Soil Organic Carbon and Nutrients Affected by Tree Species and Poultry Litter in a 17-Year Agroforestry Site
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Soil Sampling and Analysis
2.3. Statistical Analysis
3. Results and Discussion
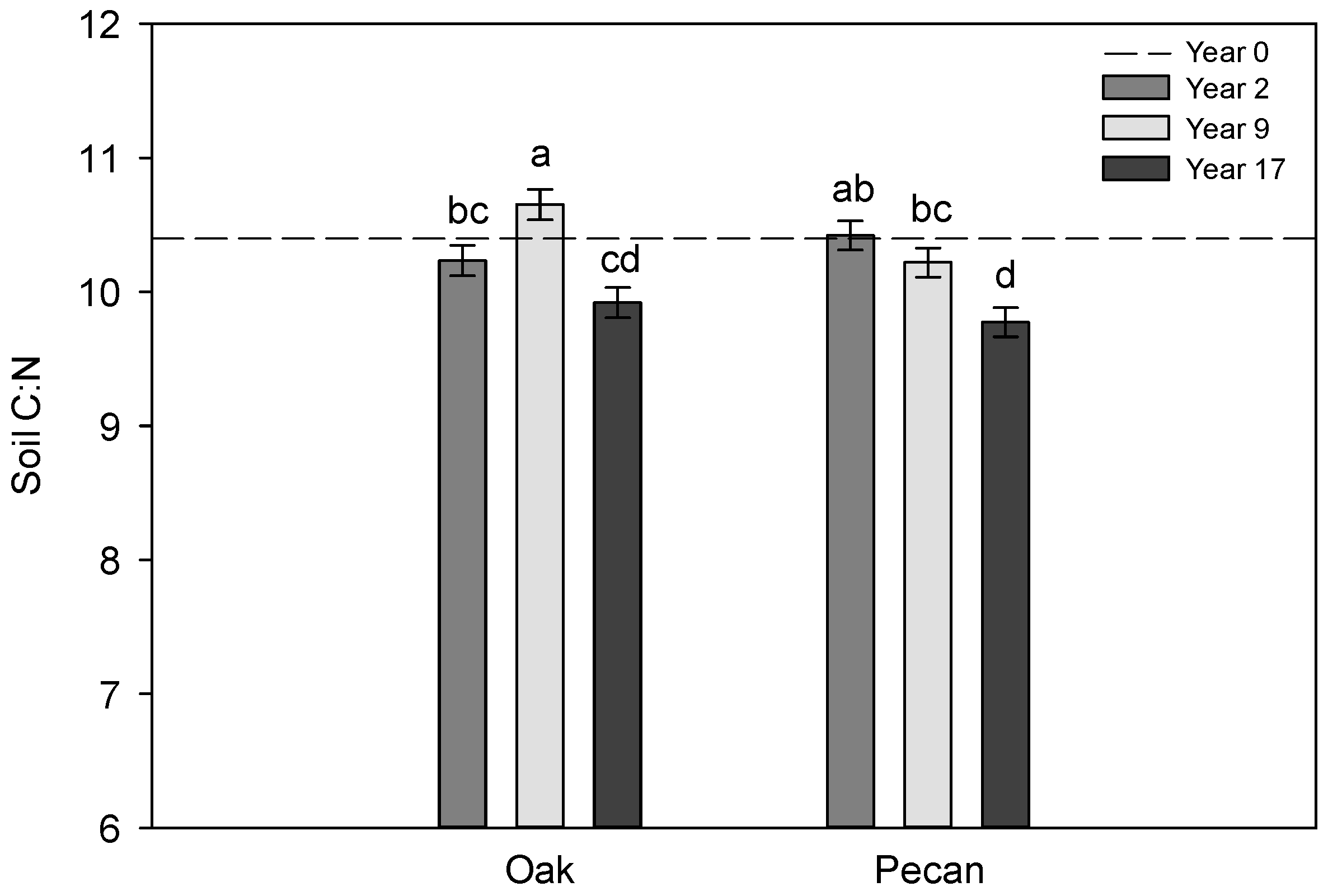
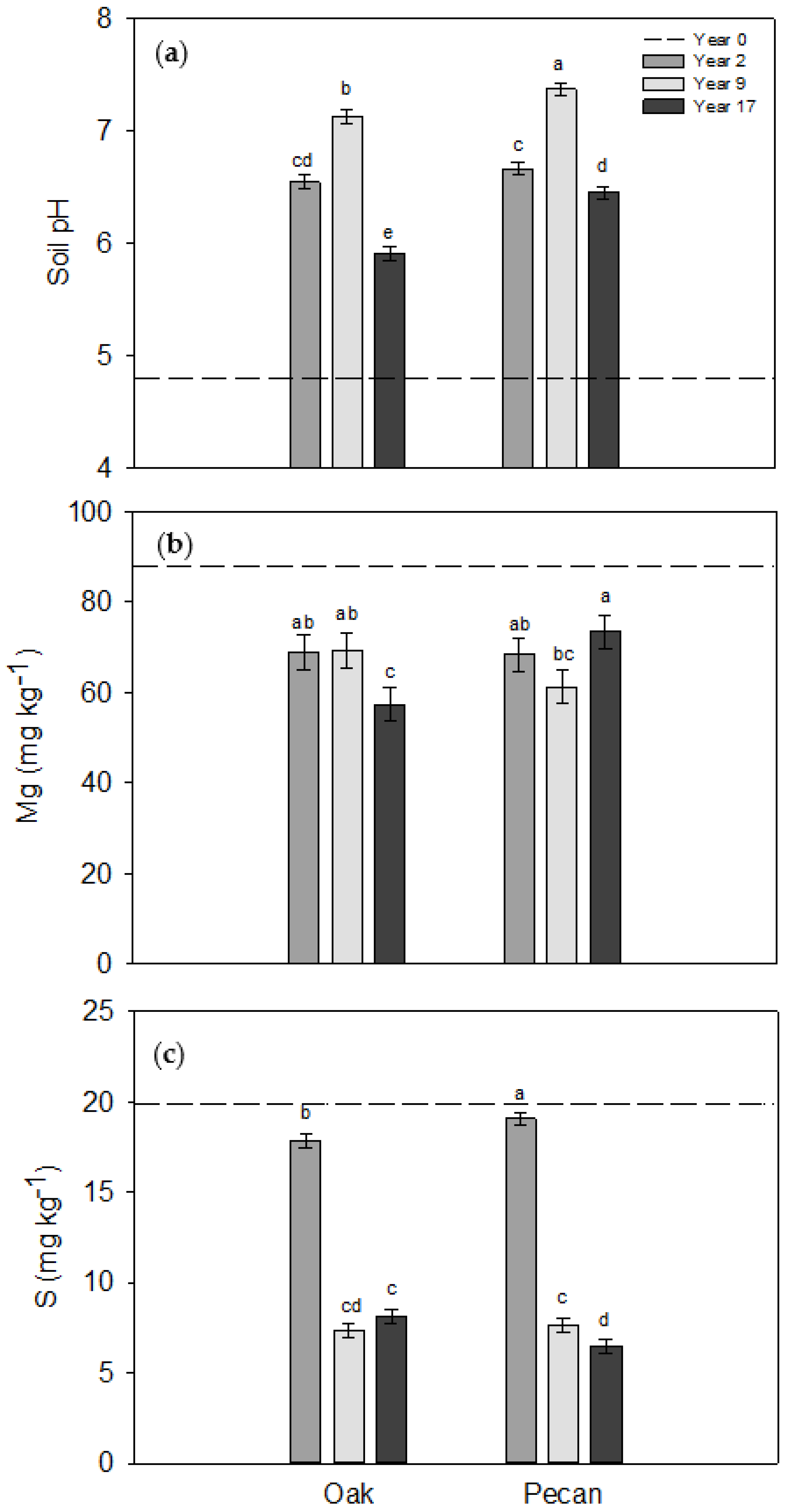
3.1. Trends in Soil Properties across Years
3.2. Soil Properties as Affected by Tree Species and Fertilization Sources after 17 Years
3.3. Soil Properties in 2016: Linkages
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Udawatta, R.P.; Jose, S. Agroforestry strategies to sequester carbon in temperate North America. Agrofor. Syst. 2012, 86, 225–242. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R. Soil organic carbon sequestration in agroforestry systems. A review. Agron. Sustain. Dev. 2014, 34, 443–454. [Google Scholar] [CrossRef] [Green Version]
- Schoeneberger, M.M. Agroforestry: Working trees for sequestering carbon on agricultural lands. Agrofor. Syst. 2009, 75, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Dollinger, J.; Jose, S. Agroforestry for soil health. Agrofor. Syst. 2018, 92, 213–219. [Google Scholar] [CrossRef] [Green Version]
- Gelaw, A.M.; Singh, B.R.; Lal, R. Soil quality indices for evaluating smallholder agricultural land uses in northern ethiopia. Sustainability 2015, 7, 2322–2337. [Google Scholar] [CrossRef] [Green Version]
- Jose, S. Agroforestry for ecosystem services and environmental benefits: An overview. Agrofor. Syst. 2009, 76, 1–10. [Google Scholar] [CrossRef]
- Nair, P.K.R. Agroforestry Systems and Environmental Quality: Introduction. J. Environ. Qual. 2011, 40, 784. [Google Scholar] [CrossRef] [Green Version]
- De Stefano, A.; Jacobson, M.G. Soil carbon sequestration in agroforestry systems: A meta-analysis. Agrofor. Syst. 2018, 92, 285–299. [Google Scholar] [CrossRef]
- Muchane, M.N.; Sileshi, G.W.; Gripenberg, S.; Jonsson, M.; Pumarino, L.; Barrios, E. Agroforestry boosts soil-mediated ecosystem services in the humid and sub- humid tropics: A meta-analysis. Agric. Ecosyst. Environ. 2020, 295, 106899. [Google Scholar] [CrossRef]
- Waldron, A.; Garrity, D.; Malhi, Y.; Girardin, C.; Miller, D.C.; Seddon, N. Agroforestry Can Enhance Food Security While Meeting Other Sustainable Development Goals. Trop. Conserv. Sci. 2017, 10, 1940082917720667. [Google Scholar] [CrossRef] [Green Version]
- Gurmessa, B.; Ashworth, A.J.; Yang, Y.; Adhikari, K.; Savin, M.; Owens, P.; Sauer, T.; Pedretti, E.F.; Cocco, S.; Corti, G. Soil Bacterial Diversity Based on Management and Topography in a Silvopastoral System. Appl. Soil Ecol. 2021, 163, 103918. [Google Scholar] [CrossRef]
- Niyigena, V.; Ashworth, A.J.; Nieman, C.; Acharya, M.; Coffey, K.P.; Philipp, D.; Meadors, L.; Sauer, T.J. Factors Affecting Sugar Accumulation and Fluxes in Warm- and Cool-Season Forages Grown in a Silvopastoral System. Agronomy 2021, 11, 354. [Google Scholar] [CrossRef]
- Augusto, L.; De Schrijver, A.; Vesterdal, L.; Smolander, A.; Prescott, C.; Ranger, J. Influences of evergreen gymnosperm and deciduous angiosperm tree species on the functioning of temperate and boreal forests. Biol. Rev. 2015, 90, 444–466. [Google Scholar] [CrossRef]
- Hoosbeek, M.R.; Remme, R.P.; Rusch, G.M. Trees enhance soil carbon sequestration and nutrient cycling in a silvopastoral system in south-western Nicaragua. Agrofor. Syst. 2018, 92, 263–273. [Google Scholar] [CrossRef] [Green Version]
- Baah-Acheamfour, M.; Carlyle, C.N.; Bork, E.W.; Chang, S.X. Trees increase soil carbon and its stability in three agroforestry systems in central Alberta, Canada. For. Ecol. Manag. 2014, 328, 131–139. [Google Scholar] [CrossRef]
- Casals, P.; Romero, J.; Rusch, G.M.; Ibrahim, M. Soil organic C and nutrient contents under trees with different functional characteristics in seasonally dry tropical silvopastures. Plant Soil 2014, 374, 643–659. [Google Scholar] [CrossRef]
- Zinn, Y.L.; Guerra, A.R.; Silva, C.A.; Faria, J.A.; Silva, T.A.C. Soil organic carbon and morphology as affected by pine plantation establishment in Minas Gerais, Brazil. For. Ecol. Manag. 2014, 318, 261–269. [Google Scholar] [CrossRef]
- Udawatta, R.P.; Gantzer, C.J.; Jose, S. Agroforestry Practices and Soil Ecosystem Services. In Soil Health and Intensification of Agroecosystems; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 305–333. ISBN 9780128054017. [Google Scholar]
- Nair, V.D.; Graetz, D.A. Agroforestry as an approach to minimizing nutrient loss from heavily fertilized soils: The Florida experience. Agrofor. Syst. 2004, 61, 269–279. [Google Scholar] [CrossRef]
- Tripathi, G.; Deora, R.; Singh, G. The influence of litter quality and micro-habitat on litter decomposition and soil properties in a silvopasture system. Acta Oecol. 2013, 50, 40–50. [Google Scholar] [CrossRef]
- Stigter, K.C.J. Agroforestry and (Micro) Climate Change. In Tree-Crops Interactions, Agroforestry in a Changing Climate, 2nd ed.; CABI: Selangor, Malaysia, 2015; pp. 119–145. [Google Scholar]
- Sauer, T.J.; Coblentz, W.K.; Thomas, A.L.; Brye, K.R.; Brauer, D.K.; Skinner, J.V.; Van Brahana, J.; DeFauw, S.L.; Hays, P.D.; Moffitt, D.C.; et al. Nutrient cycling in an agroforestry alley cropping system receiving poultry litter or nitrogen fertilizer. Nutr. Cycl. Agroecosyst. 2014, 101, 167–179. [Google Scholar] [CrossRef]
- Gaston, L.; Blazier, M.; Beasley, J.; Dodla, S.; Felicien, W.; Clason, T. Silvopasture Switchgrass Fertilized with Poultry Litter: Nutrient Removal, Soil Fertility, and Runoff Water Quality. Commun. Soil Sci. Plant Anal. 2019, 50, 948–958. [Google Scholar] [CrossRef]
- NOAA National Oceanic And Atmospheric Administration Net Work, Weather Station at the Northwest Arkansas Regional Airport. Available online: https://www.noaa.gov/opportunity-type/network (accessed on 25 February 2022).
- Dold, C.; Thomas, A.L.; Ashworth, A.J.; Philipp, D.; Brauer, D.K.; Sauer, T.J. Carbon sequestration and nitrogen uptake in a temperate silvopasture system. Nutr. Cycl. Agroecosyst. 2019, 114, 85–98. [Google Scholar] [CrossRef]
- Rhoades, C.C.; Rhodes, C. Single-tree influences on soil properties in agroforestry: Lessons from natural forest and savanna ecosystems. Agrofor. Syst. 1997, 35, 71–94. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Blake, G.R.; Hartge, K.H. Bulk Density. In Methods of Soil Analysis, Part 1: Physical and Mineralogical Methods; American Society of Agronomy—Soil Science Society of America: Madison, WI, USA, 1986; Volume 9, pp. 363–375. ISBN 9780891188643. [Google Scholar]
- Fialho, R.C.; Zinn, Y.L. Changes in soil organic carbon under eucalyptus plantations in brazil: A comparative analysis. Land Degrad. Dev. 2014, 437, 428–437. [Google Scholar] [CrossRef]
- Saxton, A.M. A macro for converting mean separation output to letter groupings in Proc Mixed. In Proceedings of the 23rd SAS Users Group International, Nashville, TN, USA, 22–25 March 1998; pp. 1243–1246. [Google Scholar]
- Nair, P.K.R.; Kumar, B.M.; Nair, V.D. Agroforestry as a strategy for carbon sequestration. J. Plant Nutr. Soil Sci. 2009, 172, 10–23. [Google Scholar] [CrossRef]
- West, T.O.; Post, W.M. Soil Organic Carbon Sequestration Rates by Tillage and Crop Rotation: A Global Data Analysis. Soil Sci. Soc. Am. J. 1997, 66, 1930–1946. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Chen, H.Y.H.; Bork, E.W.; Carlyle, C.N.; Chang, S.X. Carbon accumulation in agroforestry systems is affected by tree species diversity, age and regional climate: A global meta-analysis. Glob. Ecol. Biogeogr. 2020, 29, 1817–1828. [Google Scholar] [CrossRef]
- Ashworth, A.J.; Adams, T.; Kharel, T.; Philipp, D.; Owens, P.; Sauer, T. Root decomposition in silvopastures is influenced by grazing, fertility, and grass species. Agrosyst. Geosci. Environ. 2021, 4, e20190. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Priming effects: Interactions between living and dead organic matter. Soil Biol. Biochem. 2010, 42, 1363–1371. [Google Scholar] [CrossRef]
- Adams, T.; Ashworth, A.J.; Sauer, T. Soil CO2 evolution is driven by forage species, soil moisture, grazing pressure, poultry litter fertilization, and seasonality in silvopastures. Agrosyst. Geosci. Environ. 2021, 4, e20179. [Google Scholar] [CrossRef]
- Kingery, W.L.; Wood, C.W.; Delaney, D.P.; Williams, J.C.; Mullins, G.L.; van Santen, E. Implications of Long-Term Land Application of Poultry Litter on Tall Fescue Pastures. J. Prod. Agric. 1993, 6, 390–395. [Google Scholar] [CrossRef]
- Mowrer, J.; Endale, D.M.; Schomberg, H.H.; Norris, S.E.; Woodroof, R.H. Liming potential of poultry litter in a long-term tillage comparison study. Soil Tillage Res. 2020, 196, 104446. [Google Scholar] [CrossRef]
- O’Brien, P.L.; Thomas, A.L.; Sauer, T.J.; Brauer, D.K. Foliar nutrient concentrations of three economically important tree species in an alley-cropping system. J. Plant Nutr. 2020, 43, 2557–2568. [Google Scholar] [CrossRef]
- Kharel, T.P.; Ashworth, A.J.; Owens, P.R.; Philipp, D.; Thomas, A.L.; Sauer, T.J. Teasing apart silvopasture system components using machine learning for optimization. Soil Syst. 2021, 5, 41. [Google Scholar] [CrossRef]
- Van Eekeren, N.; De Boer, H.; Bloem, J.; Schouten, T.; Rutgers, M.; de Goede, R.; Brussard, L. Soil biological quality of grassland fertilized with adjusted cattle manure slurries in comparison with organic and inorganic fertilizers. Biol Fertil. Soils 2009, 45, 595–608. [Google Scholar] [CrossRef]
- Ozlu, E.; Sandhu, S.S.; Kumar, S.; Arriaga, F.J. Soil health indicators impacted by long-term cattle manure and inorganic fertilizer application in a corn-soybean rotation of South Dakota. Sci. Rep. 2019, 9, 11776. [Google Scholar] [CrossRef] [Green Version]
- Ylagan, S.; Amorim, H.C.S.; Ashworth, A.J.; Sauer, T.; Wienhold, B.J.; Owens, P.R.; Zinn, Y.L.; Brye, K.R. Soil quality assessment of an agroforestry system following long-term management in the Ozark Highlands. Agrosyst. Geosci. Environ. 2021, 4, e20194. [Google Scholar] [CrossRef]
- Ashworth, A.J.; Allen, F.L.; Wight, J.P.; Saxton, A.M.; Tyler, D.D.; Sams, C.E. Soil Organic Carbon Sequestration Rates under Crop Sequence Diversity, Bio-Covers, and No-Tillage. Soil Sci. Soc. Am. J. 2014, 78, 1726–1733. [Google Scholar] [CrossRef]
- Blazier, M.A.; Gaston, L.A.; Clason, T.R.; Farrish, K.W.; Oswald, B.P.; Evans, H.A. Nutrient Dynamics and Tree Growth of Silvopastoral Systems: Impact of Poultry Litter. J. Environ. Qual. 2008, 37, 1546–1558. [Google Scholar] [CrossRef]
- Berg, B. Litter decomposition and organic matter turnover in northern forest soils. For. Ecol. Manag. 2000, 133, 13–22. [Google Scholar] [CrossRef]

| Source of Variation | Soil Properties † | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| pH | P | K | Ca | Mg | S | SOC | N | C:N | |
| p-value ‡ | |||||||||
| Year | <0.01 | <0.01 | 0.77 | <0.01 | 0.60 | <0.01 | 0.66 | 0.09 | <0.01 |
| Tree Species | <0.01 | <0.01 | 0.53 | 0.21 | 0.42 | 0.82 | 0.25 | 0.09 | 0.16 |
| Year × Tree Species | <0.01 | 0.36 | 0.39 | 0.71 | <0.01 | <0.01 | 0.43 | 0.20 | 0.03 |
| Fertilization | 0.09 | <0.01 | <0.01 | 0.98 | <0.01 | 0.13 | 0.75 | 0.41 | <0.01 |
| Year × Fertilization | 0.10 | <0.01 | <0.01 | 0.71 | 0.03 | <0.01 | 0.82 | 0.98 | 0.11 |
| Tree Species × Fertilization | <0.01 | <0.01 | 0.19 | <0.01 | 0.84 | <0.01 | <0.01 | <0.01 | 0.04 |
| Year × Tree Species × Fertilization | 0.73 | 0.69 | 0.18 | 0.58 | 0.08 | 0.82 | 0.79 | 0.66 | 0.29 |
| Source of Variation | Soil Properties † | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bd | pH | P | K | Ca | Mg | S | SOC | N | C:N | SOC (Mg ha−1) | N (Mg ha−1) | |
| p-value ‡ | ||||||||||||
| Tree Species | 0.36 | <0.01 | <0.01 | 0.69 | 0.51 | 0.42 | 0.04 | 0.59 | 0.12 | 0.01 | 0.81 | 0.14 |
| Fertilization | 0.72 | 0.32 | <0.01 | 0.11 | 0.69 | <0.01 | 0.64 | 0.96 | 0.47 | 0.06 | 0.68 | 0.68 |
| Tree Species × Fertilization | 0.76 | 0.12 | <0.01 | 0.28 | 0.01 | 0.06 | 0.09 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Depth | <0.01 | 0.20 | <0.01 | <0.01 | <0.01 | <0.01 | 0.04 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Tree Species × Depth | 0.56 | 0.76 | 0.02 | 0.54 | 0.28 | <0.01 | 0.53 | 0.17 | 0.37 | 0.01 | 0.22 | 0.52 |
| Fertilization × Depth | 0.57 | 0.99 | <0.01 | 0.36 | 0.43 | 0.67 | 0.15 | 0.35 | 0.34 | 0.69 | 0.59 | 0.69 |
| Tree Species × Fertilization × Depth | 0.63 | 0.51 | 0.13 | 0.62 | <0.01 | 0.09 | 0.83 | 0.06 | 0.14 | 0.98 | 0.39 | 0.60 |
| Depth | Soil Properties † | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bd | pH | P | K | Ca | Mg | S | SOC | N | C: N | SOC | N | |
| cm | Mg dm−3 | 1:1 H2O | mg kg−1 | % | Mg ha−1 | |||||||
| 0–15 | 1.16 b ‡ | 6.18 a | 49.6 a | 101.6 a | 1421.4 a | 63.8 a | 7.2 a | 1.50 a | 0.15 a | 9.8 a | 25.8 a | 2.6 a |
| 15–30 | 1.47 a | 6.12 a | 16.1 b | 47.6 b | 1016.1 b | 42.0 b | 6.7 b | 0.70 b | 0.08 b | 8.7 b | 15.4 b | 1.8 b |
| Tree Species | Fertilization Source † | Soil Properties ‡ | ||||||
|---|---|---|---|---|---|---|---|---|
| P | Ca | SOC | N | C: N | SOC | N | ||
| mg kg−1 | % | Mg ha−1 | ||||||
| Oak | Control | 42.1 a ¶ | 1302.5 ab | 1.19 a | 0.12 ab | 9.9 a | 22.4 a | 2.24 ab |
| PL | 39.3 a | 1067.1 b | 0.98 b | 0.11 b | 9.2 b | 18.6 b | 2.01 b | |
| Pecan | Control | 9.7 b | 1098.4 b | 1.01 b | 0.11 b | 8.9 b | 19.2 b | 2.09 b |
| PL | 40.3 a | 1407.2 a | 1.22 a | 0.13 a | 9.1 b | 22.2 a | 2.39 a | |
| Soil Properties † (0–15 cm) | |||||||||
| pH | P | K | Ca | Mg | S | SOC | N | C: N | |
| Bd | −0.24 ‡ | −0.35 | −0.25 | −0.55 | −0.35 | −0.07 | −0.57 | −0.61 | −0.10 |
| pH | −0.03 | −0.07 | 0.55 | 0.33 | −0.43 | 0.25 | 0.27 | 0.01 | |
| P | 0.36 | 0.55 | 0.21 | 0.32 | 0.61 | 0.60 | 0.22 | ||
| K | 0.27 | 0.29 | 0.06 | 0.19 | 0.25 | −0.07 | |||
| Ca | 0.44 | −0.10 | 0.71 | 0.71 | 0.29 | ||||
| Mg | −0.42 | 0.34 | 0.40 | −0.03 | |||||
| S | 0.37 | 0.30 | 0.34 | ||||||
| SOC | 0.96 | 0.49 | |||||||
| N | 0.25 | ||||||||
| Soil Properties (15–30 cm) | |||||||||
| pH | P | K | Ca | Mg | S | SOC | N | C: N | |
| Bd | −0.12 | −0.17 | 0.11 | −0.26 | 0.11 | −0.19 | −0.41 | −0.43 | −0.17 |
| pH | 0.15 | −0.14 | 0.67 | −0.02 | −0.58 | 0.32 | 0.35 | 0.09 | |
| P | 0.22 | 0.58 | 0.27 | 0.05 | 0.57 | 0.56 | 0.25 | ||
| K | 0.26 | 0.59 | 0.07 | 0.31 | 0.40 | −0.07 | |||
| Ca | 0.39 | −0.26 | 0.71 | 0.69 | 0.36 | ||||
| Mg | −0.08 | 0.24 | 0.25 | 0.08 | |||||
| S | 0.21 | 0.12 | 0.28 | ||||||
| SOC | 0.93 | 0.60 | |||||||
| N | 0.27 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amorim, H.C.S.; Ashworth, A.J.; Zinn, Y.L.; Sauer, T.J. Soil Organic Carbon and Nutrients Affected by Tree Species and Poultry Litter in a 17-Year Agroforestry Site. Agronomy 2022, 12, 641. https://doi.org/10.3390/agronomy12030641
Amorim HCS, Ashworth AJ, Zinn YL, Sauer TJ. Soil Organic Carbon and Nutrients Affected by Tree Species and Poultry Litter in a 17-Year Agroforestry Site. Agronomy. 2022; 12(3):641. https://doi.org/10.3390/agronomy12030641
Chicago/Turabian StyleAmorim, Helen C. S., Amanda J. Ashworth, Yuri L. Zinn, and Thomas J. Sauer. 2022. "Soil Organic Carbon and Nutrients Affected by Tree Species and Poultry Litter in a 17-Year Agroforestry Site" Agronomy 12, no. 3: 641. https://doi.org/10.3390/agronomy12030641
APA StyleAmorim, H. C. S., Ashworth, A. J., Zinn, Y. L., & Sauer, T. J. (2022). Soil Organic Carbon and Nutrients Affected by Tree Species and Poultry Litter in a 17-Year Agroforestry Site. Agronomy, 12(3), 641. https://doi.org/10.3390/agronomy12030641







