3.1. Parameters and Genetic Diversity
The genetic parameters were estimated using the F test and no interaction between genotypes and nutrients was detected. The experimental coefficient of variation (CVe) was below 20% for almost all nutrients and organs studied. This low value indicates the low environmental influence and high experimental precision.
On the other hand, an environmental dependence of some nutrients was also observed in some exceptional cases, for example: for nutrient Fe in flowers (45.06%); B in grain (22.41%); N (27.91%), Fe (53.02%), and B (24.15%) in husks; and Fe (20.21%), Cu (25.64%), and B (24.98%) in leaves (
Table 2).
The coefficient of genetic variation (CVg) indicates how far the result is influenced by the genetics of a plant—i.e., the higher the coefficient is, the greater the genetic influence is. For all nutrients and evaluated organs, values > 1 were observed; in flowers, the lowest value was found for N (4.26%) and the highest for Fe (45.25%). In coffee beans, the lowest value was found for Fe (3.92%) and the highest for S (19.30%). For husk, the lowest percentage was 3.47% for nutrient S and the highest was 24.48% for Mn. For leaves, the CVg was lowest for P (1.88%) and highest for Fe (29.13%) (
Table 2).
The heritability index varied among nutrients and plant organs; values above 80 are considered optimal for selection based on heritability. In coffee flowers, H
2 ranged from 41.99% for nutrient P to 82.49% for Mg. For grains, the lowest value was found for Fe (25.97%) and the highest for Ca (89.20%), while the H
2 of other nutrients such as K, Mg, S, and Mn also exceeded 80%. In husk, the lowest value was observed for N (21.61%) and the highest for Mn (93.98%), while the values for P, K, and Mg exceeded 80%. In leaves, P had the lowest H
2 index (12.93%) and Mn the highest (95.48%). The values of K, Ca, Mg, and Fe exceeded 80% (
Table 2).
3.2. Groupings of Genotypes and Genetic Contribution
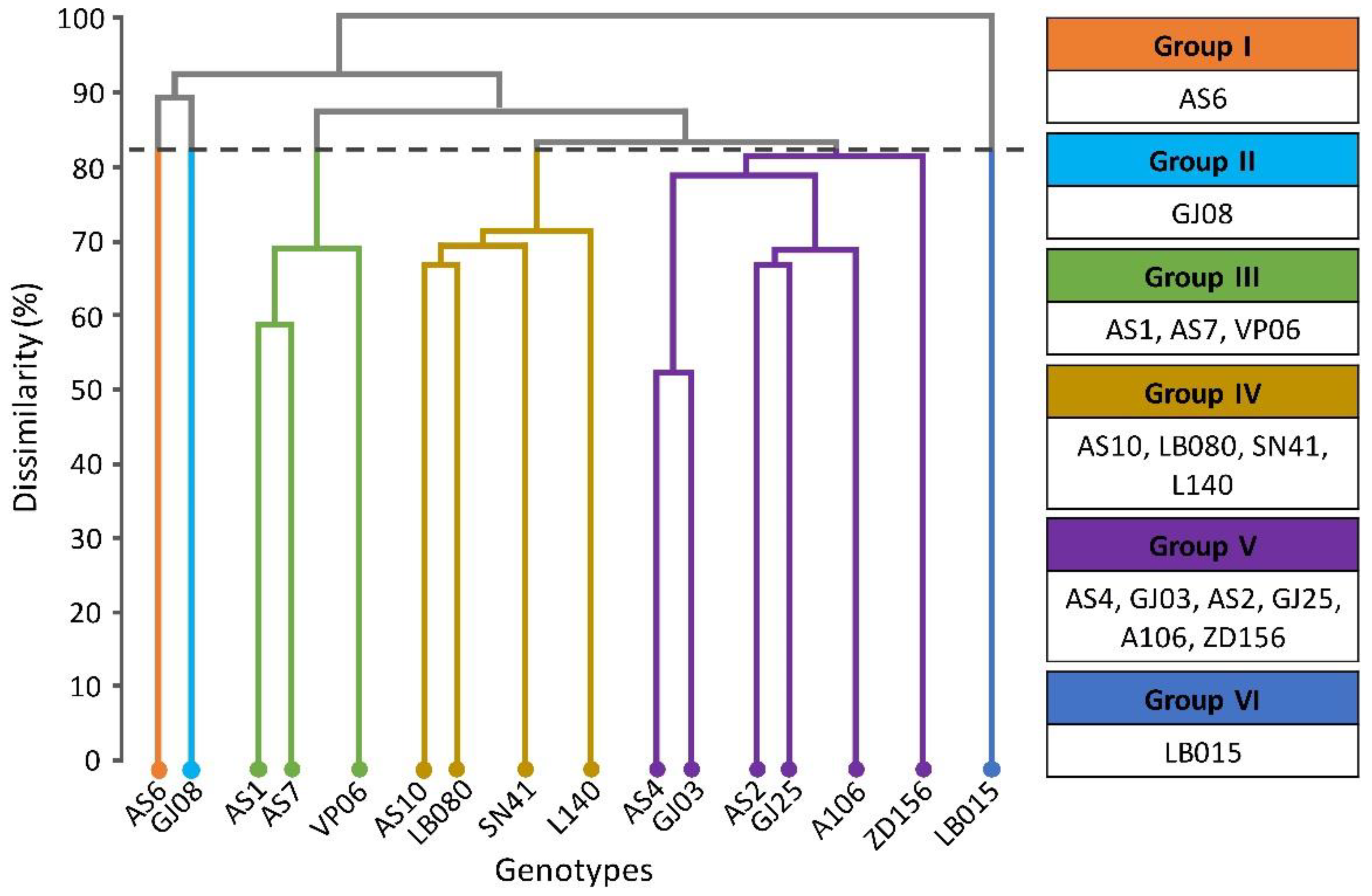
With the UPGMA hierarchical method, using the Euclidean distance as a measure of dissimilarity and based on the nutrient concentrations in the flowers, grains, husks and leaves, the genotypes were grouped according to the genetic distances among them. At a cut-off level of 82%, six distinct groups were observed (
Figure 1). Groups I, II, and VI contained only one genotype (AS6, GJ08, and LB015, respectively), and Group III contained the genotypes AS1, AS7, and VP06. Group IV consisted of AS10, LB080, SN41, and L140 and group V was the most populous, with six genotypes (AS4, GJ03, AS2, GJ25, A106, and ZD156) (
Figure 1).
Subtle differences between the clustering methods were observed. Tocher’s method divided the genotypes into seven groups, of which four contained only one genotype. Using the UPGMA method, genotype GJ25 was assigned to group V, and using the Tocher method it was assigned to group II. The UPGMA method grouped genotype ZD156 in V together with five others, and by the Tocher method it was classified alone in group V (
Table 3). Although not allocated to an isolated group by the UPGMA method, genotype ZD 156 was also considered to be divergent by this method due to its high degree of dissimilarity; it was not grouped separately only because of the maximum dissimilarity threshold (82%) adopted in the dendrogram.
To determine the relative contribution of macro- and micronutrient concentrations in different plant organs to the genetic diversity in 16
C. canephora genotypes, the method of Singh (1981) and mean Euclidean distance were used. A range of 69.93% to 0.12% was observed. The micronutrient Fe in leaves (69.93%) and flowers (21.47%) contributed most to the genetic diversity (
Table 4).
3.3. Nutrient Concentration in Flowers, Grain and Husk
The N, K, and S concentrations in the flowers of Robusta coffee were not influenced by the genotypes, which were all grouped together by the Scott–Knott test (
Table 5). For P and Ca, the genotype means were divided into two and for Mg into three dissimilarity groups, among which genotype GJ08 accounted for a group of its own. Genotype GJ25 was allocated to the group with the highest means for all macronutrients (
Table 5).
The micronutrients Fe, Zn, Mn, and B were divided into two mean groups. The genotypes AS1, L140, and LB080 were practically always in the group with the highest micronutrient means. Micronutrient Cu was divided into three mean groups, of which genotype GJ08 represented a group of its own (
Table 5).
For macronutrient P in the Robusta coffee beans, the genotypes had no influence on the nutrient concentration and were all grouped together by the Scott–Knott test (
Table 6). For Mg, the genotypes were divided into two groups, where SN41, GJ08, and LB015 were isolated in the 2nd group, while for nitrogen two groups were formed as well, although with more balanced compositions. The nutrients K, Ca, and S were clustered in three groups of means. For K, genotype ZD156 had the highest mean and was allocated alone in the first group, while for Ca and S, genotype VP06 was grouped together with the genotypes with the highest means (
Table 6).
The Fe and B concentrations in Robusta coffee grain did not differ among the genotypes, which were all clustered together. For the Zn and Cu concentrations, two mean groups were formed and three were formed for Mn. Genotype VP06 had the highest mean for Mn and was grouped separately, but was assigned to the groups with the highest means along with A106 for the other micronutrients (
Table 6).
The N and S concentrations in the husk of Robusta coffee beans were not influenced by the genotypes, which were all allocated together by the Scott–Knott test (
Table 6). The variation in means was greatest for nutrient P, for which the genotypes were separated in four groups, and genotype VP06 had the lowest observed mean (0.90). The macronutrients K and Ca formed two mean groups and for Mg the genotypes were divided into three groups. Genotype AS10 was assigned to the group with the highest means for practically all macronutrients.
The Fe concentration in the husk of Robusta coffee grain was not influenced by the genotypes, resulting in a single group according to the Scott–Knott test (
Table 6). Zn, Cu, and B were divided into two mean groups, and for these nutrients the genotypes AS2, GJ25, and LB015 were grouped similarly in the groups with the highest means. The genotype means of micronutrient Mn were the most irregular, forming five groups. The first group consisted of genotype VP06; the second of the genotypes AS6 and AS4; the third of the genotypes AS1, LB015, and GJ03; the fourth of the genotypes AS2, GJ25, and ZD156; and the fifth of the genotypes A106, AS7, SN41, AS10, L140, GJ08, and LB080 (
Table 6).
3.4. Nutrient Concentrations in Coffee Leaves in Two Sampling Periods
The nutrient concentrations in the leaves of the genotypes at pre-flowering and grain filling were compared. For N and P, there was no difference between genotypes in the two evaluation periods (
Table 7). For nutrient K in pre-flowering, the genotypes VP06, AS1, AS7, SN41, ZD156, AS4, L140, GJ08, and GJ03 were grouped together and A106, AS2, GJ25, AS6, AS10, LB080, and LB015 were grouped together in the second group of means. However, four mean groups were observed for this nutrient in the second evaluation during the grain-filling period—i.e., genotypes AS1 and L140 in the first; VP06 alone in the second; AS2, GJ25, AS7, ZD156, AS10, GJ08, LB080, and GJ03 in the third; and A106, SN41, AS6, AS4, and LB015 in the fourth (
Table 7).
For nutrient Ca, an irregular concentration among the genotypes and between the evaluation periods was observed. In the pre-flowering period, the genotypes were grouped into four mean groups: the first with genotypes L140 and LB080; the second with genotypes AS2, GJ25, and LB015; the third with most of the genotypes—namely, A106, AS1, SN41, AS6, ZD156, AS10, AS4, GJ08, and GJ03; and the fourth with the genotypes VP06 and AS7. For the grain-filling period, however, the genotypes were grouped into three mean groups. The first comprised the genotypes SN41, AS6, AS4, LB080, LB015, and GJ03; the second comprised the genotypes GJ25, ZD156, AS10, L140, and GJ08; and the third comprised the genotypes A106, AS2, VP06, AS1, and AS7 (
Table 7).
For Mg in the pre-flowering period, the genotypes were grouped into three groups. The first clustered genotypes AS2 and LB080; the second clustered genotypes A106, GJ25, L140, and LB015; and the third clustered the genotypes with the lowest means—i.e., VP06, AS1, AS7, SN41, AS6, ZD156, AS10, AS4, GJ08, and GJ03. In the grain-filling period, the 16 genotypes were also divided into three groups, but differently. The first group consisted of genotypes SN41, AS6, AS4, and LB015, the second of genotypes AS2, GJ25, AS1, ZD156, AS10, GJ08, LB080, and GJ03; and the third of genotypes A106, VP06, AS7, and L140 (
Table 7).
For nutrient S in the pre-flowering period, the 16 genotypes were divided into two mean groups. The first group clustered genotypes AS2, AS6, ZD156, AS10, AS4, and GJ08 and the second clustered genotypes A106, GJ25, VP06, AS1, AS7, SN41, L140, LB080, LB015, and GJ03. For the grain-filling period, the genotypes were also grouped into two mean groups; however, genotype GJ03 with the highest mean was grouped alone in the first group and the others without statistical difference in the second (
Table 7).
An evaluation of the interaction of genotypes with nutrients showed that the means of genotype GJ08 were equal in all periods and for all nutrients. Genotype LB015 differed only for N, where the mean during the pre-flowering period was higher than that in the grain-filling period. On the other hand, genotype ZD156 differed only for nutrient S; during the pre-flowering period, it had a higher concentration than in the grain-filling period (
Table 7). The genotypes A106, AS2, GJ25, AS1, L140, and LB080 had lower means of Ca and Mg in the grain-filling period. For nutrient N, the means of the genotypes AS6, LB015, and GJ03 were irregular, with the highest mean occurring in the grain-filling period.
The performance pattern of the micronutrients differed from that of the macronutrients. For Fe during pre-flowering, the genotypes were grouped into two clusters with similar means within the groups, while for the grain-filling period this pattern was different and the 16 genotypes were divided into four groups. The genotypes LB015 and GJ03 were assigned to the group with the highest means in both physiological plant stages. For the pre-flowering stage, micronutrient Zn was divided into two groups. Similarly, for grain-filling the genotypes were divided into two groups, but differently. Genotype A106 was always in the group with the highest means in both tested phenological stages of the coffee tree (
Table 8).
For both phases, the Cu concentrations were separated into three mean groups. Genotypes AS6 and ZD156s were assigned to the group with the highest means during pre-flowering. During grain-filling, the only genotype with a higher mean was LB015. For Mn, the 16 genotypes were divided into four mean groups for the pre-flowering stage and three for the grain-filling stage. The LB015 genotype presented the highest average in both phenological stages and was grouped separately (
Table 8).
For B concentrations during pre-flowering and grain-filling, the genotypes were divided into three groups in both stages, although the clusters had different structures. Genotypes GJ25 and VP06 were allocated in the group with the highest mean in both stages; however, in the grain-filling period, another 13 genotypes had similar means (
Table 8). The means of genotypes AS1, AS7, and LB080 were not influenced by the evaluation period for any micronutrient. In turn, at least four of the five micronutrient concentrations in genotypes GJ08, SN41, and LB015 were not influenced by the assessment period (
Table 8).
3.5. Correlation between Nutrient Concentrations of Flowers, Grain, Husk, and Leaves during Pre-Flowering and during Grain-Filling
Using Spearman’s correlation coefficients for macro- and micronutrient concentrations in the flowers, grains, husks, and leaves of 16 genotypes, a total of 50 significant correlations were detected, 7 of which were negative. Positive and significant correlations were confirmed for the relationships Flower × Grain for the nutrients K, Ca, and Mg and micronutrients Cu, Mn, and B. For the relationship Flower × Husk, significance was only observed for K. For the correlation between Flower × Leaf in the pre-flowering period, positive correlations were observed for macronutrient Mg and for the micronutrients Fe and Mn. The correlation Flower × Leaf in the grain-filling period was only positive for the micronutrients Fe and Zn, and the correlation Flower × Leaf was only positive in both periods for Fe (
Table 9).
For the relationships of grain with the other plant parts, only husk had a strong and positive correlation for the macronutrients P, K, and Ca and for the micronutrient Mn. For the relationships of husk with the other organs, only Husk × Leaf had a weak positive correlation for nutrient P during grain-filling, and only Husk × Leaf had a weak positive correlation for micronutrient Cu in both periods (
Table 9).
When correlating the two leaf sampling periods (leaf in pre-flowering × leaf in grain-filling), positive correlations were observed for nutrients K and Fe only. However, when correlating pre-flowering and grain-filling with the mean of the two periods, positive correlations were found for all nutrients (
Table 9).
Negative correlations were observed between Flower × Husk for the micronutrient Fe and Flower × Leaf in the pre-flowering period for N. Negative correlations with grain were observed for Grain × Leaf in the grain-filling period for Mg and Cu. For Fe, negative values were observed for all correlations of husk with the other organs (
Table 9).