Cereal and Pulse Crops with Improved Resistance to Pratylenchus thornei Are Needed to Maximize Wheat Production and Expand Crop Sequence Options
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Site
2.2. Field Experiment and Design
2.3. Soil Sampling
2.4. Nematode Extraction and Soil Water
2.5. Statistical Analysis
3. Results
3.1. Grain Yield of the Phase 1 Crop Cultivars
3.2. Nematode Population Densities and Soil Water before Sowing the Phase 2 Wheat Cultivars
3.3. Biomass of the Phase 2 Wheat
3.4. Regression Analysis
4. Discussion
4.1. Crop Rotation to Manage P. thornei
4.2. Growth of Phase 1 Crops and Residual P. thornei Population Densities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castillo, P.; Vovlas, N. Pratylenchus (Nematoda: Pratylenchidae): Diagnosis, biology, pathogenicity and management. In Biologyy and Ecology of Pratylenchus, Nematology Monographs and Perspectives 6; Series 9; Hunt, D.J., Perry, R.N., Eds.; Brill: Leiden, The Netherlands; Boston, MA, USA, 2007. [Google Scholar]
- Thompson, J.P.; Clewett, T.G.; O’Reilly, M.M. Temperature response of root-lesion nematode (Pratylenchus thornei) reproduction on wheat cultivars has implications for resistance screening and wheat production. Ann. Appl. Biol. 2015, 167, 1–10. [Google Scholar] [CrossRef]
- Talavera, M.; Vanstone, V.A. Monitoring Pratylenchus thornei densities in soil and roots under resistant (Triticum turgidum durum) and susceptible (Triticum aestivum) wheat cultivars. Phytoparasitica 2001, 29, 29–35. [Google Scholar] [CrossRef]
- Whish, J.P.M.; Thompson, J.P.; Clewett, T.G.; Wood, J.; Rostad, H.E. Predicting the slow decline of root lesion nematodes (Pratylenchus thornei) during host-free fallows to improve farm management decisions. Europ. J. Agron. 2017, 91, 44–53. [Google Scholar] [CrossRef]
- Thompson, J.P.; Mackenzie, J.; Sheedy, G.H. Root-lesion nematode (Pratylenchus thornei) reduces nutrient response, biomass and yield of wheat in sorghum-fallow-wheat cropping systems in a subtropical environment. Field Crops Res. 2012, 137, 126–140. [Google Scholar] [CrossRef]
- Whish, J.P.M.; Thompson, J.P.; Clewett, T.G.; Lawrence, J.L.; Wood, J. Pratylenchus thornei populations reduce water uptake in intolerant wheat cultivars. Field Crops Res. 2014, 161, 1–10. [Google Scholar] [CrossRef]
- Owen, K.J.; Clewett, T.G.; Bell, K.B.; Thompson, J.P. Wheat biomass and yield increased when populations of the root-lesion nematode (Pratylenchus thornei) were reduced through sequential rotation with partially resistant winter and summer crops. Crop Pasture Sci. 2014, 65, 227–241. [Google Scholar] [CrossRef]
- Thompson, J.P.; Clewett, T.G. Impacts of root-lesion nematode (Pratylenchus thornei) on plant nutrition, biomass, grain yield and yield components of susceptible/intolerant wheat cultivars determined by nematicide applications. Agronomy 2021, 11, 296. [Google Scholar] [CrossRef]
- Owen, K.J.; Fanning, J.P.; Reeves, K.L.; Hollaway, G.J. Consistent responses of yield and resistance of wheat cultivars to the root-lesion nematode, Pratylenchus thornei, in the Australian northern subtropical region, but not in the temperate southern region. Plant Pathol. 2021, 70, 1790–1806. [Google Scholar] [CrossRef]
- Thompson, J.P.; Clewett, T.G.; Sheedy, J.G.; Reen, R.A.; O’Reilly, M.M.; Bell, K.L. Occurrence of root-lesion nematodes (Pratylenchus thornei and P. neglectus) and stunt nematode (Merlinius brevidens) in the northern grain region of Australia. Australas. Plant Pathol. 2010, 39, 254–264. [Google Scholar] [CrossRef]
- Starr, L.J.; Bridge, J.; Cook, R. Resistance to plant-parasitic nematodes: History, current use and future potential. In Plant Resistance to Parasitic nematodes; Starr, J.L., Cook, R., Bridge, J., Eds.; CABI Publishing: Wallingford, UK, 2002; pp. 1–22. [Google Scholar]
- Roberts, P.A. Concepts and consequences of resistance. In Plant Resistance to Parasitic nematodes; Starr, J.L., Cook, R., Bridge, J., Eds.; CABI Publishing: Wallingford, UK, 2002; pp. 23–41. [Google Scholar]
- GRDC. 2022 Queensland Wheat Varieties Guide; Grains Research and Development: Kingston, ACT, Australia, 2021; ISSN 2652-3590. [Google Scholar]
- Thompson, J.P.; Sheedy, J.G.; Robinson, N.A.; Clewett, T.G. Tolerance of wheat (Triticum aestivum) genotypes to root-lesion nematode (Pratylenchus thornei) in the subtropical grain region of eastern Australia. Euphytica 2021, 217, 48. [Google Scholar] [CrossRef]
- Thompson, J.P.; Sheedy, J.G.; Robsinson, N.A. Resistance of wheat genotypes to root-lesion nematode (Pratylenchus thornei) can be used to predict final nematode population densities, crop greenness, and grain yield in the field. Phtyopathology 2020, 110, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Murray, G.M.; Brennan, J.P. Estimating disease losses to the Australian wheat industry. Australas. Plant Pathol. 2009, 38, 558–570. [Google Scholar] [CrossRef]
- Murray, G.M.; Brennan, J.P. Estimating disease losses to the Australian barley industry. Australas. Plant Pathol. 2010, 39, 85–96. [Google Scholar] [CrossRef]
- Murray, G.M.; Brennan, J.P. The Current and Potential Costs from Diseases of Pulse Crops in Australia; Grains Research and Development Corporation: Kingston, ACT, Australia, 2012; ISBN 978-1-921779-19-0. [Google Scholar]
- Webb, A.A.; Grundy, M.L.; Powell, B.; Littleboy, M. The Australian subtropical cereal belt: Soils, climate and agriculture. In Sustainable Crop Production in the Sub-Tropics—An Australian Perspective; Clarke, A.L., Wylie, P.B., Eds.; Queensland Department of Primary Industries: Brisbane, Australia, 1997; pp. 8–23. [Google Scholar]
- Cox, H.W.; Kelly, R.M.; Strong, W.M. Pulse crops in rotation with cereals can be a profitable alternative to nitrogen fertiliser in central Queensland. Crop Pasture Sci. 2010, 61, 752–762. [Google Scholar] [CrossRef]
- Angus, J.F.; Kirkegaard, J.A.; Hunt, J.R.; Ryan, M.H.; Ohlander, L.; Peoples, M.B. Break crops and rotations for wheat. Crop Pasture Sci. 2015, 66, 523–552. [Google Scholar] [CrossRef]
- Thompson, J.P.; Brennan, P.S.; Clewett, T.G.; Sheedy, J.G.; Seymour, N.P. Progress in breeding wheat for tolerance and resistance to root-lesion nematode (Pratylenchus thornei). Australas. Plant Pathol. 1999, 28, 45–52. [Google Scholar] [CrossRef]
- Isbell, R.F. The Australian Soil Classification, Revised ed.; CSIRO Publishing: Melbourne, Australia, 1996. [Google Scholar]
- Beckmann, G.C.; Thompson, C.H. Soils and land use in the Kurrawa Area, Darling Downs, Queensland. In CSIRO Division of Soils, Soils and Land Use Series No. 37; CSIRO Publishing: Melbourne, Australia, 1960. [Google Scholar]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weeds Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Whitehead, A.G.; Hemming, J. A comparison of some quantitative methods of extracting small vermiform nematodes from the soil. Ann. App. Biol. 1965, 55, 25–38. [Google Scholar] [CrossRef]
- Fortuner, R. Pratylenchus thornei. C.I.H. Description of Plant-Parasitic Nematodes; Set 7; Commonwealth Institute of Helminthology: St Albans, UK, 1997; No. 93. [Google Scholar]
- Siddiqi, M.R. Merlinius brevidens. C.I.H. Description of Plant-Parasitic Nematodes, Set 1; Commonwealth Institute of Helminthology: St Albans, UK, 1972; No. 8. [Google Scholar]
- Reen, R.A.; Thompson, J.P.; Clewett, T.G.; Sheedy, J.G.; Bell, K.L. Yield response in chickpea cultivars and wheat following crop rotation affecting population densities of Pratylenchus thornei and arbuscular mycorrhizal fungi. Crop Pasture Sci. 2014, 65, 428–441. [Google Scholar] [CrossRef]
- VSN International. Genstat for Windows, 18th ed.; VSN International: Hemel Hempstead, UK, 2015; Available online: https://vsni.co.uk/software/genstat (accessed on 30 June 2017).
- Proctor, J.R.; Marks, C.F. Determination of normalizing transformations for nematode count data from soil samples and of efficient sampling schemes. Nematologica 1974, 20, 395–406. [Google Scholar] [CrossRef]
- Berry, D.A. Logarithmic transformations in ANOVA. Biometrics 1987, 43, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Van Gundy, S.D.; Jose Gustavo Perez, B.; Stolzy, L.H.; Thomason, I.J. A pest management approach to the control of Pratylenchus thornei on wheat in Mexico. J. Nematol. 1974, 6, 107–116. [Google Scholar] [PubMed]
- Bell, M.J.; Seymour, N.P.; Stirling, G.R.; Stirling, A.M.; Van Zwieten, L.; Vancov, T.; Sutton, G.; Moody, P. Impacts of management of soil biota in Vertosols supporting the broadacre grains industry in northern Australia. Aust. J. Soil Res. 2006, 44, 433–451. [Google Scholar] [CrossRef]
- Elliott, V.; Taylor, P.; Ford, R. Pathogenic variation within the 2009 Australian Ascochyta rabiei population and implications for future disease management strategy. Australas. Plant Pathol. 2011, 40, 568–574. [Google Scholar] [CrossRef]
- Reen, R.A.; Mumford, M.H.; Thompson, J.P. Novel sources of resistance to root-lesion nematode (Pratylenchus thornei) in a new collection of wild Cicer species (C. reticulatum and C. echinospermum) to improve resistance in cultivated chickpea (C. arietinum). Phytopathol 2019, 109, 1270–1279. [Google Scholar] [CrossRef]
- Hollaway, G.; Taylor, S.P.; Eastwood, R.F.; Hunt, C. Effect of field crops on density of Pratylenchus in South eastern Australia; Part 2: P. thornei. Supp. J. Nematol. 2000, 32, 600–608. [Google Scholar]
- Hollaway, G.J. Effect of oat (Avena sativa) on the population density of Pratylenchus thornei in the field Australas. Plant Pathol. 2002, 31, 147–149. [Google Scholar] [CrossRef]
- Thompson, J.P. Concurrent management of symbiotic VAM fungi and pathogenic root-lesion nematodes through crop rotation. In Soil Biota—Management in Sustainable Farming Systems; Pankhurst, C.E., Doube, B.M., Gupta, V.V.S.R., Grace, P.R., Eds.; CSIRO Publishing: Melbourne, Australia, 1994; pp. 119–120. [Google Scholar]
- Owen, K.J.; Clewett, T.G.; Thompson, J.P. Pre-cropping with canola decreased Pratylenchus thornei populations, arbuscular mycorrhizal fungi, and yield of wheat. Crop Pasture Sci. 2010, 61, 399–410. [Google Scholar] [CrossRef]
- Sheedy, J.G.; Clewett, T.G.; Thompson, J.P.; Paull, J. Resistance to root-lesion nematode of Australian faba bean cultivars. In Pratylenchus thornei. Plant Disease Management Reports; The American Phytopathological Society: St. Paul, MN, USA, 2008; Volume 3, p. N035. [Google Scholar]
- Paull, J.; (University of Adelaide, Adelaide, South Australia, Australia). Personal communication, 2017.
- Doyle, A.D.; McLeod, R.W.; Wong, P.T.W.; Hetherington, S.E.; Southwell, R.J. Evidence for the involvement of the root lesion nematode Pratylenchus thornei in wheat yield decline in northern New South Wales. Aust. J. Exp. Agric. 1987, 27, 563–570. [Google Scholar] [CrossRef]
- Smiley, R.W.; Sheedy, J.G.; Easley, S.A. Vertical distribution of Pratylenchus spp. in silt loam soil and Pacific Northwest dryland crops. Plant Dis. 2008, 92, 1662–1668. [Google Scholar] [CrossRef]
- Rahaman, M.M.; Zwart, R.S.; Rupasinghe, T.W.T.; Hayden, H.L.; Thompson, J.P. Metabolomic profiling of wheat genotypes resistant and susceptible to root-lesion nematode Pratylenchus thornei. Plant Mol. Biol. 2021, 106, 381–406. [Google Scholar] [CrossRef] [PubMed]
- Dalal, R.C.; Strong, W.M.; Weston, E.J.; Cooper, J.E.; Thomas, G.A. Prediction of grain protein in wheat and barley in a subtropical environment from available water and nitrogen in Vertisols at sowing. Aust. J. Exp. Agric. 1997, 37, 351–357. [Google Scholar] [CrossRef]
- Di Vito, M.; Zaccheo, G.; Catalano, F. Effect of Pratylenchus neglectus and P. thornei on the growth of faba bean. Nematol. Mediterr. 2000, 28, 261–265. [Google Scholar]
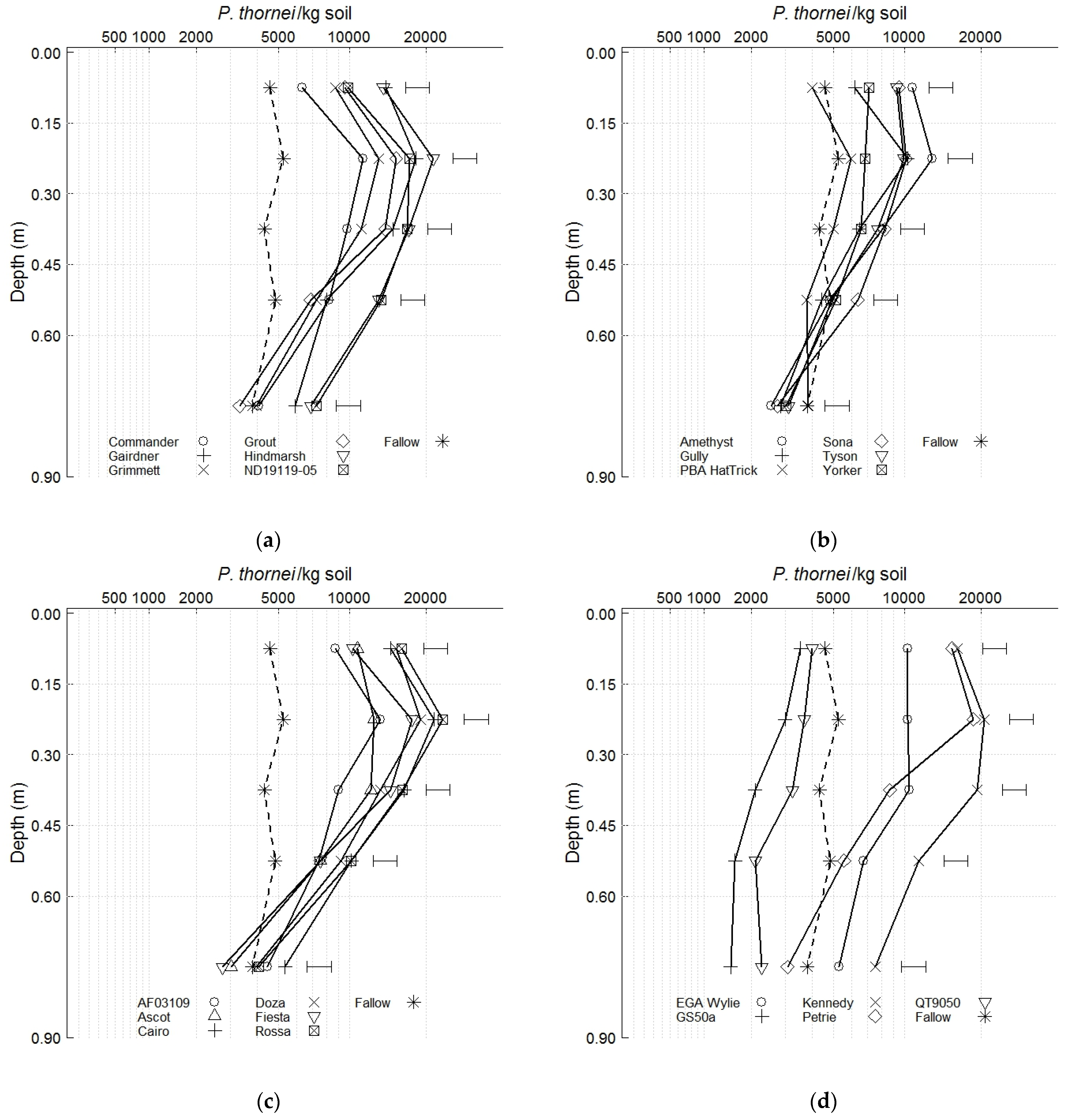
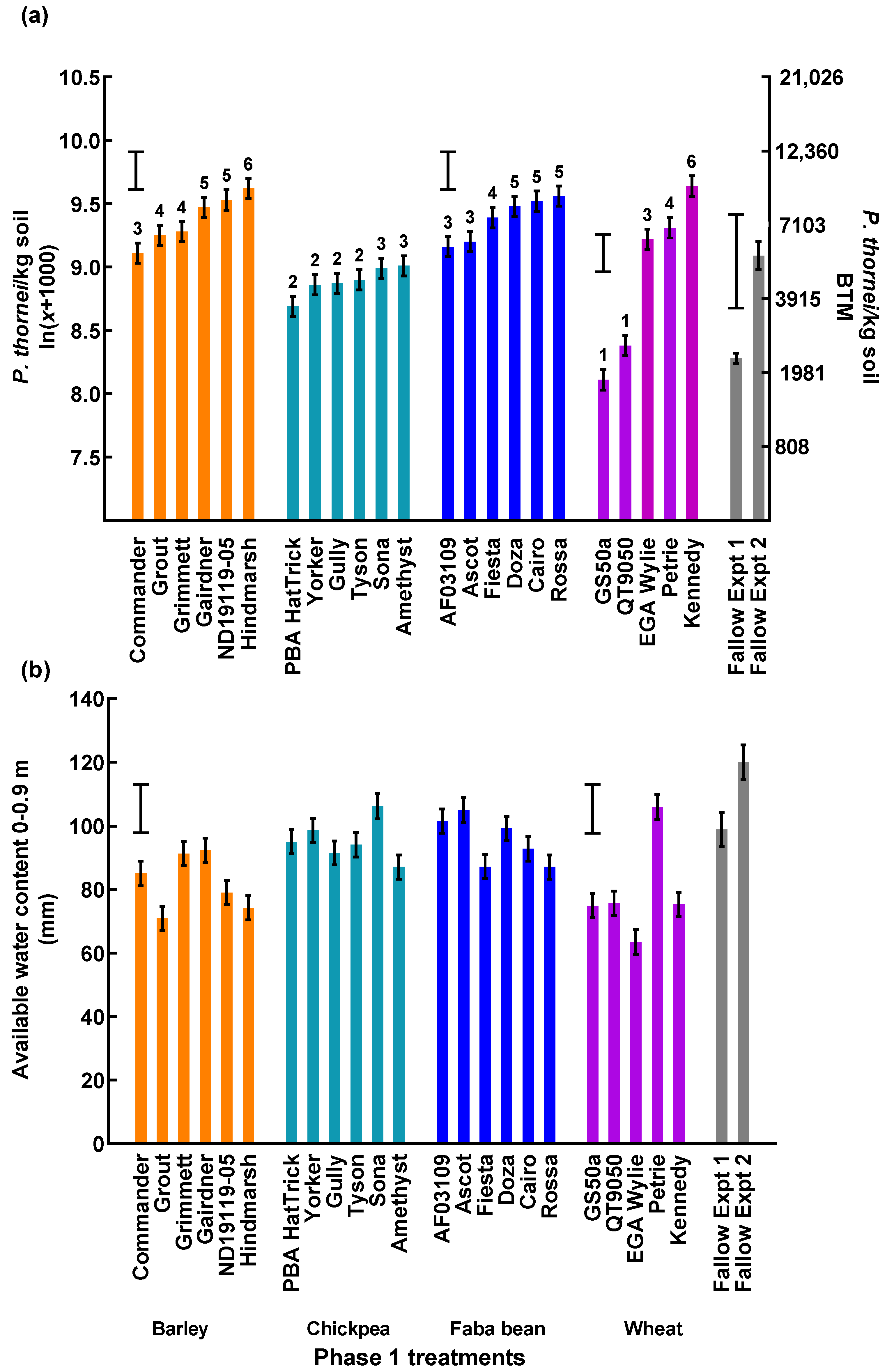

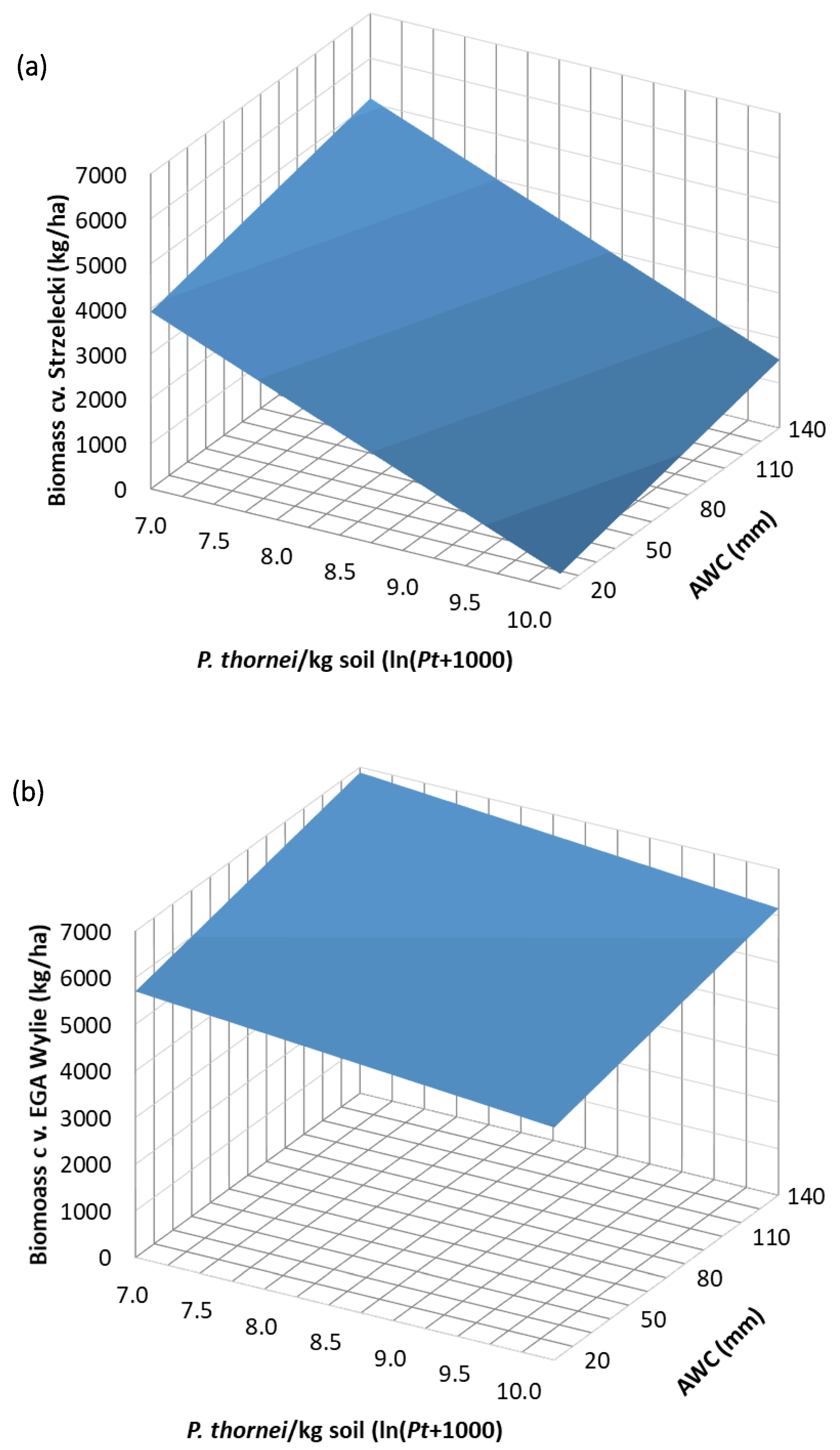
| Experiment 1 | Experiment 2 | |
|---|---|---|
| Previous cropping history 1 (most recent at top) | Sorghum (LF) Wheat (SF) Wheat (LF) Sorghum (LF) Wheat (SF) | Wheat (SF) Sorghum (LF) Wheat (SF) Wheat (LF) Sorghum (LF) |
| P. thornei/kg soil | 2490 | 8150 |
| Available water content (mm) | 110 | 98 |
| Crop | Phase 1 Cultivars |
|---|---|
| Barley (Hordeum vulgare) | Commander |
| Gairdner | |
| Grimmett | |
| Grout | |
| ND-19119-05a | |
| Hindmarsh | |
| Chickpea (Cicer arietinum) | Amethyst |
| Gully | |
| PBA HatTrick | |
| Sona | |
| Tyson | |
| Yorker | |
| Faba bean (Vicia faba) | AF03109 |
| Ascot | |
| Cairo | |
| Doza | |
| Fiesta | |
| Rossa | |
| Wheat (Triticum aestivum) | EGA Wylie |
| GS50a | |
| Kennedy | |
| Petrie | |
| QT9050 |
| Phase 1 | Phase 2 | |
|---|---|---|
| Sown | 10 June 2009 | 15 July 2010 |
| Biomass collection | ND 1 | 20 October 2010 |
| Grain harvest | November 2009 | ND 2 |
| Soil collection | May 2009 | May 2010 |
| 3 Annual rainfall (mm) | 373 | 885 |
| In-crop rainfall (mm) | 87 | 202 |
| Fallow rainfall (mm) (7 months) | 298 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owen, K.J.; Clewett, T.G.; Bell, K.L.; Thompson, J.P. Cereal and Pulse Crops with Improved Resistance to Pratylenchus thornei Are Needed to Maximize Wheat Production and Expand Crop Sequence Options. Agronomy 2022, 12, 573. https://doi.org/10.3390/agronomy12030573
Owen KJ, Clewett TG, Bell KL, Thompson JP. Cereal and Pulse Crops with Improved Resistance to Pratylenchus thornei Are Needed to Maximize Wheat Production and Expand Crop Sequence Options. Agronomy. 2022; 12(3):573. https://doi.org/10.3390/agronomy12030573
Chicago/Turabian StyleOwen, Kirsty J., Timothy G. Clewett, Kerry L. Bell, and John P. Thompson. 2022. "Cereal and Pulse Crops with Improved Resistance to Pratylenchus thornei Are Needed to Maximize Wheat Production and Expand Crop Sequence Options" Agronomy 12, no. 3: 573. https://doi.org/10.3390/agronomy12030573
APA StyleOwen, K. J., Clewett, T. G., Bell, K. L., & Thompson, J. P. (2022). Cereal and Pulse Crops with Improved Resistance to Pratylenchus thornei Are Needed to Maximize Wheat Production and Expand Crop Sequence Options. Agronomy, 12(3), 573. https://doi.org/10.3390/agronomy12030573






