1. Introduction
Most walnut plants produced worldwide arise from grafting under seedling rootstocks, with the associated genetic variability derived from their seed origin. Given the limited ability to develop adventitious roots, characteristic of the
Juglans genotypes [
1], clonal walnut propagation is a long-pursued goal with scarce results so far. Attempts to overcome this recalcitrant behavior were made by using stool layering [
2] and hardwood or softwood cuttings [
3,
4]. In fact, while these are the most traditional and cheapest systems for walnut cloning, by no means are they used for large-scale propagation. Better results were attained using in vitro propagation protocols [
5,
6,
7,
8,
9,
10,
11], which have allowed for the commercial-scale production of clonal rootstocks in some laboratories around the world. Even with the availability of clonal rootstocks, the production of grafted walnut plants by traditional grafting methods is a long, seasonal, and time-consuming process [
12,
13,
14,
15].
All the above-mentioned problems can be minimized using micrografting. Rehman and Gill [
16] defined micrografting as the placement of a maintained scion onto an in vitro grown rootstock, under aseptic conditions. In turn, Hartmann et al. [
17] had previously provided a broader definition, which encompasses procedures developed under in vitro or ex vitro conditions. In the first scenario, scion and rootstock are taken from plant material growing under aseptic conditions and grafted under the same conditions. In the second, grafting can be performed during the transition from in vitro to ex vitro, either during or after the acclimatization phase of the rootstock. The technique can be used to produce virus-free plants, as well as to study scion–rootstock interactions, long-distance signaling, and virus indexing, but its potential for large-scale plant propagation is frequently neglected [
18].
Murashige et al. [
19], Navarro et al. [
20], and Navarro and Juarez [
21] were pioneers of using micrografting for virus cleaning of citrus cultivars. More recently, Martínez-Gómez and Gradziel [
22] demonstrated the usefulness of the technique in an almond breeding program where micrografting was used to early evaluate the compatibility between different cultivars and rootstocks. Few reports demonstrate the applicability of this technique to large-scale plant propagation, but a noteworthy example is the work of Bandeira et al. [
23]. A survival rate of 87% for grafted plants was registered seventy days after grafting (DAG), using seedlings of
Eucalyptus grandis and
Eucalyptus urophylla germinated in vitro as rootstocks and shoot apices of two
E. urophylla × E. grandis micropropagated clones as scions.
Concerning micrografting applied to
Juglans sp., two references could be found in the literature. The first one refers to the work of Wang et al. [
24], who used seedling rootstocks and grafted them ex vitro using scions from three walnut (
J. regia) cultivars produced in vitro. Parameters such as scion genotype, seedling age, and the use of plant growth regulators were evaluated. Ninety percent of grafting survival was achieved with the best combination of factors.
The second one refers to the work of Liu et al. [
25], who used in vitro micrografting to evaluate possible mRNA transfer between the two grafted bionts. An autografting was performed using in vitro cultured ‘Paradox’ explants, which resulted from the evolution of somatic embryos. Only the initial stages of grafting were followed, with callus production and grafting consolidation being recorded on 95% of the grafted explants. Neither rooting nor acclimatization was the goal of this work, and no data on these items were provided.
The micrografting technique here presented was used for the first time in in vitro grown walnut explants of adult origin for both scions and rootstocks. A cleft graft, being made on the transition from in vitro to ex vitro conditions, before adventitious root development on the rootstock, was used and, to the best of our knowledge, never tested before. The presence of leaves on both the scion and the rootstock, and its effect on rooting and grafting success, was also analyzed and discussed.
2. Materials and Methods
2.1. Plant Materials
All scions (‘Chandler’) and rootstocks (‘Vlach’) used in the experiments were obtained from in vitro precultured materials. Initial explants were taken from stem cuttings collected in 5-year-old trees growing in a private walnut orchard in Estremoz (Portugal) and multiplied in vitro for at least 10 subcultures.
The rootstocks were prepared from the bottom part of in vitro cultured ‘Vlach’ shoots, approximately 30 to 40 mm high, and scions for cleft grafting were prepared from the upper part of cv. ‘Chandler’ shoots, 20 to 50 mm high.
2.2. Grafting Procedure
Micrografting was performed in a horizontal laminar flow cabinet. This is not a grafting requisite, because the procedure can be performed in nonaseptic conditions, but it was used to allow the aseptic preparation of uninodal microcuttings, needed to maintain the multiplication phase of the ‘Chandler’ cultivar, that were taken from the explants not selected for grafting (see schema of
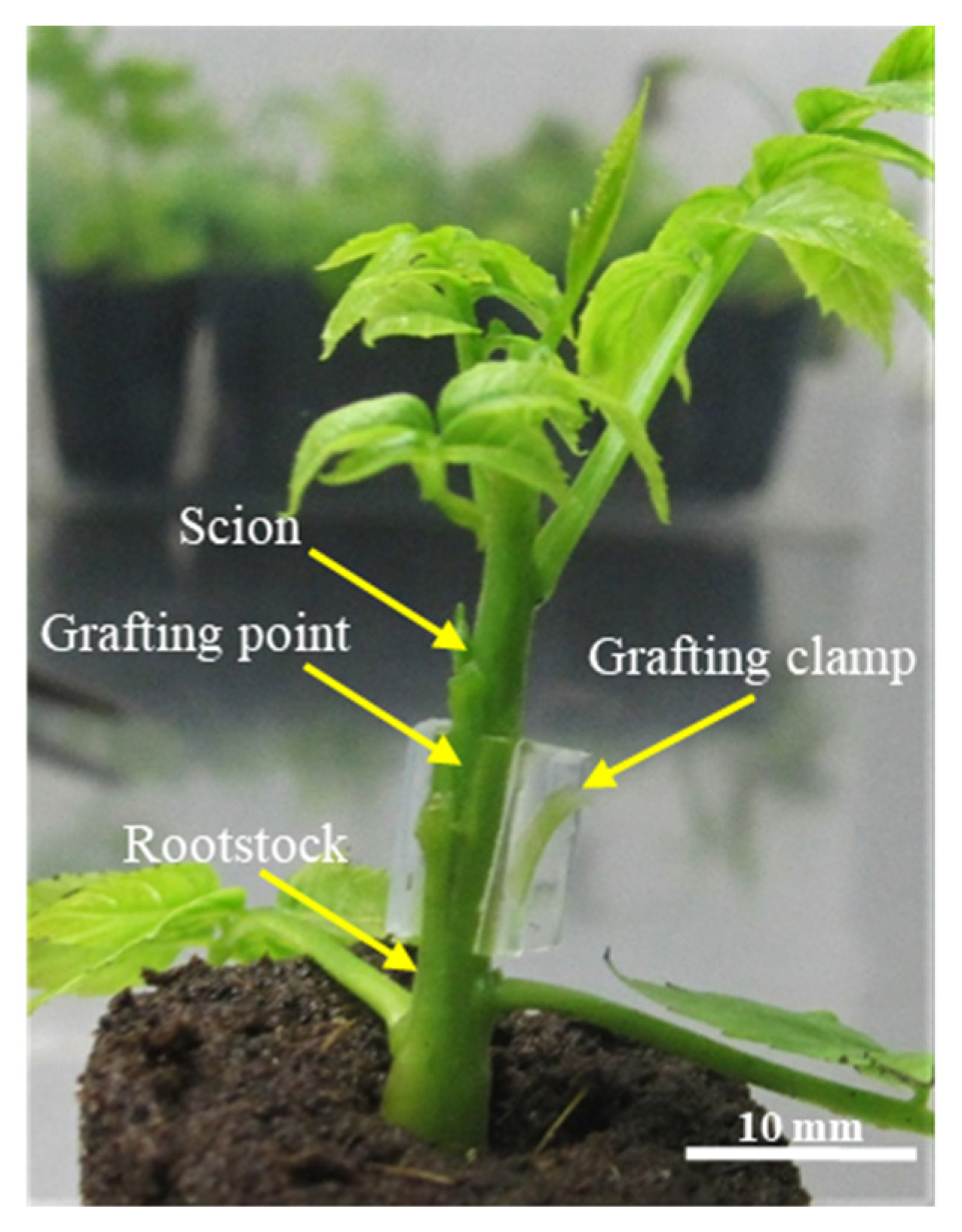
Figure 1). To prepare the scions, the basal portion of the explant was cut with a razor blade in a double long bevel shape. To prepare the rootstocks, a vertical incision was made on the top, to accommodate the scion. The graft was fixed with micrografting plastic clamps 2.5 mm in diameter (Brinkman model PT3). Explants used as scions and rootstocks were chosen with diameters as similar as possible (
Figure 1).
Depending on the treatment in use, fully expanded leaves on both bionts (two on the rootstock and two to three on the scion) were maintained or removed. For the grafting procedure, rootstocks were selected from explants from the in vitro root expression medium, while scions were prepared from explants grown in in vitro multiplication medium (see
Section 2.3 for details on media formulations).
2.3. Culture Media and Substrates
As previously stated, scions and rootstocks were obtained from explants growing in vitro conditions (
Figure 2), following the procedure previously proposed by Peixe et al. [
11], with slight modifications as described below.
Multiplication: uninodal explants were cultured in Driver and Kuniyuki medium (DKW) [
5] with 1.5× micronutrients and 96 mg L
−1 Fe-EDDHA, supplemented with 1 mg L
−1 BAP, 0.01 mg L
−1 IBA, 30 g L
−1 sucrose, and 200 mg L
−1 hydrolyzed casein and gelled with 2.5 g L
−1 Gellan Gum
®. The pH was adjusted to 5.5 before autoclaving.
Root induction: ‘Vlach’ explants collected during the multiplication phase were cultured in DKW medium [
5], with ½ macronutrients and 96 mg L
−1 Fe-EDDHA, added with 3 mg L
−1 IBA, 30 g L
−1 sucrose, and 100 mg L
−1 hydrolyzed casein and gelled with 2.5 g L
−1 Gellan Gum
®. The pH was adjusted to 5.5 before autoclaving.
Root expression,
grafting, and acclimatization: After 7 days in the root induction medium, the explants were cleft grafted and transferred to organic substrate for root expression, graft consolidation, and the beginning of the acclimatization phase. Irradiated Jiffy Preformas
® 30 mm in diameter were used as substrate on the first stage of this phase (
Figure 3A,B), while Jiffy Grow Blocks
® were used on the second stage of the acclimatization process (
Figure 3C,D) (see
Section 2.5 for details on the process phases).
2.4. Containers for Plant Multiplication, Root Induction, and Root Expression
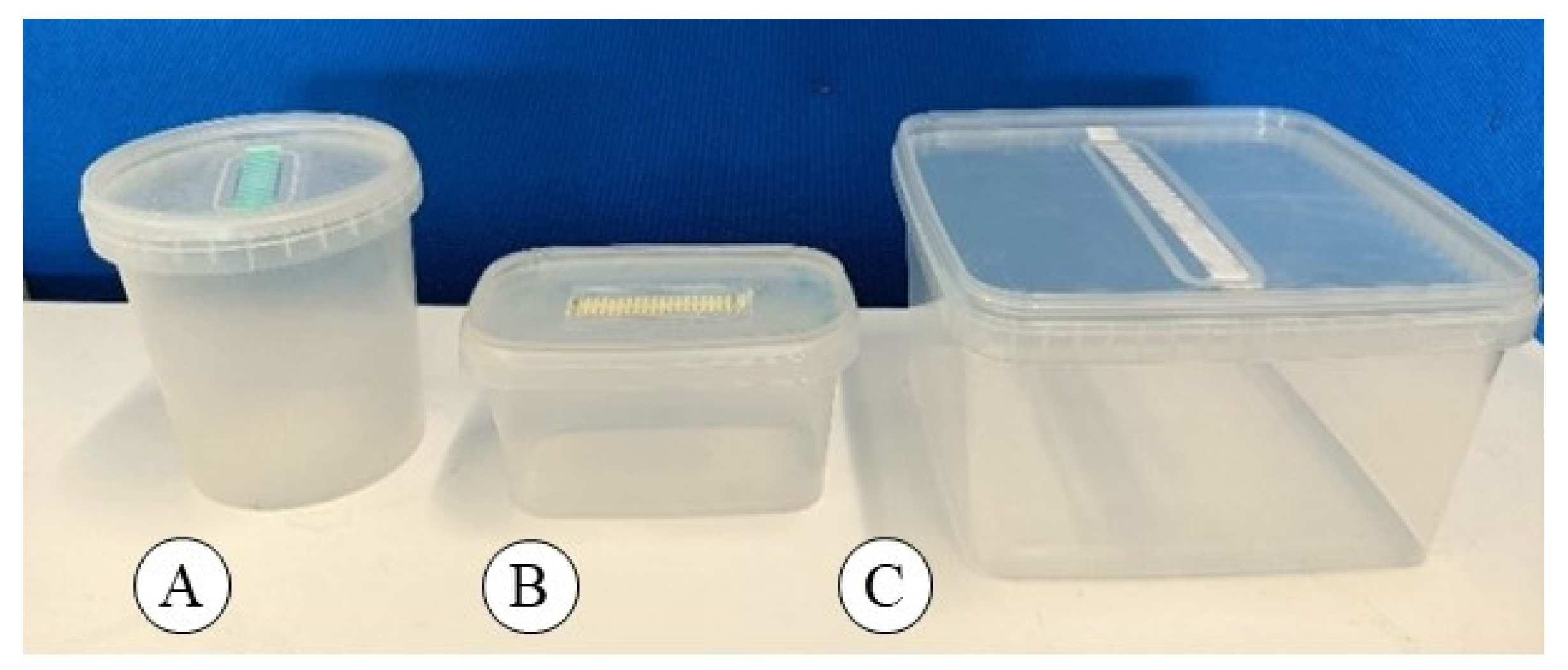
Polypropylene boxes, SacO
2® model O118/120 + OD118 (
Figure 4A), with 90 mL of culture medium and 9 explants/box, were used for the multiplication phase. For root induction, polypropylene boxes, SacO
2® model OV80 + OVD80 (
Figure 4B), were used, containing 80 mL of culture medium and 25 explants/box. For root expression and acclimatization, polypropylene boxes, SacO
2® model TP4000 + TPD4000 (
Figure 4C), containing 20 plants/box in acclimatization Stage 1 and 9 plants/box in Stage 2, were used. The number of plants/box from acclimatization Stage 1 to acclimatization Stage 2 was reduced from 20 to 9, due to the plant transfer from Jiffy Preformas
® to Jiffy Grow Blocks
® substrate.
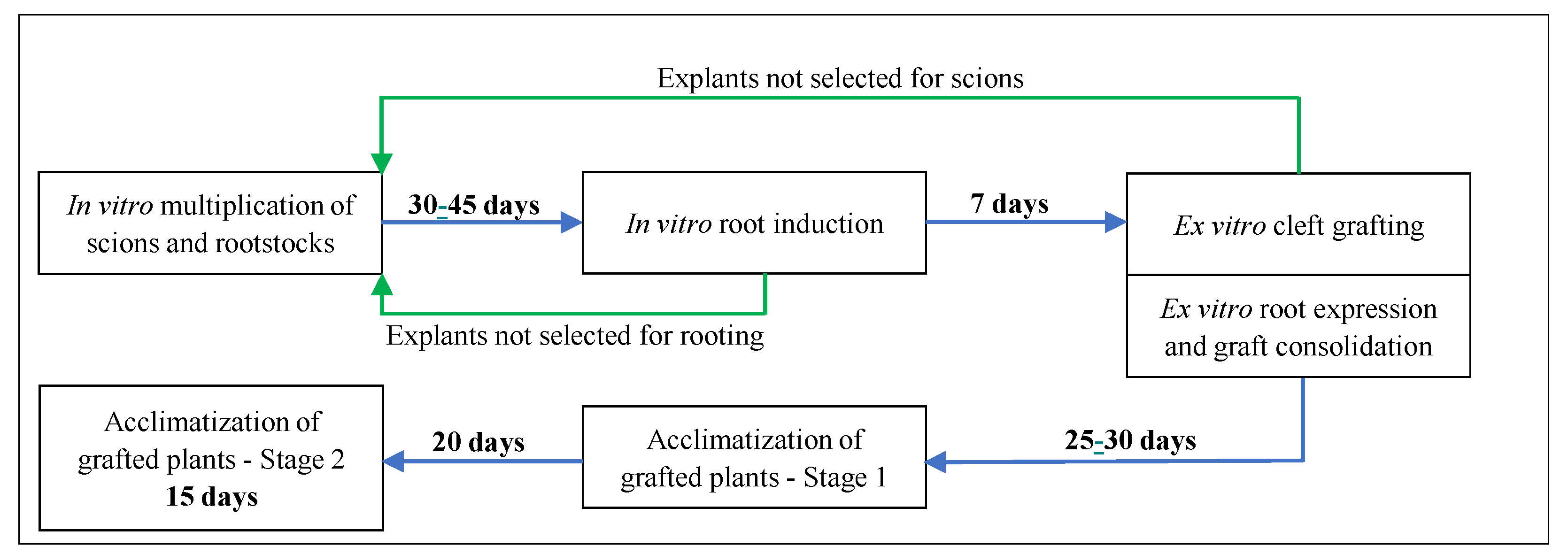
2.5. Process Phases and Experimental Conditions
The multiplication, root induction, root expression/graft consolidation phases, and the two stages of plant acclimatization were all accomplished in plant growth chambers according to the scheme presented in
Figure 5.
Multiplication phase (30–45 days): Accomplished in a growth chamber (Fitoclima 12000—Aralab®, Portugal), programmed with 24/22 °C day/night temperature for the rootstocks and 28/24 °C for the scions. A 16 h photoperiod and 80 µmol m−2 s−1 of light intensity, provided by Osram L fluorescent tubes 36W/840, were used for both explants.
Root induction phase (7 days): The selected ‘Vlach’ explants were transferred into another growth chamber (Fitoclima 750E—Aralab®, Portugal) and kept for 7 days in the dark under a constant temperature of 23 °C. Explants not developed enough to be used as rootstocks were fragmented into uninodal microcuttings, to maintain the multiplication process.
Root expression/graft consolidation phase (25–30 days): Selected explants were cleft grafted and placed in a growth chamber (Fitoclima 1200—Aralab®, Portugal), programmed for 28/26 °C day/night temperature, 16 h photoperiod, and 120 µmol m−2 s−1 of light intensity, provided by Osram L 36W/840 fluorescent tubes. ‘Chandler’ explants not used for scion production were also fragmented into uninodal microcuttings, to maintain the multiplication process. At the end of this phase, all explants with a consolidated graft union were kept in the same growth chamber to start the acclimatization process, which took place in two stages (see below).
Acclimatization process: Stage 1 (20 days): The plant containers remained closed with the original lid, and inner humidity was kept at approximately 100%. These conditions are similar to those used in the previous phase. Stage 2 (15 days): After this period, the lid was replaced by transparent adherent film, which was gradually and carefully removed. This was accomplished in another 15-day period, where the humidity was gradually lowered from 100% to 60%.
During the acclimatization phase, the growth chamber temperature was maintained at 28/26 °C day/night, the humidity at 60%, and the light intensity was increased to 240 µmol m−2 s−1 in Stage 1 and 400 µmol m−2 s−1 in Stage 2.
The overall process, from an in vitro uninodal explant until a fully acclimatized grafted plant that is ready for transfer into a greenhouse, takes approximately 90 days.
2.6. Experimental Design and Data Analysis
Two separate trials were developed. The first one aimed at testing the efficiency of the proposed methodology and evaluating the influence of the presence of leaves on the rootstock rooting ability and on the consolidation of the graft union. The goal of the second trial was to evaluate the reproducibility of the best treatment used during the first trial.
The four possible scion/rootstock combinations tested in the first trial are presented in
Table 1. Each combination was replicated six times with 10 plants, and the graft procedure was made by a different operator on each replicate.
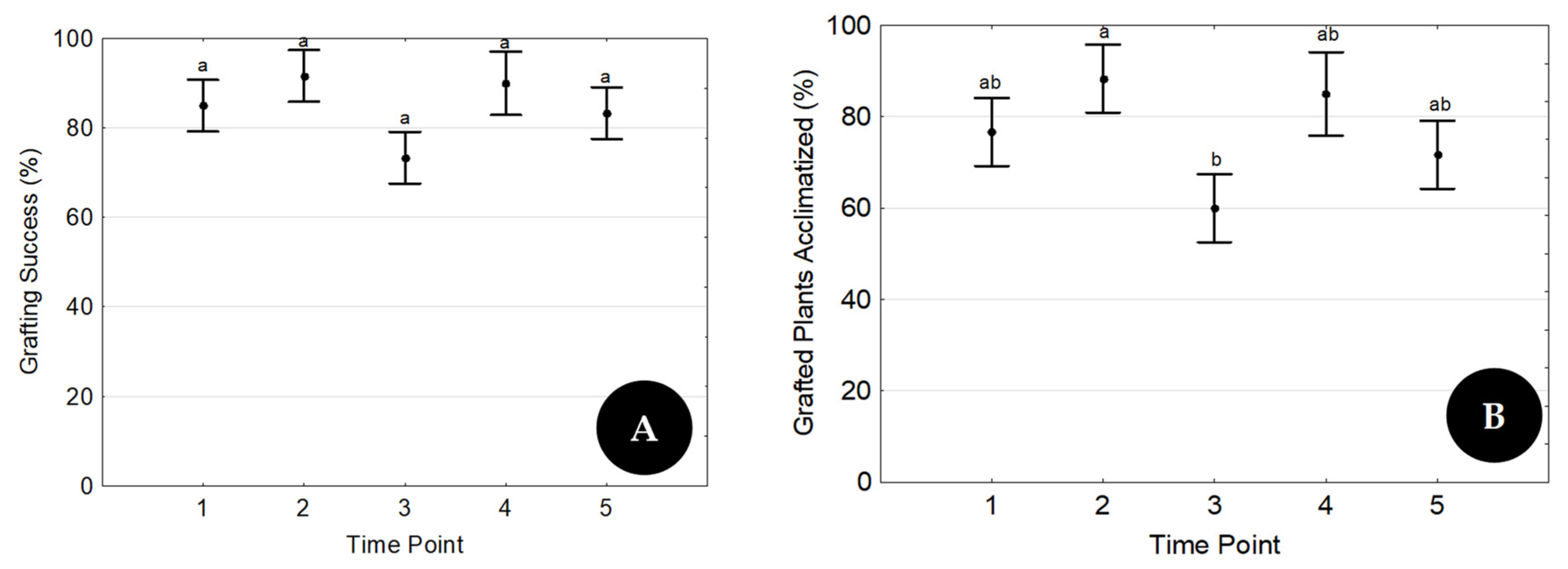
The treatment with the highest success rate in Trial 1 was repeated five times throughout the year (five time points), one each month starting in February, to evaluate its reproducibility (Trial 2). At each time point, 3 replicates of 20 grafts were prepared by three different operators.
Data analysis was performed using STATISTICA 12.0. Data concerning rooting, grafting success and final acclimatization of the grafted plants were recorded at 90 days after grafting (90 DAG). All data were tested for normality (by Shapiro–Wilk test) and submitted to analysis of variance (ANOVA), followed by Fisher’s (LSD) post hoc test. Significant differences were recorded for p ≤ 0.05. Values in percentage were transformed by arcsine of the square root before analysis.
4. Discussion
A micrografting procedure involving graft union consolidation and simultaneous rootstock rooting, using explants in transition from in vitro to ex vitro conditions, is presented. The trials followed an innovative protocol for ex vitro rooting and acclimatization of the walnut hybrid ‘Paradox’ cl. ’Vlach’, developed in 2015 by this research team [
11]. When compared with the protocol proposed by Jay-Allemand et al. [
6], where explants are rooted in vitro and transferred for acclimatization with bare roots, this protocol presents a completely different approach for the production of in vitro self-rooted walnut plants. It proposes ex vitro rooting after a seven-day root induction period, followed by explant transfer with balled roots throughout the subsequent plant production phases.
This protocol was reevaluated here not only for certifying its efficiency on ex vitro explant rooting but also for testing the possibility of simultaneous explant micrografting. The importance of the presence of leaves on scions and rootstocks to the rooting and grafting success rates was also evaluated.
Since the second half of the last century, when semi-hardwood and softwood cuttings became the standard for large-scale propagation of fruit species, it was noticed the importance of leaves on adventitious root formation [
17,
26,
27]. This effect became even more evident when in vitro cultured explants started to be used, because due to their nature and small size, the level of reserves at explant cutting-off is particularly low and usually a limiting factor [
17]. Leaves activate the movement of solutes and water, as well as plant hormones, through photosynthesis and transpiration. In such situations, the ability to photosynthesize during propagation seems to be the main governing factor of explant survival and growth [
27].
Scion origin, scion length, rootstock age, culture media, and graft method were referred to by [
16] as major factors affecting micrografting success, but, unlike with adventitious rooting, the presence of leaves as a key factor for grafting success is usually ignored. In both processes (rooting and grafting), the interruption of the plant vascular continuity results in the disruption of the molecular content of sugars, phytohormones, and other molecules that are transported through the vascular tissues [
27]. Young leaves and apical meristems are primary sites for auxin synthesis, and auxins are known as key regulators of cell proliferation and differentiation, activation of cambium proliferation, and regulation of vascular differentiation [
28,
29]. Those are morphological events fundamental for adventitious root development and graft union consolidation, thus justifying the importance of testing the presence of leaves to the rooting and graft consolidation rates under the conditions here evaluated.
Another drawback of the research conducted on micrografting processes refers to the use of seedling rootstocks. In fruit trees micrografting most authors used seedling rootstocks produced in vivo: Abousalim and Mantell [
30] and Onay et al. [
31] with pistachio, Fengtong et al. [
32] with mulberry or in vitro, Yildirim et al. [
33,
34] with almond, Naz et al. [
35] with citrus, and Hassanen [
36] with pear.
As previously referred, micrografting was only tested as a vegetative propagation tool in walnut by Wang et al. [
24], also using seedling rootstocks. The use of seedling rootstocks is not an issue when micrografting aims at producing virus-free plants or testing long-distance signaling. However, if the aim is to be an alternative tool for vegetative propagation, then the use of clonal scions and rootstocks is mandatory.
Even so, those experiments proved that micrografting is possible in vitro, as well as ex vitro, for several fruit species, including walnut. Data now presented confirmed this hypothesis also when in vitro grown explants of adult origin are used as rootstocks and scions.
The similarity between the work of Wang et al. [
24] and the work presented here lies in the scion’s origin and the grafting method. In both cases, scions were prepared from in vitro growing shoots, 25 to 50 mm long, cut in a double long bevel shape and the cleft grafted on the rootstock. Wang et al. [
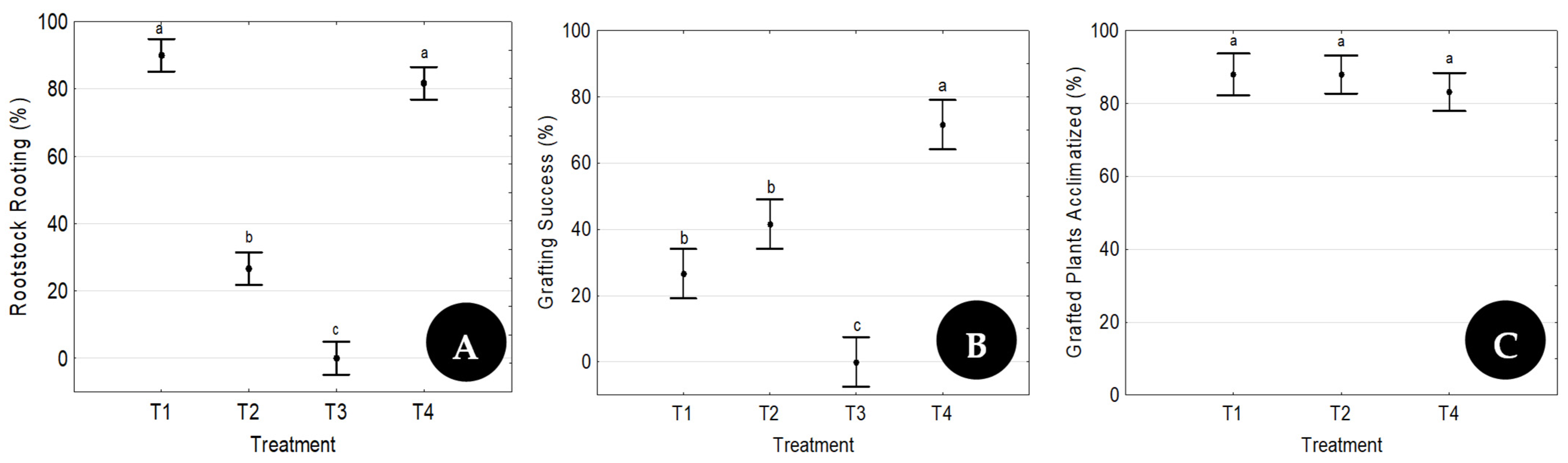
24] evaluated parameters such as the scion genotype, the seedling age, and the use of plant growth regulators and reported a grafting survival rate of 90% as the best combination of factors. In the present research, with the best factor combination (T4), a grafting survival rate of 70% was achieved in Trial 1. Similar values to those attained by Wang and coworkers (90%) were also observed in two time points of Trial 2, whose goal was to evaluate the reproducibility of the technique.
Unlike most micrografting reports, where values of grafted plant acclimatization are omitted, Wang et al. [
24] reported an acclimatization success rate of 80% for the best factor combination. Here, an 84% of plant acclimatization success for the best factor combination was attained. The data here presented and reported by Wang et al. [
24] are very good, because, as stated by Payghamzadeh and Kazemitabar [
37], it is known that acclimatization is one of the most sensitive steps of in vitro walnut plant production.
Difficulties in acclimatation of in vitro produced walnut plantlets are related to the morphological characteristics of leaves, such as the lower thickness of the epidermis, higher density of stomas per unit of leaf area, and greater stomatal opening [
38]. All those factors lead to high transpiration rates and increased explant susceptibility to leaf dehydration, which can only be efficiently controlled by using plant growth chambers. The high success rate of acclimatized grafted plants shown here (
Figure 6C and
Figure 7B) proves that environmental conditions were successfully controlled.
Overall, the data presented confirmed the efficacy of the ex vitro rooting procedure proposed by Peixe et al. [
11] and proved that the presence of leaves in both explants is a key factor for good rooting and grafting results. The reproduction of the best treatment at five time points throughout the year, always with constant results, proved the reproducibility of the proposed rooting/micrografting methodology.
5. Conclusions
An innovative approach on the use of ex vitro rooting and micrografting was presented, based on Juglans sp. adult plant material multiplied in vitro.
The data attained here allowed validation of the protocol as an alternative to produce self-rooted and grafted walnut plants, because even the plants where the graft failed but the rootstock rooted successfully can be sold as self-rooted clonal rootstocks. Plants ready for orchard planting can be obtained in less than one year, significantly reducing the time required when using traditional rooting and grafting procedures (2–3 years).
Concerning the micrografting technique implementation, it was found that one of the determining aspects was the speed of execution to avoid dehydration of the in vitro produced explants. Thus, grafting mechanization must be considered in future research, by testing for instance devices for softwood grafting, already used in horticultural species such as tomato or watermelon. In addition, rigorous control of the environmental conditions during acclimatization (which is dependent on the use of plant growth chambers) has also proved to be a determining factor for the success of the technique.
Choosing simultaneous rooting and grafting at the time of explant transfer from in vitro to ex vitro conditions may not seem the most evident choice. Grafting over in vitro rooted rootstocks would probably be more consensual. Nevertheless, throughout the in vitro explant production process this is the time point when scion and rootstocks explants can be found with similar diameters, an extremely important factor when cleft grafting is used.