliar application, after which it drains to the root, where the excess solution is recovered (Figure 2b).
- Aero-Gro system: The nutrient solution is injected onto the roots directly through finely separated droplets at low pressure, avoiding clogging problems in pipes and spray nozzles (Figure 2c).
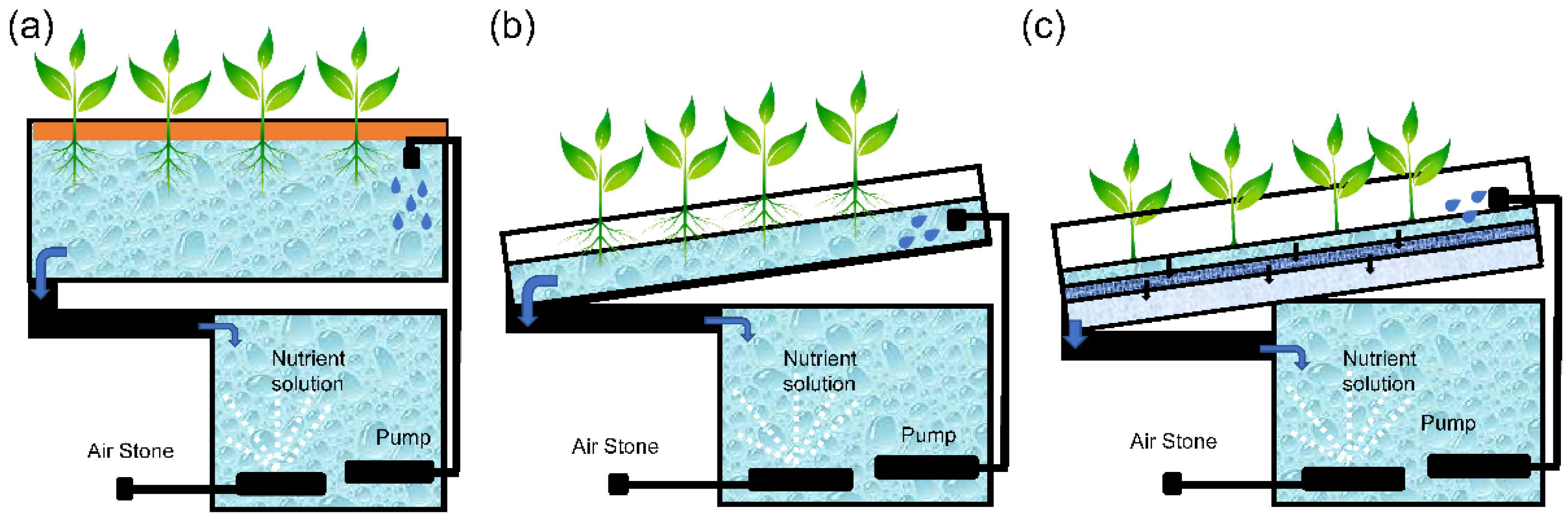
2.1.2. Hydroponic Cultivation
- Deep floating technique (DFT): It incorporates perforated polystyrene sheets as growing units that are placed on top of the tanks filled with the nutrient solution. The aerial part of the plants grows on these sheets with their roots submerged in the tank solution. These systems have an air pump that aerates the nutrient solution (Figure 3a).
- Nutrient film technique (NFT): This system is based on pumping a thin layer of nutrient solution onto the root system through constant flow. This is achieved by placing a small channel with a 1% slope to ensure that the nutritive solution reaches the roots by laminar flow. The excess solution drains into a collecting tank where the conductivity and pH values are restored and the nutrient solution can be pumped back to the top of the channel (Figure 3b).
- New growing system technique (NGST): This system is based on a channel formed by polyethylene bags located internally in three interconnected layers and wrapped by a layer of black polyethylene, which prevents direct contact of light with the root system. The entire system is suspended in the air and leveled to collect drainage at the end of the growing line. The irrigation system is in continuous operation and the drained solution reaches a tank where the nutrient levels are adjusted, heated, and pumped back into the system. The irrigation pipe is located close the root system to facilitate heating of the roots [11] (Figure 3c).
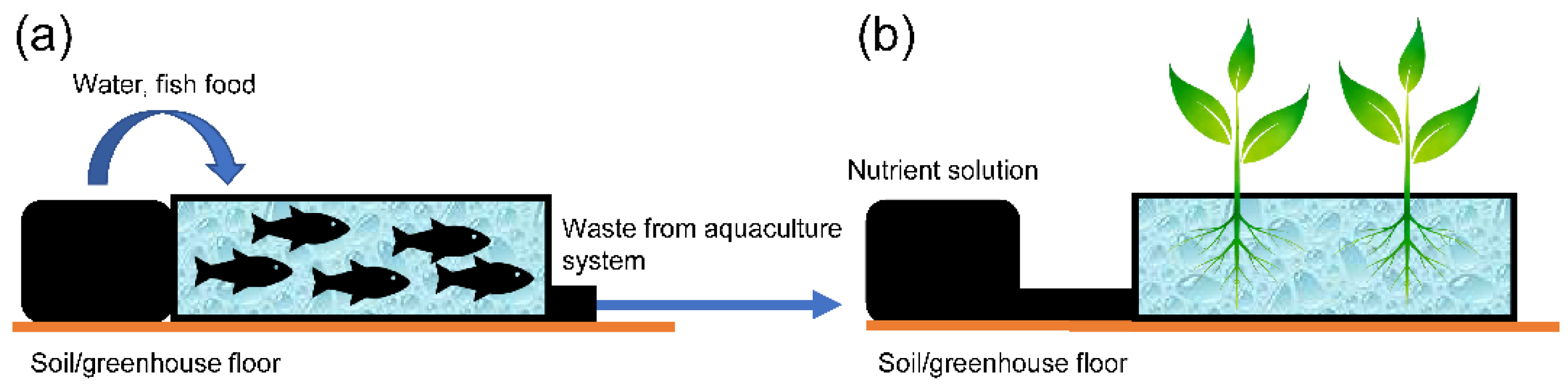
2.1.3. Aquaponic Cultivation
2.1.4. Cultivation in Organic and Inorganic Substrates
- Growing directly on substrate: These systems are delimited by a thick polyethylene mat that prevents the nutrient solution from leaking into the soil. The irrigation system utilized is drip irrigation, and the excess nutrient solution is sent to a tank where the appropriate adjustments will be made for reusing the nutrient solution (Figure 5a,b).
- Growing in bags or containers: The root volume is delimited by elongated two-color polyethylene bags closed at the ends and with two drainage holes filled with substrate. The plants will grow in these bags, the nutritive solution will be dripped in, and the excess solution will be channeled to a tank for further adjustment and reuse [11] (Figure 5c).
- Single unit culture systems: These systems were developed due to the need to control the transmission of fungal diseases in the continuous systems. In this case, the container is the basic cultivation unit and is placed parallel to the drip line. This allows for better control of individual plants, but this system in large-scale production could be prohibitively expensive (Figure 5d,e).
2.2. Physiological Disorders in Soilless Cropping Systems
3. Physiological Disorders: Blossom-End Rot (BER) and Tipburn (TB)
3.1. Abiotic Factors Influencing BER and TB
3.1.1. Drought
3.1.2. High Temperature
3.1.3. Salinity
3.2. Physiological Factors Influencing BER and TB Incidence
3.3. Genetic Factors Influencing BER and TB Incidence
4. Solutions to Alleviate Ca-Related Disorders in Soilless Production Systems
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security and Greenhouse Gas Fluxes in Terrestrial Ecosystems. Available online: https://www.ipcc.ch/srccl/ (accessed on 24 February 2022).
- Climate Change: Atmospheric Carbon Dioxide. Available online: https://www.climate.gov/news-features/understanding-climate (accessed on 24 February 2022).
- Reidsma, P.; Ewert, F.; Lansink, A.O.; Leemans, R. Adaptation to Climate Change and Climate Variability in European Agriculture: The Importance of Farm Level Responses. Eur. J. Agron. 2010, 32, 91–102. [Google Scholar] [CrossRef]
- Hamidov, A.; Helming, K.; Bellocchi, G.; Bojar, W.; Dalgaard, T.; Ghaley, B.B.; Hoffmann, C.; Holman, I.; Holzkämper, A.; Krzeminska, D.; et al. Impacts of Climate Change Adaptation Options on Soil Functions: A Review of European Case-Studies. Land Degrad. Dev. 2018, 29, 2378–2389. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Iqbal, K.; Showket, A.; Prasanto, M.; Negi, A.K. A Review On The Science Of Growing Crops Without Soil (Soilless Culture)—A Novel Alternative For Growing Crops. Int. J. Agric. Crop Sci. 2014, 7, 833–842. [Google Scholar]
- Beltrano, J.; Gimenez, D.O. Cultivo En Hidroponía, 1st ed.; Edulp integra la Red de Editoriales Universitarias Nacionales (REUN): La Plata, Argentina, 2015. [Google Scholar]
- Gruda, N.; Bisbis, M.; Tanny, J. Impacts of Protected Vegetable Cultivation on Climate Change and Adaptation Strategies for Cleaner Production—A Review. J. Clean. Prod. 2019, 225, 324–339. [Google Scholar] [CrossRef]
- Martinez-Mate, M.A.; Martin-Gorriz, B.; Martínez-Alvarez, V.; Soto-García, M.; Maestre-Valero, J.F. Hydroponic System and Desalinated Seawater as an Alternative Farm-Productive Proposal in Water Scarcity Areas: Energy and Greenhouse Gas Emissions Analysis of Lettuce Production in Southeast Spain. J. Clean. Prod. 2018, 172, 1298–1310. [Google Scholar] [CrossRef]
- Urrestarazu, M.; Postigo, A.; Salas, M.; Sánchez, A.; Carrasco, G. Nitrate Accumulation Reduction Using Chloride in the Nutrient Solution on Lettuce Growing by NFT in Semiarid Climate Conditions. J. Plant Nutr. 1998, 21, 1705–1714. [Google Scholar] [CrossRef]
- Baixauli Soria, C.; Aguilar Olivert, J.M. Cultivo Sin Suelo de Hortalizas: Aspectos Práticos y Experiencias; Generalitat Valenciana, Conselleria de Agricultura, Pesca y Alimentación: Valencia, Spain, 2002. [Google Scholar]
- Maluin, F.N.; Hussein, M.Z.; Nik Ibrahim, N.N.L.; Wayayok, A.; Hashim, N. Some Emerging Opportunities of Nanotechnology Development for Soilless and Microgreen Farming. Agronomy 2021, 11, 1213. [Google Scholar] [CrossRef]
- Goddek, S.; Joyce, A.; Kotzen, B.; Burnell, G.M. Aquaponics Food Production Systems, Combined Aquaculture and Hydroponic Production Technologies for the Future; Springer Nature Switzerland AG: Cham, Switzerland, 2020. [Google Scholar]
- Maucieri, C.; Nicoletto, C.; Junge, R.; Schmautz, Z. Hydroponic Systems and Water Management in Aquaponics: A Review. Ital. J. Agron. 2018, 13, 1012. [Google Scholar] [CrossRef]
- Lennard, W.; Ward, J. A Comparison of Plant Growth Rates between an NFT Hydroponic System and an NFT Aquaponic System. Horticulturae 2019, 5, 27. [Google Scholar] [CrossRef]
- Hagassou, D.; Francia, E.; Ronga, D.; Buti, M. Blossom End-Rot in Tomato (Solanum lycopersicum L.): A Multi-Disciplinary Overview of Inducing Factors and Control Strategies. Sci. Hortic. 2019, 249, 49–58. [Google Scholar] [CrossRef]
- Saure, M.C. Causes of the Tipburn Disorder in Leaves of Vegetables. Sci. Hortic. 1998, 76, 131–147. [Google Scholar] [CrossRef]
- Adams, P.; Ho, L.C. Effects of Environment on the Uptake and Distribution of Calcium in Tomato and on the Incidence of Blossom-End Rot. Plant Soil 1993, 154, 127–132. [Google Scholar] [CrossRef]
- Saure, M. Blossom-End Rot of Tomato (Lycopersicon esculentum Mill.)—A Calcium or a Stress-Related Disorder? Sci. Hortic. 2001, 90, 193–208. [Google Scholar] [CrossRef]
- Ho, L.C. The Physiological Basis for Improving Tomato Fruit Quality. Acta Hortic. 1999, 487, 33–40. [Google Scholar] [CrossRef]
- Ho, L.C.; White, P.J. A Cellular Hypothesis for the Induction of Blossom-End Rot in Tomato Fruit. Ann. Bot. 2005, 95, 571–581. [Google Scholar] [CrossRef]
- Camacho Ferre, F.; Cánovas, M.F.; Magán, C.J. Técnicas de Producción En Cultivos Protegidos-Cultivos Sin Suelo, 2nd ed.; Caja Rural Intermediterránea, Cajamar.: Barcelona, Spain, 2003. [Google Scholar]
- Mendelsohn, R.; Dinar, A. Climate Change, Agriculture and Developing Countries: Does Adaptation Matter? World Bank Res. Obs. 1999, 14, 277–293. [Google Scholar] [CrossRef]
- Casey Barickman, T.; Kopsell, D.A.; Sams, C.E. Foliar Applications of Abscisic Acid Decrease the Incidence of Blossom-End Rot in Tomato Fruit. Sci. Hortic. 2014, 179, 356–362. [Google Scholar] [CrossRef]
- Bisbis, M.B.; Gruda, N.; Blanke, M. Potential Impacts of Climate Change on Vegetable Production and Product Quality—A Review. J. Clean. Prod. 2018, 170, 1602–1620. [Google Scholar] [CrossRef]
- Wedgworth, H.H.; Neal, D.C.; Wallace, J.M.; Ricks, J.R. Wilt and Blossom-End Rot of the Tomato. Bulletins 1927, 247, 1–18. [Google Scholar]
- Saure, M.C. Why Calcium Deficiency Is Not the Cause of Blossom-End Rot in Tomato and Pepper Fruit—A Reappraisal. Sci. Hortic. 2014, 174, 151–154. [Google Scholar] [CrossRef]
- Reitz, N.F.; Shackel, K.A.; Mitcham, E.J. Differential Effects of Excess Calcium Applied to Whole Plants vs. Excised Fruit Tissue on Blossom-End Rot in Tomato. Sci. Hortic. 2021, 290, 110514. [Google Scholar] [CrossRef]
- Sonneveld, C.; van den Ende, J. The Effect of Some Salts on Head Weight and Tipburn of Lettuce and on Fruit Production and Blossom-End Rot of Tomatoes. Neth. J. Agric. Sci. 1975, 23, 191–201. [Google Scholar] [CrossRef]
- Teichmann, C.; Bülow, K.; Otto, J.; Pfeifer, S.; Rechid, D.; Sieck, K.; Jacob, D. Avoiding Extremes: Benefits of Staying below +1.5 °C Compared to +2.0 °C and +3.0 °C Global Warming. Atmosphere 2018, 9, 115. [Google Scholar] [CrossRef]
- Lynch, J.P.; Chimungu, J.G.; Brown, K.M. Root Anatomical Phenes Associated with Water Acquisition from Drying Soil: Targets for Crop Improvement. J. Exp. Bot. 2014, 65, 6155–6166. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Penella, C.; Hernández, J.A.; Díaz-Vivancos, P.; Sánchez-Blanco, M.J.; Navarro, J.M.; Gómez-Bellot, M.J.; Barba-Espín, G. Towards a Sustainable Agriculture: Strategies Involving Phytoprotectants Against Salt Stress. Agronomy 2020, 10, 194. [Google Scholar] [CrossRef]
- Karni, L.; Aloni, B.; Bar-Tal, A.; Moreshet, S.; Keinan, M.; Yao, C. The Effect of Root Restriction on the Incidence of Blossom-End Rot in Bell Pepper (Capsicum annuum L.). J. Hortic. Sci. Biotechnol. 2000, 75, 364–369. [Google Scholar] [CrossRef]
- Sun, Y.; Feng, H.; Liu, F. Comparative Effect of Partial Root-Zone Drying and Deficit Irrigation on Incidence of Blossom-End Rot in Tomato under Varied Calcium Rates. J. Exp. Bot. 2013, 64, 2107–2116. [Google Scholar] [CrossRef]
- Périard, Y.; Caron, J.; Lafond, J.A.; Jutras, S. Root Water Uptake by Romaine Lettuce in a Muck Soil: Linking Tip Burn to Hydric Deficit. Vadose Zone J. 2015, 14, vzj2014.10.0139. [Google Scholar] [CrossRef]
- Kuronuma, T.; Ando, M.; Watanabe, H. Tipburn Incidence and Ca Acquisition and Distribution in Lisianthus (Eustoma grandiflorum (Raf.) Shinn.) Cultivars under Different Ca Concentrations in Nutrient Solution. Agronomy 2020, 10, 216. [Google Scholar] [CrossRef]
- Ee, R.; Eriksen, L.; Knepper, C.; Cahn, M.D.; Mou, B. Screening of Lettuce Germplasm for Agronomic Traits under Low Water Conditions. HortScience 2016, 51, 669–679. [Google Scholar]
- Barta, D.J.; Tibbitts, T.W. Calcium Localization in Lettuce Leaves with and without Tipburn: Comparison of Controlled-Environment and Field-Grown Plants. HortScience 1991, 116, 870–875. [Google Scholar] [CrossRef]
- Millones-Chanamé, C.E.; de Oliveira, A.M.S.; de Castro, E.M.; Maluf, W.R. Inheritance of Blossom End Rot Resistance Induced by Drought Stress and of Associated Stomatal Densities in Tomatoes. Euphytica 2019, 215, 120. [Google Scholar] [CrossRef]
- Adams, S.R.; Cockshull, K.E.; Cave, C.R.J. Effect of Temperature on the Growth and Development of Tomato Fruits. Ann. Bot. 2001, 88, 869–877. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat Tolerance in Plants: An Overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Sattar, F.A.; Hamooh, B.T.; Wellman, G.; Ali, M.A.; Shah, S.H.; Anwar, Y.; Mousa, M.A.A. Growth and Biochemical Responses of Potato Cultivars under in Vitro Lithium Chloride and Mannitol Simulated Salinity and Drought Stress. Plants 2021, 10, 924. [Google Scholar] [CrossRef]
- Kałużewicz, A.; Krzesiński, W.; Knaflewski, M. Effect of Temperature on the Yield and Quality of Broccoli Heads. J. Fruit Ornam. Plant Res. 2009, 71, 51–58. [Google Scholar] [CrossRef]
- Mølmann, J.A.B.; Steindal, A.L.H.; Bengtsson, G.B.; Seljåsen, R.; Lea, P.; Skaret, J.; Johansen, T.J. Effects of Temperature and Photoperiod on Sensory Quality and Contents of Glucosinolates, Flavonols and Vitamin C in Broccoli Florets. Food Chem. 2015, 172, 47–55. [Google Scholar] [CrossRef]
- Wiebe, H.-J. The Morphological Development of Cauliflower and Broccoli Cultivars Depending on Temperature. Sci. Hortic. 1975, 3, 95–101. [Google Scholar] [CrossRef]
- Tibbitts, T.W.; Theodore, G.F. Effects of Relative Humidity and Root Temperature on Calcium Concentration and Tipburn Development in Lettuce. J. Am. Soc. Hortic. Sci. 1984, 109, 128–131. [Google Scholar]
- Tonetto de Freitas, S.; McElrone, A.J.; Shackel, K.A.; Mitcham, E.J. Calcium Partitioning and Allocation and Blossom-End Rot Development in Tomato Plants in Response to Whole-Plant and Fruit-Specific Abscisic Acid Treatments. J. Exp. Bot. 2014, 65, 235–247. [Google Scholar] [CrossRef]
- Lee, J.G.; Choi, C.S.; Jang, Y.A.; Jang, S.W.; Lee, S.G.; Um, Y.C. Effects of Air Temperature and Air Flow Rate Control on the Tipburn Occurrence of Leaf Lettuce in a Closed-Type Plant Factory System. Hortic. Environ. Biotechnol. 2013, 54, 303–310. [Google Scholar] [CrossRef]
- Gonzalo, M.J.; Li, Y.-C.; Chen, K.-Y.; Gil, D.; Montoro, T.; Nájera, I.; Baixauli, C.; Granell, A.; Monforte, A.J. Genetic Control of Reproductive Traits in Tomatoes Under High Temperature. Front. Plant Sci. 2020, 11, 326. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.Y.; Paek, K.Y.; Lee, Y.B. Effect of Air Temperature on Tipburn Incidence of Butterhead and Leaf Lettuce in a Plant Factory. In Transplant Production in the 21st Century; Springer: Dordrecht, The Netherlands, 2000; pp. 166–171. [Google Scholar]
- Tonetto de Freitas, S.; Padda, M.; Wu, Q.; Park, S.; Mitcham, E.J. Dynamic Alternations in Cellular and Molecular Components during Blossom-End Rot Development in Tomatoes Expressing SCAX1, a Constitutively Active Ca2+/H+ Antiporter from Arabidopsis. Plant Physiol. 2011, 156, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Jenni, S.; Truco, M.J.; Michelmore, R.W. Quantitative Trait Loci Associated with Tipburn, Heat Stress-Induced Physiological Disorders, and Maturity Traits in Crisphead Lettuce. Theor. Appl. Genet. 2013, 126, 3065–3079. [Google Scholar] [CrossRef]
- Ruan, C.-J.; Da Silva, J.A.T.; Mopper, S.; Qin, P.; Lutts, S. Halophyte Improvement for a Salinized World. Crit. Rev. Plant Sci. 2010, 29, 329–359. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant Responses to Salt Stress: Adaptive Mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Liu, X.; Baird, W.V. Identification of a Novel Gene, HaABRC5, from Helianthus annuus (Asteraceae) That Is Upregulated in Response to Drought, Salinity, and Abscisic Acid. Am. J. Bot. 2004, 91, 184–191. [Google Scholar] [CrossRef]
- Xu, C.; Mou, B. Evaluation of Lettuce Genotypes for Salinity Tolerance. Hortscience 2015, 50, 1441–1446. [Google Scholar] [CrossRef]
- Alam, M.S.; Tester, M.; Fiene, G.; Mousa, M.A.A. Early Growth Stage Characterization and the Biochemical Responses for Salinity Stress in Tomato. Plants 2021, 10, 712. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Z.; Zhang, Z.; Guo, X.; Wu, M.; Rasool, G.; Qiu, R.; Wang, X. Effects of Uneven Vertical Distribution of Soil Salinity on Blossom-End Rot of Tomato Fruit. HortScience 2017, 52, 958–964. [Google Scholar] [CrossRef]
- Al-Maskri, A.; Al-Klharusi, L.; Al-Miqbali, H.; Khran, M.M. Effects of Salinity Stress on Growth of Lettuce (Lactuca sativa) under Closed-Recycle Nutrient Film Technique. Agric. Biol. 2010, 12, 377–380. [Google Scholar]
- Zhai, Y.; Yang, Q.; Hou, M. The Effects of Saline Water Drip Irrigation on Tomato Yield, Quality, and Blossom-End Rot Incidence–A 3a Case Study in the South of China. PLoS ONE 2015, 10, e0142204. [Google Scholar] [CrossRef] [PubMed]
- Zushi, K.; Matsuzoe, N. Utilization of Correlation Network Analysis to Identify Differences in Sensory Attributes and Organoleptic Compositions of Tomato Cultivars Grown under Salt Stress. Sci. Hortic. 2011, 129, 18–26. [Google Scholar] [CrossRef]
- Kang, S.M.; Shahzad, R.; Bilal, S.; Khan, A.L.; Park, Y.G.; Lee, K.E.; Asaf, S.; Khan, M.A.; Lee, I.J. Indole-3-Acetic-Acid and ACC Deaminase Producing Leclercia Adecarboxylata MO1 Improves Solanum lycopersicum L. Growth and Salinity Stress Tolerance by Endogenous Secondary Metabolites Regulation. BMC Microbiol. 2019, 19, 80. [Google Scholar] [CrossRef] [PubMed]
- Akram, W.; Aslam, H.; Ahmad, S.R.; Anjum, T.; Yasin, N.A.; Khan, W.U.; Ahmad, A.; Guo, J.; Wu, T.; Luo, W.; et al. Bacillus Megaterium Strain A12 Ameliorates Salinity Stress in Tomato Plants through Multiple Mechanisms. J. Plant Interact. 2019, 14, 506–518. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.D.C.; Duan, J.; DiBernardo, M.; Zetter, E.; Campos-García, J.; Glick, B.R.; Santoyo, G. The Production of ACC Deaminase and Trehalose by the Plant Growth Promoting Bacterium Pseudomonas sp. UW4 Synergistically Protect Tomato Plants against Salt Stress. Front. Microbiol. 2019, 10, 1392. [Google Scholar] [CrossRef] [PubMed]
- Vaishnav, A.; Singh, J.; Singh, P.; Rajput, R.S.; Singh, H.B.; Sarma, B.K. Sphingobacterium sp. BHU-AV3 Induces Salt Tolerance in Tomato by Enhancing Antioxidant Activities and Energy Metabolism. Front. Microbiol. 2020, 11, 443. [Google Scholar] [CrossRef]
- Ha-Tran, D.M.; Nguyen, T.T.M.; Hung, S.-H.; Huang, E.; Huang, C.-C. Roles of Plant Growth-Promoting Rhizobacteria (PGPR) in Stimulating Salinity Stress Defense in Plants: A Review. Int. J. Mol. Sci. 2021, 22, 3154. [Google Scholar] [CrossRef]
- Adams, P. Plant Nutrition Demystified. Acta Hortic. 1999, 481, 341–344. [Google Scholar] [CrossRef]
- Kanai, S.; Moghaieb, R.E.; El-Shemy, H.A.; Panigrahi, R.; Mohapatra, P.K.; Ito, J.; Nguyen, N.T.; Saneoka, H.; Fujita, K. Potassium Deficiency Affects Water Status and Photosynthetic Rate of the Vegetative Sink in Green House Tomato Prior to Its Effects on Source Activity. Plant Sci. 2011, 180, 368–374. [Google Scholar] [CrossRef]
- Pujos, A.; Morard, P. Effects of Potassium Deficiency on Tomato Growth and Mineral Nutrition at the Early Production Stage. Plant Soil 1997, 189, 189–196. [Google Scholar] [CrossRef]
- Kataoka, K.; Sugimoto, K.; Ohashi, H.; Yamada, H. Effect of Organo-Mineral Fertilizer on Tomato Fruit Production and Incidence of Blossom-End Rot under Salinity. Hortic. J. 2017, 86, 357–364. [Google Scholar] [CrossRef]
- Ronga, D.; Caradonia, F.; Setti, L.; Hagassou, D.; Azevedo, C.V.G.; Milc, J.; Pedrazzi, S.; Allesina, G.; Arru, L.; Francia, E. Effects of Innovative Biofertilizers on Yield of Processing Tomato Cultivated in Organic Cropping Systems in Northern Italy. Acta Hortic. 2019, 1233, 129–135. [Google Scholar] [CrossRef]
- Reitz, N.F.; Mitcham, E.J. Validation and Demonstration of a Pericarp Disc System for Studying Blossom-End Rot of Tomatoes. Plant Methods 2021, 17, 28. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Cheng, N.H.; Pittman, J.K.; Yoo, K.S.; Park, J.; Smith, R.H.; Hirschi, K.D. Increased Calcium Levels and Prolonged Shelf Life in Tomatoes Expressing Arabidopsis H+/Ca2+ Transporters. Plant Physiol. 2005, 139, 1194–1206. [Google Scholar] [CrossRef] [PubMed]
- Corriveau, J.; Gaudreau, L.; Caron, J.; Jenni, S.; Gosselin, A. Testing Irrigation, Day/Night Foliar Spraying, Foliar Calcium and Growth Inhibitor as Possible Cultural Practices to Reduce Tipburn in Lettuce. Can. J. Plant Sci. 2012, 92, 889–899. [Google Scholar] [CrossRef][Green Version]
- de Freitas, S.T.; Handa, A.K.; Wu, Q.; Park, S.; Mitcham, E.J. Role of Pectin Methylesterases in Cellular Calcium Distribution and Blossom-End Rot Development in Tomato Fruit. Plant J. 2012, 71, 824–835. [Google Scholar] [CrossRef]
- Fenn, M.A.; Giovannoni, J.J. Phytohormones in Fruit Development and Maturation. Plant J. 2021, 105, 446–458. [Google Scholar] [CrossRef]
- Jiroutova, P.; Oklestkova, J.; Strnad, M. Crosstalk between Brassinosteroids and Ethylene during Plant Growth and under Abiotic Stress Conditions. Int. J. Mol. Sci. 2018, 19, 3283. [Google Scholar] [CrossRef]
- Bielach, A.; Hrtyan, M.; Tognetti, V.B. Plants under Stress: Involvement of Auxin and Cytokinin. Int. J. Mol. Sci. 2017, 18, 1427. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Li, W.; Nguyen, K.H.; Fujita, M.; Tran, L.-S.P. Strigolactones in Plant Adaptation to Abiotic Stresses: An Emerging Avenue of Plant Research. Plant Cell Environ. 2018, 41, 2227–2243. [Google Scholar] [CrossRef] [PubMed]
- de Jong, M.; Mariani, C.; Vriezen, W.H. The Role of Auxin and Gibberellin in Tomato Fruit Set. J. Exp. Bot. 2009, 60, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Castro, P.R.C. Plant Growth Regulators in Tomato Crop Production. Acta Hortic. 1980, 100, 99–104. [Google Scholar] [CrossRef]
- Bangerth, F. Calcium Related Physiological Disorders of Plants. Annu. Rev. Phytopathol. 1979, 17, 97–122. [Google Scholar] [CrossRef]
- de Freitas, S.; Shackel, K.A.; Mitcham, E.J. Abscisic Acid Triggers Whole-Plant and Fruit-Specific Mechanisms to Increase Fruit Calcium Uptake and Prevent Blossom End Rot Development in Tomato Fruit. J. Exp. Bot. 2011, 62, 2645–2656. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, S.T.; Martinelli, F.; Feng, B.; Reitz, N.F.; Mitcham, E.J. Transcriptome Approach to Understand the Potential Mechanisms Inhibiting or Triggering Blossom-End Rot Development in Tomato Fruit in Response to Plant Growth Regulators. J. Plant Growth Regul. 2018, 37, 183–198. [Google Scholar] [CrossRef]
- Kuronuma, T.; Watanabe, H. Identification of the Causative Genes of Calcium Deficiency Disorders in Horticulture Crops: A Systematic Review. Agriculture 2021, 11, 906. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Wei, Q.; Li, B.; Zhong, X.; Hu, T.; Hu, H.; Bao, C. Transcriptome-Wide Identification and Characterization of Circular RNAs in Leaves of Chinese Cabbage (Brassica rapa L. ssp. pekinensis) in Response to Calcium Deficiency-Induced Tip-Burn. Sci. Rep. 2019, 9, 14544. [Google Scholar]
- Gaion, L.A.; Muniz, J.C.; Barreto, R.F.; D’Amico-Damião, V.; de Mello Prado, R.; Carvalho, R.F. Amplification of Gibberellins Response in Tomato Modulates Calcium Metabolism and Blossom End Rot Occurrence. Sci. Hortic. 2019, 246, 498–505. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Calcium in Plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- Barker, A.V.; Ready, K.M. Ethylene Evolution by Tomatoes Stressed by Ammonium Nutrition. J. Am. Soc. Hortic. Sci. 1994, 119, 706–710. [Google Scholar] [CrossRef]
- Besada, C.; Gil, R.; Bonet, L.; Quiñones, A.; Intrigliolo, D.; Salvador, A. Chloride Stress Triggers Maturation and Negatively Affects the Postharvest Quality of Persimmon Fruit. Involvement of Calyx Ethylene Production. Plant Physiol. Biochem. 2016, 100, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, S.; Targolli, J.; Liu, L.F.; Ho, T.H.D.; Wu, R. Transgenic Approaches to Increase Dehydration-Stress Tolerance in Plants. Mol. Breed. 1999, 5, 493–503. [Google Scholar] [CrossRef]
- Birlanga, V.; Acosta-Motos, J.R.; Pérez-Pérez, J.M. Genotype-Dependent Tipburn Severity during Lettuce Hydroponic Culture Is Associated with Altered Nutrient Leaf Content. Agronomy 2021, 11, 616. [Google Scholar] [CrossRef]
- Jenni, S.; Hayes, R.J. Genetic Variation, Genotype × Environment Interaction, and Selection for Tipburn Resistance in Lettuce in Multi-Environments. Euphytica 2010, 171, 427–439. [Google Scholar] [CrossRef]
- Jenni, S.; Yan, W. Genotype by Environment Interactions of Heat Stress Disorder Resistance in Crisphead Lettuce. Plant Breed. 2009, 128, 374–380. [Google Scholar] [CrossRef]
- Koyama, R.; Sanada, M.; Itoh, H.; Kanechi, M.; Inagaki, N.; Uno, Y. In Vitro Evaluation of Tipburn Resistance in Lettuce (Lactuca sativa L.). Plant Cell Tissue Organ Cult. 2012, 108, 221–227. [Google Scholar] [CrossRef]
- Ryder, E.J.; Waycott, W. Crisphead Lettuce Resistant to Tipburn Cultivar Tiber and Eight Breeding Lines. HortScience 1998, 33, 903–904. [Google Scholar] [CrossRef]
- Macias-González, M.; Truco, M.J.; Bertier, L.D.; Jenni, S.; Simko, I.; Hayes, R.J.; Michelmore, R.W. Genetic Architecture of Tipburn Resistance in Lettuce. Theor. Appl. Genet. 2019, 132, 2209–2222. [Google Scholar] [CrossRef]
- Macias-González, M.; Truco, M.J.; Han, R.; Jenni, S.; Michelmore, R.W. High Resolution Genetic Dissection of the Major QTL for Tipburn Resistance in Lettuce, Lactuca sativa. G3 Genes Genomes Genet. 2021, 11, jkab097. [Google Scholar] [CrossRef]
- Holmes, S.C.; Wells, D.E.; Pickens, J.M.; Kemble, J.M. Selection of Heat Tolerant Lettuce (Lactuca sativa L.) Cultivars Grown in Deep Water Culture and Their Marketability. Horticulturae 2019, 5, 50. [Google Scholar] [CrossRef]
- Carassay, L.R.; Bustos, D.A.; Golberg, A.D.; Taleisnik, E. Tipburn in Salt-Affected Lettuce (Lactuca sativa L.) Plants Results from Local Oxidative Stress. J. Plant Physiol. 2012, 169, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Martins, L.B.; Sermons, S.; Balint-Kurti, P. Genetic and Physiological Characterization of a Calcium Deficiency Phenotype in Maize. G3 Genes Genomes Genet. 2020, 10, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Uozumi, A.; Ikeda, H.; Hiraga, M.; Kanno, H.; Nanzyo, M.; Nishiyama, M.; Kanahama, K.; Kanayama, Y. Tolerance to Salt Stress and Blossom-End Rot in an Introgression Line, IL8-3, of Tomato. Sci. Hortic. 2012, 138, 1–6. [Google Scholar] [CrossRef]
- Watanabe, T.; Tomizaki, R.; Watanabe, R.; Maruyama, H.; Shinano, T.; Urayama, M.; Kanayama, Y. Ionomic Differences between Tomato Introgression Line IL8–3 and Its Parent Cultivar M82 with Different Trends to the Incidence of Blossom-End Rot. Sci. Hortic. 2021, 287, 110266. [Google Scholar] [CrossRef]
- Changmai, T.; Gertphol, S.; Chulak, P. Smart Hydroponic Lettuce Farm Using Internet of Things. In Proceedings of the 2018 10th International Conference on Knowledge and Smart Technology: Cybernetics in the Next Decades, KST 2018, Chiangmai, Thailand, 21 January–3 February 2018; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2018; pp. 231–236. [Google Scholar]
- Duarte-Galvan, C.; Romero-Troncoso, R.d.J.; Torres-Pacheco, I.; Guevara-Gonzalez, R.G.; Fernandez-Jaramillo, A.A.; Contreras-Medina, L.M.; Carrillo-Serrano, R.V.; Millan-Almaraz, J.R. FPGA-Based Smart Sensor for Drought Stress Detection in Tomato Plants Using Novel Physiological Variables and Discrete Wavelet Transform. Sensors 2014, 14, 18650–18669. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, R.; Lowenberg-Deboer, J. Precision Agriculture and Sustainability. Precis. Agric. 2004, 5, 359–387. [Google Scholar] [CrossRef]
- Sambo, P.; Nicoletto, C.; Giro, A.; Pii, Y.; Valentinuzzi, F.; Mimmo, T.; Lugli, P.; Orzes, G.; Mazzetto, F.; Astolfi, S.; et al. Hydroponic Solutions for Soilless Production Systems: Issues and Opportunities in a Smart Agriculture Perspective. Front. Plant Sci. 2019, 10, 923. [Google Scholar] [CrossRef]
- Yin, H.; Cao, Y.; Marelli, B.; Zeng, X.; Mason, A.J.; Cao, C. Soil Sensors and Plant Wearables for Smart and Precision Agriculture. Adv. Mater. 2021, 33, 2007764. [Google Scholar] [CrossRef]
- Srivani, P.; Yamuna Devi, C.; Manjula, S.H. A Controlled Environment Agriculture with Hydroponics: Variants, Parameters, Methodologies and Challenges for Smart Farming. In Proceedings of the 2019 Fifteenth International Conference on Information Processing (ICINPRO), Bengaluru, India, 20–22 December 2019; pp. 1–8. [Google Scholar]
- Hasan, M.; Tanawala, B.; Patel, K.J. Deep Learning Precision Farming: Tomato Leaf Disease Detection by Transfer Learning. In Proceedings of the 2nd International Conference on Advanced Computing and Software Engineering, Sultanpur, India, 8–9 February 2019; pp. 171–175. [Google Scholar]
- Rubanga, D.P.; Hatanaka, K.; Shimada, S. Development of a Simplified Smart Agriculture System for Small-Scale Greenhouse Farming. Sens. Mater. 2019, 31, 831–843. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Y.; Wang, T.; Pan, J.; Zhou, B.; Muhammad, T.; Zhou, C.; Li, Y. Micro-Nano Bubble Water Oxygation: Synergistically Improving Irrigation Water Use Efficiency, Crop Yield and Quality. J. Clean. Prod. 2019, 222, 835–843. [Google Scholar] [CrossRef]
- Wilson, M.A.; Tran, N.H.; Milev, A.S.; Kannangara, G.S.K.; Volk, H.; Lu, G.Q.M. Nanomaterials in Soils. Geoderma 2008, 146, 291–302. [Google Scholar] [CrossRef]
- Corradini, E.; de Moura, M.R.; Mattoso, L.H.C. A Preliminary Study of the Incorparation of NPK Fertilizer into Chitosan Nanoparticles. Express Polym. Lett. 2010, 4, 509–515. [Google Scholar] [CrossRef]
- Manjunatha, S.B.; Biradar, D.P.; Aladakatti, Y.R. Nanotechnology and Its Applications in Agriculture: A Review. J. Farm Sci. 2016, 29, 1–13. [Google Scholar]
- Lai, F.; Wissing, S.A.; Müller, R.H.; Fadda, A.M. Artemisia arborescens L Essential Oil-Loaded Solid Lipid Nanoparticles for Potential Agricultural Application: Preparation and Characterization. AAPS PharmSciTech 2006, 7, E10. [Google Scholar] [CrossRef]
- Liu, R.; Lal, R. Potentials of Engineered Nanoparticles as Fertilizers for Increasing Agronomic Productions. Sci. Total Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef]
- Khodakovskaya, M.V.; Kim, B.S.; Kim, J.N.; Alimohammadi, M.; Dervishi, E.; Mustafa, T.; Cernigla, C.E. Carbon Nanotubes as Plant Growth Regulators: Effects on Tomato Growth, Reproductive System, and Soil Microbial Community. Small 2013, 9, 115–123. [Google Scholar] [CrossRef]
- Lucas García, J.A.; Probanza, A.; Ramos, B.; Palomino, M.; Gutiérrez Mañero, F.J. Effect of Inoculation of Bacillus licheniformis on Tomato and Pepper. Agron. EDP Sci. 2004, 24, 169–176. [Google Scholar]
- Sheikhalipour, P.; Bolandndnaza, S.A.; Panahandeh, J. Influence of KSB, PSB and NFB on Fruit Quality and Potassium Contents in Tomato. Int. J. Adv. Biol. Biomed. Res. 2016, 4, 170–178. [Google Scholar]
- Lee, S.W.; Ahn, I.P.; Sim, S.Y.; Lee, S.Y.; Seo, M.W.; Kim, S.; Park, S.Y.; Lee, Y.H.; Kang, S. Pseudomonas sp. LSW25R, Antagonistic to Plant Pathogens, Promoted Plant Growth, and Reduced Blossom-End Rot of Tomato Fruits in a Hydroponic System. Eur. J. Plant Pathol. 2010, 126, 1–11. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J. Beneficial Bacteria and Fungi in Hydroponic Systems: Types and Characteristics of Hydroponic Food Production Methods. Sci. Hortic. 2015, 195, 206–215. [Google Scholar] [CrossRef]

| Classification | Categories | Characteristics |
|---|---|---|
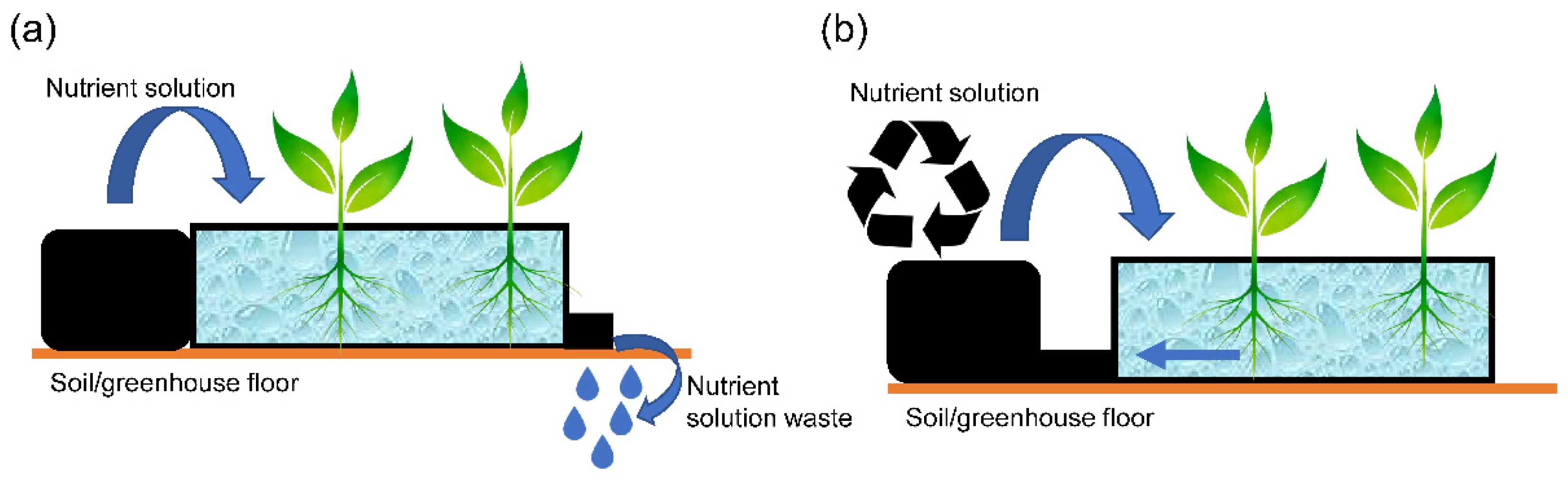
| Nutrient solution use | Open-loop systems | The used nutrient solution is discarded |
| Closed-loop systems | Nutrient solution is reformulated and returned to the system | |
| Physical state of root growth media | Gaseous (aeroponic cultivation) | Spray column system |
| Schwalbach system | ||
| Aero-Gro system | ||
| Liquid (hydroponic cultivation) | Deep floated technique | |
| Nutrient film technique | ||
| New growing system technique | ||
| Liquid (aquaponic cultivation) | Nutrient solution is derived from waste from fish production | |
| Solid (substrate cultivation) | Directly in substrate | |
| Systems of cultivation in bags or containers | ||
| Single unit culture systems |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birlanga, V.; Acosta-Motos, J.R.; Pérez-Pérez, J.M. Mitigation of Calcium-Related Disorders in Soilless Production Systems. Agronomy 2022, 12, 644. https://doi.org/10.3390/agronomy12030644
Birlanga V, Acosta-Motos JR, Pérez-Pérez JM. Mitigation of Calcium-Related Disorders in Soilless Production Systems. Agronomy. 2022; 12(3):644. https://doi.org/10.3390/agronomy12030644
Chicago/Turabian StyleBirlanga, Virginia, José Ramón Acosta-Motos, and José Manuel Pérez-Pérez. 2022. "Mitigation of Calcium-Related Disorders in Soilless Production Systems" Agronomy 12, no. 3: 644. https://doi.org/10.3390/agronomy12030644
APA StyleBirlanga, V., Acosta-Motos, J. R., & Pérez-Pérez, J. M. (2022). Mitigation of Calcium-Related Disorders in Soilless Production Systems. Agronomy, 12(3), 644. https://doi.org/10.3390/agronomy12030644








